Abstract
The aim of the study was to develop a method for fast and reliable diagnosis of peroxisomal diseases and to facilitate differential diagnosis of cholestatic hepatopathy. For the quantification of bile acids and their conjugates as well as C27 precursors di- and trihydroxycholestanoic acid (DHCA, THCA), in small pediatric blood samples we combined HPLC separation on a reverse-phase C18 column with ESI-MS/MS analysis in the negative ion mode. Analysis was done with good precision (CV 3,7%–11.1%) and sufficient sensitivity (LOQ: 11–91 nmol/L) without derivatization. Complete analysis of 17 free and conjugated bile acids from dried blood spots and 10 µL serum samples, respectively, was performed within 12 min. Measurement of conjugated primary bile acids plus DHCA and THCA as well as ursodeoxycholic acid was done in 4.5 min. In blood spots of healthy newborns, conjugated primary bile acids were found in the range of 0.01 to 2.01 µmol/L. Concentrations of C27 precursors were below the detection limit in normal controls. DHCA and THCA were specifically elevated in cases of peroxysomal defects and one Zellweger patient.
Synthesis of bile acids, their hepatic secretion, and intestinal reabsorption are part of a complex multistep system susceptible to functional and structural defects. Dysfunction is observed at any age but is most frequent in newborns, especially preterms. Degradation of cholesterol to chenodeoxycholic and cholic acid is performed in a process involving at least 14 enzymes. Several inborn errors of bile acid biogenesis are known (1). Quantifying individual bile acids and their conjugates as well as bile acid precursors for the diagnosis of such diseases is of high diagnostic value and may improve the diagnostic process. Unfortunately, in most cases in pediatric patients there are only very small sample volumes available for analysis.
Some infants suffering from cholestasis are treated with ursodeoxycholic acid (UDC) (2). Defects in bile acid formation may be treated with chenodeoxycholic acid (CDC) (2, 3). For effective surveillance of such a therapy, the measurement of total bile acids, as often performed, is only of limited value. Detailed information on the concentrations of primary and secondary bile acids and their conjugates, however, will allow precise monitoring of therapeutic interferences.
Due to the complex nature of bile acids and their low physiological concentration, accurate and sensitive quantification in small biological samples has remained a difficult task. Techniques like nuclear magnetic resonance (4), gas chromatography, high-performance liquid chromatography (5), supercritical fluid chromatography (6), gas chromatography-mass spectrometry (7), and liquid chromatography-mass spectrometry (8–10) are available for instrumental analysis but have not been developed for very small samples. Gustafsson et al. (7) measured lithocholic acid (LC), cholic acid (CA), and chenodeoxycholic acid (CDC) from dried blood spots of newborns (mean in µmol/L: 0.08, 1.7, 1.8) with GC-MS technique and found increased levels for LC, CA, and CDCA (mean in µmol/L: 0.11, 15.6, 7.4) caused by extrahepatic biliary artresia. For the determination of dihydroxcholestanoic acid (DHCA) and trihydroxycholestanoic acid (THCA) in small volumes of plasma and in 3 mm blood spots, Johnson et al. (11) esterified the C27 bile acid precursors with dimethylaminoethanol. This method is also applicable to free C24 bile acids but does not include conjugates which, in cases of hepatobiliary disease, are generally accumulated at much higher concentrations than the free acids.
A rapid method for the quantitative analysis of bile acids and their conjugates was used by Tagliacozzi et al. (12). Prior to electrospray tandem mass spectrometry, they extracted plasma with acetonitrile and separated the target substances by HPLC on a C18 column. They state that in a single chromatographic run of 20 min they were able to measure all isomeric forms of unconjugated, taurine or glycine conjugated C24 bile acids. The precursors DHCA and THCA were not included. A drawback of this method is a sample volume of 250 µl plasma. This volume is very large compared with the 10 to 12 µl serum in a 6 mm blood spot. For establishing a profile of conjugated bile acids, Perwaiz et al. (13) extracted gallbladder bile directly with a C18 reverse-phase column and achieved full separation and quantitation in only 5 min MS/MS run time. Neither free acids nor DHCA or THCA were included. A similar technique was used by Tessier et al. (14) for the determination of ursodeoxycholic acid and its conjugates. Sample volume here was 300 µL. With the aim to develop a method suitable for mass screening for early detection of cholestasis, Mills et al. (15) used methanol to elute conjugated bile acids from blood spots. Glycine and taurine conjugates of the bile acids were measured after reconstitution in acetonitrile/water and injected into a tandem mass spectrometer. This is a fast and simple method, worth further testing for the inclusion of unconjugated and nonphysiological bile acids like UDC. C27 bile acids, however were not included. The method has been applied by the same group [Mushtaq et al. (16)] to test the possibility of screening for cholestatic hepatobiliary disease in newborns.
Considering these experiences, we developed a method for measuring a broad spectrum of primary and secondary bile acids without derivatization. Bile acid conjugates and C27 bile acids were included. The method was conceived for dried blood spots or small volumes of serum. Dried blood spots are the standard samples for metabolic newborn screening and can also be used for selective screening in symptomatic infants.
MATERIALS AND METHODS
Methanol, water (E.Merck, Germany), and acetonitrile (Appli Chem, Germany) were ultra HPLC grade; ammonium acetate (Sigma, Deisenhofen, Germany) and formic acid (AppliChem, Darmstadt, Germany) were of highest purity.
Sodium taurodeoxycholate hydrate (TDC), deoxycholic acid (DC), sodium glycochenodeoxycholate (GCDC), chenodeoxycholic acid (CDC), sodium glycodeoxycholate (GDC), sodium taurolithocholate (TLC), and sodium glycocholate hydrate (GC) were purchased at Sigma (Deisenhofen). Sodium taurocholate hydrate (TC), lithocholic acid (LC), cholic acid (CA), and ursodeoxycholic acid (UDC) were delivered by Fluka (Deisenhofen, Germany). Tauroursodeoxycholic acid sodium salt (TUDC), taurochenodeoxycholic acid sodium salt (TCDC), glycoursodeoxycholic acid sodium salt (GUDC), and glycolithocholic acid sodium salt (GLC) were products of Calbiochem/Merck KG (Darmstadt, Germany). We bought the C27-acids DHCA and THCA from H. ten Brink (AMC Amsterdam, The Netherlands).
Internal standards
Internal standards cholic-2,2,4,4-d4 acid (d4-CA), lithocholic-2,2,4,4-d4 acid (d4-LC) und glycocholic-2,2,4,4-d4 acid (d4-GC) from CDN (Quebec, Canada), sodium taurochenodeoxycholic-2,2,4,4-d4 acid (d4-TCDC) synthesized by WITEGA (Berlin). d3-DHCA, and d3-THCA acid (H. ten Brink, AMC Amsterdam, The Netherlands) were dissolved in methanol at a final concentration of 0.16 µmol/L. The solution was kept at −20°C, it was stable for at least 6 months.
Calibrators and controls
Stock solutions of 500 µmol/L of all bile acids (DHCA, THCA: 100 µmol/L) were used to prepare calibrators with concentrations of 100, 50, 10, 1, and 0.1 µmol/L in methanol (DHCA, THCA: 20, 10, 5, 1, 0.5, 0.1, 0.08, 0.04, 0.02 0.01µmol/L). For the preparation of controls, whole blood with a hematocrit of 55% and serum were spiked to achieve final concentrations of 20, 5, 1, 0.5, 0.1 µmol/L (DHCA, THCA: 15, 7.5, 1.5, 0.3, 0.15 µmol/L). Twenty-five µl of spiked whole blood was used for the preparation of blood spot standards on filterpaper (Whatmann 903). Calibrators and controls were stored at −20°C. Unspiked whole blood spots and serum were used as blanks.
Samples
For method development serum and dried blood spots of healthy volunteers were used after obtaining informed consent. For the establishment of reference and patient data we analyzed anonymized blood spot material of our routine metabolic newborn and selective screening program as well as several serum samples or dried blood spots of known patients. Blood spots were collected on filterpaper Whatman 903. The study was approved by the Medical Ethics Review Board of Hannover Medical School.
Sample preparation
For extraction of bile acids, 150 µl methanolic standard solution were added to each 6 mm blood spot and 12.4 µl serum, respectively. After sealing with aluminum foil, the microtitre plate (ABgene, Hamburg, Germany) was shaken for 30 min. Centrifugation at 1,000 g in order to remove small particles followed. The supernatant was transferred to a second plate and evaporated to dryness at 65°C under a gentle stream of ambient air. The residue was dissolved with 200 µl methanol/water 1:1 v/v by vigorously shaking the plate for 20 min and centrifuged again at 1,000 g. Twenty µl of the supernatant was directly used for chromatography.
Liquid chromatography
HPLC was done on a Luna C18(2) column of 50 × 2.0 mm, 3µm, precolumn was C18 4 × 2.0 mm both from Phenomex (Aschaffenburg, Germany) at 23°C. The mobile phase consisted of two solvents: solvent A (HPLC grade water with 0.012% formic acid and 5 mM ammonium acetate, pH 4.50, solvent B (acetonitrile/water 97:3 v/v with 0.012% formic acid and 5 mM ammonium acetate, pH 7.31). The flow rate was 250 µl/min. In order to optimize bile acid determination for different clinical applications we developed three different variants of the HPLC part of the analysis.
Variant A
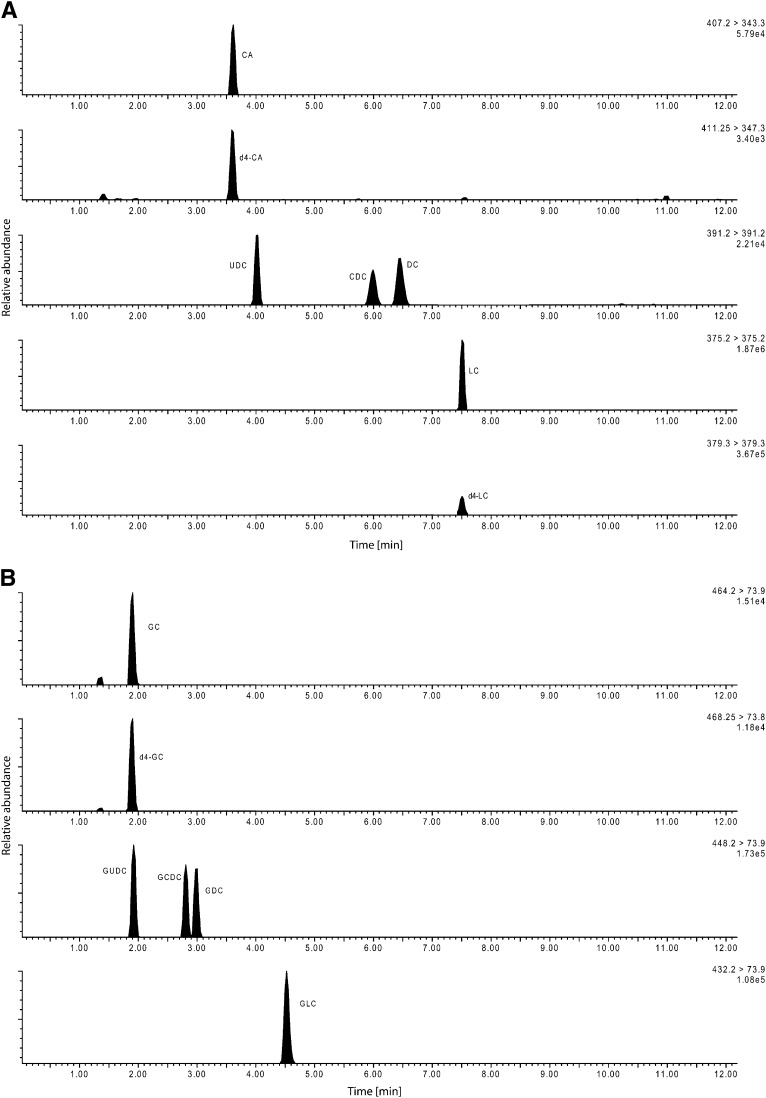
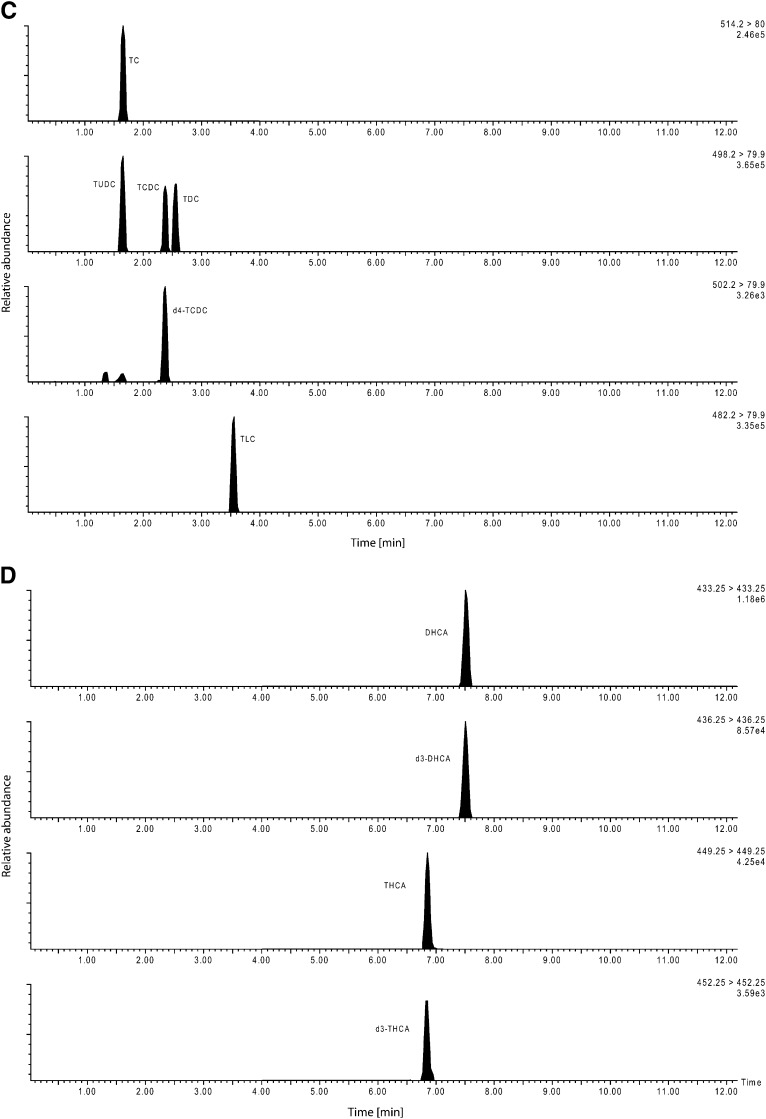
This variant was designed to yield a complete spectrum of 15 primary and secondary bile acids including their glycine and taurine conjugates plus two unconjugated C27 bile acid precursors. Chromatography started with a solvent mixture of 68.5% solvent A and 31.5% solvent B. The gradient increased up to 50% solvent B within 3.3 min, was held for 2.7 min and reached 100% B after additional 0.2 min, was again held for 1.8 min, then went back to the initial setting for a period of 4 min. The total run time was 12 min (Chromatogram, see Fig. 1).
Fig. 1.
Chromatographic separation of unconjugated bile acids (method variant A). B: Separation of the glycine conjugated bile acids (method variant A). C: Separation of the taurine conjugated bile acids (method variant A). D: Chromatographic separation of the bile acid precursors dihydroxycholestanoic acid (DHCA) and trihydroxycholestanoic acid (THCA) and the internal standards (method variant A).
Variant B
A program was conceived as extended selective screening measuring conjugated primary bile acids only plus unconjugated C27 bile acids in one run. We prefer this variant for pediatric patients showing symptoms indicative of peroxisomal dysfunction. Chromatography started with a solvent mixture of 30% solvent B. The gradient increased up to 40% solvent B within 0.7 min, was held for 1 min and increased to 100% B after 0.1 min, was again held for 0.8 min, then returned to the initial setting for a period of 1.9 min. The total run time was 4.5 min (see supplementary Fig. I).
Variant C
The third configuration allows measuring the conjugates of the primary bile acids, free and conjugated UDC, and is usable for monitoring UDC therapy applied in cholestatic diseases. For a total run time of 4.5 min, chromatographic separation started with a solvent mixture of 60% solvent A. The gradient increased up to 43% solvent B within 1.0 min and reached 100% B after 1.6 min, was held for 0.8 min and returned to the initial setting for a period of 2.1 min (see supplementary Fig. II).
Mass spectrometry conditions
All analyses were done on a QUATTRO Micro tandem mass spectrometer (Waters, Eschborn, Germany) connected to a CTC-Pal autosampler and injection system with microtitre racks (Axel Semrau, Sprockhoevel, Germany), and a binary pump 1525µ with internal solvent degasser (Waters). The instruments were controlled by the MassLynx software 4.0 (Waters); for integration and quantification the QuanLynx software (Waters) was used.
Mass spectrometry conditions were: capillary voltage 3.0 kV, cone voltage 50-90 V, collision energy 5-68 eV, source temperature 120°C, desolvation temperature 350°C, dwell time 0.05 s, argon gas cell pressure 4.2 × 10−3 mbar. The analysis was done at 23°C room temperature controlled by air conditioning. All bile acids were measured in ESI negative mode (Table 1). Optimal settings and fragments were defined after injection of methanolic solutions of bile acids by a Harvard infusion pump (Modell 11). All bile acids were quantified using external calibrators. Deuterated bile acids were used as internal standards.
TABLE 1.
Analytical settings for the detected bile acids
| Parent | Daughter | Cone | Coll | RT | Respective | ||
|---|---|---|---|---|---|---|---|
| Abk. | [m/z] | [m/z] | [Volts] | [eV] | [min] | Internal Standard | |
| TC | 514,2 | 80,0 | 70 | 64 | 1,68 | d4-GC | |
| TUDC | 498,2 | 79,9 | 80 | 62 | 1,68 | d4-TCDC | |
| GC | 464,2 | 73,9 | 50 | 34 | 1,82 | d4-GC | |
| d4-GC | 468,3 | 73,8 | 50 | 36 | 1,82 | ||
| GUDC | 448,2 | 73,9 | 50 | 34 | 1,84 | d4-GC | |
| TCDC | 498,2 | 79,9 | 80 | 62 | 2,38 | d4-TCDC | |
| d4-TCDC | 502,2 | 79,9 | 90 | 68 | 2,38 | ||
| TDC | 498,2 | 79,9 | 80 | 62 | 2,57 | d4-TCDC | |
| GCDC | 448,2 | 73,9 | 50 | 34 | 2,67 | d4-GC | |
| GDC | 448,2 | 73,9 | 50 | 34 | 2,86 | d4-GC | |
| TLC | 482,2 | 79,9 | 90 | 58 | 3,56 | d4-GC | |
| CA | 407,2 | 343,3 | 60 | 34 | 3,59 | d4-CA | |
| d4-CA | 411,3 | 347,3 | 60 | 34 | 3,59 | ||
| UDC# | 391,2 | 60 | 5 | 4,00 | d4-CA | ||
| GLC | 432,2 | 73,9 | 50 | 34 | 4,20 | d4-GC | |
| CDC# | 391,2 | 60 | 5 | 5,94 | d4-CA | ||
| DC# | 391,2 | 60 | 5 | 6,38 | d4-CA | ||
| THCA# | 449,3 | 70 | 32 | 6,85 | d4-THCA | ||
| d3-THCA# | 452,3 | 70 | 32 | 6,83 | |||
| LC# | 375,2 | 60 | 5 | 7,51 | d4-LC | ||
| d4-LC# | 379,3 | 60 | 5 | 7,51 | |||
| DHCA# | 433,3 | 65 | 32 | 7,53 | |||
| d3-DHCA | d3-DHCA# | 436,3 | 65 | 32 | 7,53 |
#, measured in single ion recording (SIR). Bold type indicates deuterated internal standards.
To determine the concentration the ratio of analyte to internal standard was used. d4-GC was used for unconjugated and glycine conjugated bile acids and d4-CA was used for calculation of CAs and unfragmented bile acids. d4-TCDC was used to determine the concentration of taurine conjugated BAs.
Precision data and statistics
Recovery given in % was calculated as the concentration of the spiked samples substracted by the blank values and divided by the calculated value of the spiked serum sample. Data analysis was done by MS-Excel® and the statistic program MedCalc® 10 (Mariakerke, Belgium). Validation was determined using the EP5-A2 guideline of the Clinical and Laboratory Standards Institute (CLSI) (17).
RESULTS
Liquid chromatography tandem mass spectrometry
Detection of qualified precursors and product ions for each bile acid and optimization of parameters were done by direct infusion of each bile acid in methanolic solution at a concentration of 10 µmol/L into the mass spectrometer. To evaluate matrix effects the postcolumn infusion method was used (18). Mainly salts, lipids, phospholipids and fatty acids coelute with the analytes and may affect the ESI droplet desolvation process and influence the ionization of the compounds of interest (19). The pKa of free bile acids is around 6 (6). Optimal separation was obtained at a pH of 4.
Method performance
Precision.
For intra-day and inter-day variation two quality control concentrations within the calibration range for each analyte were determined (Table 2, Supplementary Table I). The intra-assay coefficient of variation (CV) for dried blood material ranged from 3.7% (taurolithocholic acid) to 11.1% (lithocholic acid). Total imprecision results yielded CV of 7.1% (glycocholic acid) to 12.6% (tauro ursodeoxycholic acid) at low concentration levels and 7.7% to 13% for the same substances at higher concentrations. Recovery of all determined bile acids from dried blood spot material averaged 66%. After extraction the limit of detection (LOD) (signal/noise ratio = 3) varied between 4 and 30 nmol/L, the limit of quantification (LOQ) (signal/noise ratio = 10) between 11 and 91 nmol/L, respectively.
TABLE 2.
Variation, recovery, limit of detection (LOD), limit of quantification (LOQ) and regression data from dried blood spot material (level 1: 5 µmol/L, level 2: 20 µmol/L bile acid)
| Intra-assay |
Inter-assay |
Recovery [%] | LOQ |
LOD |
Linear Regression Parameters |
||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Level | [CV in %] n = 10 | [nmol/L] | Slope | Intercept | Regression Coefficient | |||
| TDC | 1 | 7.4 | 9.9 | 63.3 | 14.0 | 4.7 | 0.8378 | 0.0210 | 0.9983 |
| 2 | 6.0 | 8.1 | 65.7 | ||||||
| DC | 1 | 9.4 | 11.0 | 67.7 | 83.0 | 27.7 | 0.1791 | 0.0750 | 0.9977 |
| 2 | 7.8 | 8.8 | 68.1 | ||||||
| TC | 1 | 7.4 | 9.2 | 62.8 | 19.0 | 6.3 | 0.0882 | 0.0741 | 0.9992 |
| 2 | 7.4 | 8.9 | 67.8 | ||||||
| GCDC | 1 | 7.4 | 10.2 | 70.9 | 21.0 | 7.0 | 0.1082 | 0.0277 | 0.9979 |
| 2 | 6.1 | 7.9 | 69.2 | ||||||
| CDC | 1 | 7.2 | 8.7 | 65.2 | 91.0 | 30.3 | 0.1403 | 0.0232 | 0.9989 |
| 2 | 6.9 | 8.0 | 64.4 | ||||||
| GDC | 1 | 6.2 | 8.2 | 67.4 | 23.0 | 7.7 | 0.1143 | 0.0289 | 0.9966 |
| 2 | 8.0 | 9.6 | 71.8 | ||||||
| TLC | 1 | 3.7 | 10.2 | 67.1 | 11.0 | 3.7 | 0.1563 | 0.2604 | 0.9956 |
| 2 | 6.2 | 9.7 | 65.8 | ||||||
| GC | 1 | 3.9 | 7.1 | 66.8 | 26.0 | 8.7 | 0.0822 | 0.0348 | 0.9986 |
| 2 | 6.2 | 7.7 | 68.6 | ||||||
| LC | 1 | 11.1 | 12.0 | 59.5 | 14.0 | 4.7 | 0.1335 | 0.8212 | 0.9991 |
| 2 | 10.2 | 12.3 | 58.9 | ||||||
| UDC | 1 | 7.1 | 9.2 | 62.9 | 39.0 | 13.0 | 0.3015 | 0.1552 | 0.9965 |
| 2 | 6.4 | 11.1 | 64.9 | ||||||
| TUDC | 1 | 4.7 | 12.6 | 60.0 | 14.0 | 4.7 | 0.6840 | 0.5813 | 0.9961 |
| 2 | 7.3 | 13.0 | 68.7 | ||||||
| TCDC | 1 | 6.1 | 9.5 | 63.7 | 11.0 | 3.7 | 0.8343 | 0.2117 | 0.9969 |
| 2 | 7.1 | 8.8 | 61.1 | ||||||
| GUDC | 1 | 7.2 | 8.7 | 62.5 | 18.0 | 6.0 | 0.1220 | 0.0156 | 0.9982 |
| 2 | 7.5 | 9.0 | 64.4 | ||||||
| GLC | 1 | 5.1 | 7.3 | 68.9 | 45.0 | 15.0 | 0.0974 | 0.0200 | 0.9977 |
| 2 | 7.8 | 8.3 | 71.6 | ||||||
| CA | 1 | 6.8 | 9.6 | 66.9 | 78.0 | 26.0 | 0.1672 | 0.1005 | 0.9977 |
| 2 | 7.6 | 9.1 | 69.6 | ||||||
| DHCA | 1 | 5.8 | 8.4 | 59.0 | 11.0 | 3.7 | 0.7233 | 0.1156 | 0.9989 |
| 2 | 7.2 | 9.4 | 76.0 | ||||||
| THCA | 1 | 8.8 | 8.4 | 60.0 | 21.0 | 7.0 | 0.6267 | 0.1024 | 0.9965 |
| 2 | 7.6 | 10.1 | 69.0 | ||||||
Linearity.
Calibration curves of all bile acids from dried blood spots (see Table 2) were prepared 6-fold and analyzed in a single run. The ratio of each analyte to internal standard peak height was plotted against the analyte concentration. For all bile acids, the calibration curves were linear with a regression coefficient of >0.996.
Ion suppression.
Ion suppression was evaluated analyzing extracted dried blood samples injected into a flow of a bile acid standard solution inserted by a syringe pump at a constant flow rate of 10 µL/min. Slightly decreased intensity of the baseline in the mass transition of the bile acids was shown between 0.3 and 0.55 min. No ion suppression was seen at any later point of time. With the first peak eluting after 0.7 min, none of the bile acids were affected by ion suppression.
Healthy newborns (controls)
Dried blood spots from newborns (1–3 days old) without known diseases divided into gestational age groups of 20 individuals were analyzed. The medians of the groups are summarized in Table 3. The concentrations of the bile acids TCDC, GCDC, TC, and GC decreased with increasing gestational age, the remaining bile acids showed low concentrations and no correlations with the gestational age.
TABLE 3.
Medians of bile acid concentrations [µmol/L] from newborns classified in gestational groups.
| Gestational Age [week] | Number | TUDC | TCDC | TDC | GUDC | GCDC | GDC | UDC | CDC | DC | TC | GC | TLC | GLC | CA | LC | DHCA | THCA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 23–25 | 20 | 0.00 | 3.35 | 0.20 | 0.20 | 0.70 | 0.10 | 0.00 | 0.00 | 0.10 | 1.30 | 1.80 | 0.00 | 0.10 | 0.10 | 0.00 | < 0.01 | < 0.01 |
| 26–28 | 20 | 0.00 | 2.80 | 0.00 | 0.10 | 0.40 | 0.20 | 0.00 | 0.10 | 0.00 | 1.00 | 1.30 | 0.00 | 0.00 | 0.30 | 0.00 | < 0.01 | < 0.01 |
| 29–31 | 20 | 0.00 | 4.60 | 0.10 | 0.00 | 0.60 | 0.20 | 0.20 | 0.10 | 0.10 | 1.25 | 1.50 | 0.00 | 0.20 | 0.10 | 0.10 | < 0.01 | < 0.01 |
| 32–34 | 20 | 0.10 | 1.70 | 0.30 | 0.00 | 0.40 | 0.00 | 0.10 | 0.20 | 0.10 | 0.65 | 0.90 | 0.00 | 0.10 | 0.10 | 0.00 | < 0.01 | < 0.01 |
| 35–37 | 20 | 0.00 | 2.00 | 0.10 | 0.10 | 0.60 | 0.10 | 0.10 | 0.10 | 0.00 | 0.70 | 0.85 | 0.00 | 0.20 | 0.10 | 0.10 | < 0.01 | < 0.01 |
| 38–41 | 20 | 0.10 | 0.80 | 0.10 | 0.00 | 0.40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.45 | 0.70 | 0.00 | 0.00 | 0.00 | 0.00 | < 0.01 | < 0.01 |
During the first 3 days of life when the majority of blood samples are collected for newborn metabolic screening, concentrations of bile acids were mostly around the detection limit of this method. Sensitivity, however, was good enough to detect any pathologically elevated concentrations. Median and 99th percentile of free and conjugated bile acids are summarized in Table 4. A total of 310 samples of infants 3 to 81 days old were measured to calculate median and 99th percentiles. These were used as reference values in Supplementary Tables II and III.
TABLE 4.
Median and 99. percentile of unconjugated and conjugated bile acids from newborns (dried blood spots)
| Bile Acid | Free Acid [µmol/L] | Taurine Conjugate [µmol/L] | Glycine Conjugate [µmol/L] |
|---|---|---|---|
| chenodeoxycholic acid | 0.01, 0.22 | 2.01, 6.70 | 0.01, 7.00 |
| cholic acid | 0.08, 0.52 | 1.53, 5.20 | 1.43, 7.18 |
| deoxycholic acid | 0.01, 0.08 | 0.01, 0.06 | 0.03, 0.13 |
| ursodeoxycholic acid | 0.01, 0.20 | 0.01, 0.10 | 0.01, 0.08 |
| lithocholic acid | 0.01, 0.11 | 0.01, 0.02 | 0.01, 0.02 |
Patient samples
Patient samples were sent for selective screening in symptomatic patients. Measurement of bile acids was done as an additional test.
Peroxisomal defects.
Patients A–F showed increased levels of C26. In cases of peroxysomal defects (patients A–E, Table 5), DHCA and THCA were measured in significantly elevated concentrations in serum. In patient G diagnosed with X-linked adrenoleucodystrophy (X-ALD), a disease that does not affect the β-oxidation of C27 bile acid precursors, DHCA and THCA were within their normal ranges. Liver dysfunction probably caused elevated concentrations of the primary bile acids and their conjugates in five out of six of these patients.
TABLE 5.
Bile acid concentration in serum from infants with peroxysomal defects (cases A-E), genetically proven Zellweger syndrome (case F) and X-linked adrenoleukodystropy (case G); (n.d. – not detected)
| Sample | Age [days] | TCDC [µmol/l] | GCDC [µmol/l] | CDC [µmol/l] | TC [µmol/l] | GC [µmol/l] | CA [µmol/l] | DHCA [µmol/l] | THCA [µmol/l] | C26 [µmol/l] | C26/C22 [µmol/l] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | 2.01 | 1.41 | 0.01 | 1.53 | 1.43 | 0.08 | 0.01 | 0.01 | 0.2-1.6 | 0.005-0.029 | |
| A | 112 | 1.10 | 0.61 | 0.20 | 0.83 | 0.42 | n.d. | 21.2 | 3.96 | 10.7 | 0.38 |
| B | 65 | 7.43 | 4.40 | 0.91 | 4.14 | 2.10 | n.d. | 12.2 | 21.8 | 6.6 | 0.98 |
| C | 287 | 12.3 | 10.3 | 0.93 | 9.63 | 7.01 | n.d. | 4.93 | 10.9 | 3.36 | 0.40 |
| D | 10 | 8.21 | 17.8 | 1.10 | 8.52 | 11.9 | 0.42 | 0.42 | 0.71 | 19.9 | 3.15 |
| E | 193 | 17.0 | 7.10 | 1.0 | 5.44 | 2.33 | 0.10 | 10.6 | 4.56 | 11.4 | 0.85 |
| F | 111 | n.d. | n.d. | 0.9 | 0.3 | 2.91 | n.d. | 0.53 | 7.05 | 3.41 | 0.33 |
| G | 64 | 11.0 | 21.3 | 0.93 | 3.82 | 4.41 | n.d. | 0.02 | 0.01 | 2.25 | 0.38 |
Classical galactosemia.
Among a group of infants with severe liver disease selectively screened for metabolic disease there were eight patients suffering from classical galactosemia. They had not been tested for galactosemia during the first days of life. All patients listed in Supplementary Table II showed grossly elevated concentrations of conjugated CDC and in some of the samples conjugated cholic acid was increased. Newborn screening for galactosemia had not been done except for patient 8. In this case we were able to demonstrate normal bile acid concentrations at day 3 of life but elevated concentrations a few days later when liver disease became clinically apparent. Free primary and secondary bile acids as well as C27 precursors were found within the normal range in all cases. Three additional infants diagnosed with galactokinase deficiency did not exhibit increased bile acid concentrations (data not shown).
Biliary atresia.
In two cases of biliary atresia, we were able to compare bile acid concentrations in dried blood samples of the second day of life to those taken at an age of 60 and 118 days, respectively (Supplementary Table III). Although the complete spectrum of bile acids was normal in those samples collected early, concentrations of the primary bile acid conjugates were strongly elevated at the second analysis. Other bile acids were not affected.
Pediatric patients on ursodeoxycholic acid treatment.
Neonates and infants on treatment with ursodeoxycholic acid were tested for bile acids. They showed variable concentrations of primary bile acid conjugates as well as of the therapeutic agent and its conjugates (Supplementary Table IV).
DISCUSSION
The aim of this study was to provide a method for rapid quantification of C24 and C27 bile acids in small volumes of pediatric blood and serum samples. MS/MS cannot distinguish between molecules of identical mass/charge (m/z) relations, which deliver daughter ions of identical masses. As shown in Table 1, this unfortunately applies to the free acids and taurine as well as to the glycine conjugates of chenodeoxycholic acid and its secondary metabolites deoxycholic acid and ursodeoxycholic acid. Therefore, quantifying a spectrum of bile acids and bile acid conjugates cannot be done without chromatographic separation of the individual substances. For small samples of biological material, especially dried blood on filter paper, HPLC separation has proved very efficient. In combination with MS/MS, high sensitivity can be achieved even without derivatization of analytes. Our method doesn't require solid phase extraction as mentioned by Tribe et al. (20). Analysis of bile acids in serum samples shows similar recoveries compared with the data given in the method by Tribe et al. including sold phase extraction.
There are a number of pathological conditions that may cause alterations of bile acid metabolism or enterohepatic circulation. Interruptions of bile flow, reduction of intestinal bile acid absorption, or defects of bile acid metabolism can induce severe disease. Abnormal concentrations of bile acids may thus be associated with inborn errors of cholesterol degradation as well as with a number of hepatic diseases. Biliary atresia, liver tumors, or short bowel syndrome may result in mechanical inhibition of bile flow. In all these cases, fast and detailed quantitation of individual bile acids is of great interest.
In infants with inborn errors of metabolism, dried blood spot samples are regularily used to monitor the levels of key metabolites. In diseases associated with cholestasis, it may be advantageous to add bile acids to the test spectrum in order to survey liver function and bile acid circulation.
In conclusion, bile acid profiling using the method described here is useful for diagnosing and monitoring diseases affecting bile acid metabolism or circulation especially in pediatric patients. It also provides a means for the early detection of compromised bile acid metabolism including defects of the β-oxidation of bile acid precursors.
Supplementary Material
Footnotes
Abbreviations:
- CA
- cholic acid
- CDC
- chenodeoxycholate
- DC
- deoxycholate
- DHCA
- dihydroxycholestanoic acid
- GC
- glycocholate
- GCDC
- glycochenodeoxycholate
- GDC
- glycodeoxycholate
- GLC
- glycolithocholate
- GUDC
- glycoursodeoxychoate
- MRM
- multiple reaction monitoring
- SIR
- single ion recording
- TC
- taurocholate
- TCDC
- taurochenodeoxycholate
- TDC
- taurodeoxycholate
- THCA
- trihydroxycholestanoic acid
- TLC
- taurolithocholate
- TUDC
- tauroursodeoxychoate
- UDC
- ursodeoxycholate
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and three tables.
REFERENCES
- 1.Heubi J. E., Setchell K. D., Bove K. E. 2007. Inborn errors of bile acid metabolism. Semin. Liver Dis. 27: 282–294. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann A. F., Hagey L. R. 2008. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 65: 2461–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setchell K. D., Heubi J. E. 2006. Defects in bile acid biosynthesis–diagnosis and treatment. J. Pediatr. Gastroenterol. Nutr. 43(Suppl 1): S17–S22. [DOI] [PubMed] [Google Scholar]
- 4.Kunnecke B., Bruns A., von Kienlin M. 2007. Non-invasive analysis of gallbladder bile composition in cynomolgus monkeys using in vivo 1H magnetic resonance spectroscopy. Biochim. Biophys. Acta. 1771: 544–549. [DOI] [PubMed] [Google Scholar]
- 5.Budai K., Javitt N. B. 1997. Bile acid analysis in biological fluids: a novel approach. J. Lipid Res. 38: 1906–1912. [PubMed] [Google Scholar]
- 6.Scalia S. 1995. Bile acid separation. J. Chromatogr. B Biomed. Appl. 671: 299–317. [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson J., Alvelius G., Bjorkhem I., Nemeth A. 2006. Bile acid metabolism in extrahepatic biliary atresia: lithocholic acid in stored dried blood collected at neonatal screening. Ups. J. Med. Sci. 111: 131–136. [DOI] [PubMed] [Google Scholar]
- 8.Alnouti Y., Csanaky I. L., Klaassen C. D. 2008. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 873: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentayeb K., Batlle R., Sanchez C., Nerin C., Domeno C. 2008. Determination of bile acids in human serum by on-line restricted access material-ultra high-performance liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 869: 1–8. [DOI] [PubMed] [Google Scholar]
- 10.Bobeldijk I., Hekman M., de Vries-van der Weij J., Coulier L., Ramaker R., Kleemann R., Kooistra T., Rubingh C., Freidig A., Verheij E. 2008. Quantitative profiling of bile acids in biofluids and tissues based on accurate mass high resolution LC-FT-MS: compound class targeting in a metabolomics workflow. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 871: 306–313. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D. W., ten Brink H. J., Schuit R. C., Jakobs C. 2001. Rapid and quantitative analysis of unconjugated C(27) bile acids in plasma and blood samples by tandem mass spectrometry. J. Lipid Res. 42: 9–16. [PubMed] [Google Scholar]
- 12.Tagliacozzi D., Mozzi A. F., Casetta B., Bertucci P., Bernardini S., Di Ilio C., Urbani A., Federici G. 2003. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin. Chem. Lab. Med. 41: 1633–1641. [DOI] [PubMed] [Google Scholar]
- 13.Perwaiz S., Tuchweber B., Mignault D., Gilat T., Yousef I. M. 2001. Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry. J. Lipid Res. 42: 114–119. [PubMed] [Google Scholar]
- 14.Tessier E., Neirinck L., Zhu Z. 2003. High-performance liquid chromatographic mass spectrometric method for the determination of ursodeoxycholic acid and its glycine and taurine conjugates in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 798: 295–302. [DOI] [PubMed] [Google Scholar]
- 15.Mills K. A., Mushtaq I., Johnson A. W., Whitfield P. D., Clayton P. T. 1998. A method for the quantitation of conjugated bile acids in dried blood spots using electrospray ionization-mass spectrometry. Pediatr. Res. 43: 361–368. [DOI] [PubMed] [Google Scholar]
- 16.Mushtaq I., Logan S., Morris M., Johnson A. W., Wade A. M., Kelly D., Clayton P. T. 1999. Screening of newborn infants for cholestatic hepatobiliary disease with tandem mass spectrometry. BMJ. 319: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tholen D. W., et al. National Committee for Clinical Laboratory Standards. 2004. Evaluation of precision performance of quantitative measurement methods: approved guideline. 2nd edition NCCLS, Wayne, PA. [Google Scholar]
- 18.Bonfiglio R., King R. C., Olah T. V., Merkle K. 1999. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 13: 1175–1185. [DOI] [PubMed] [Google Scholar]
- 19.Van Eeckhaut A., Lanckmans K., Sarre S., Smolders I., Michotte Y. 2009. Validation of bioanalytical LC-MS/MS assays: evaluation of matrix effects. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2198–2207. [DOI] [PubMed] [Google Scholar]
- 20.Tribe R. M., Dann A. T., Kenyon A. P., Seed P., Shennan A. H., Mallet A. 2009. Longitudinal Profiles of 15 Serum Bile Acids in Patients With Intrahepatic Cholestasis of Pregnancy. Am. J. Gastroenterol. 10.1038/ajg.2009.633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.