Abstract
The aim of this study was to develop an enzymatic cholesterol staining method to determine HDL subclasses in a polyacrylamide gradient gel electrophoresis, which further allows staining by protein in the same electrophoresis lane. HDLs from 120 healthy individuals were separated through nondenaturing PAGE. HDLs were stained for cholesterol using an enzymatic semisolid mixture. Once the gels were unstained, they were stained again for proteins with Coomassie blue. The proportions of HDL subclasses were determined by densitometry. HDL subclasses were transformed to concentrations using as reference HDL-cholesterol plasma levels. This method is comparable in linearity and reproducibility to Coomassie blue staining, although it provides quantitative data. As expected, HDL size distribution shifted toward larger particles when determined by cholesterol as compared with protein. With this method, we observed different proportions of HDL subclasses between men and women as compared with Coomassie blue staining. We described a method to determine HDL size distribution by enzymatic cholesterol staining on polyacrylamide gels. The method allows the quantification of the cholesterol plasma concentration of each HDL subclass with the possibility to further stain the protein in the same sample. The combination of HDL staining by cholesterol and protein on electrophoresis gels provides information that may have clinical relevance.
Keywords: high-density lipoprotein subclasses, enzymatic staining, optical densitometry analysis, HDL composition, cardiovascular risk
Several epidemiological studies have demonstrated a negative correlation between HDL-cholesterol (HDL-C) and the development of coronary heart disease (CHD) (1). The causal relationship between plasma HDL-C concentration and CHD has been explained by the role played by these lipoproteins in reverse cholesterol transport, as well as by the potentially anti-atherogenic properties of HDL, such as their anti-inflammatory, anti-oxidative, anti-aggregatory, anti-coagulant, and pro-fibrinolytic effects [for review see Ref. (2)].
HDLs comprise a heterogeneous group of lipoproteins that may be classified by decreasing size in HDL2b, HDL2a, HDL3a, HDL3b, and HDL3c, as assessed by nondenaturing PAGE in conjunction with automated densitometry (3, 4). However, HDLs have been classified by other approaches, such as selective precipitation (5, 6), ultracentrifugation (7), NMR (8), electronic microscopy (9), and two-dimensional electrophoresis (10). All these strategies recognize the existence of HDL particles of different sizes or densities that might have different properties against atherosclerosis development.
Nevertheless, it is still a controversial issue as to which HDL fraction confers better cardiovascular protection; it has been postulated that the large HDL fraction is the most atheroprotective, because CHD patients have lower levels of these particles than controls, as assessed by selective precipitation or NMR (6, 11). In contrast, small HDL particles are the best acceptors of cholesterol from peripheral tissues (12) and also have better antioxidant properties than large HDL (13, 14). Moreover, thiazolidinediones as well as fibrates both antiatherogenic drugs that increase HDL-cholesterol plasma levels, shift HDL size distribution toward small HDL particles (15, 16). In addition, some subjects with severe hypoalphalipoproteinemia who do not develop CHD have a high proportion of small HDL (9), suggesting an atheroprotective role of these particles.
The existing methods for the estimation of HDL subclasses involve the determination of at least one of their components. NMR, vertical autoprofile-II (VAP-II), as well as selective precipitation are based on HDL lipid content for estimating subclasses (5, 8, 17, 18), whereas protein is the HDL component determined in other methods, such as two-dimensional electrophoresis (10) and PAGE in native conditions (17). Because small HDL particles are protein rich and lipid poor, whereas large particles are the contrary, the relative proportion of HDL subclasses is dependent on the component determined for the quantification. The wide variety of methods used for determining HDL subclasses may explain, at least in part, the apparent controversy concerning which is the most antiatherogenic fraction of HDL. Therefore, the aim of this study was to develop an enzymatic cholesterol staining method to determine HDL subclasses in a polyacrylamide gradient electrophoresis gel, which further allows staining by protein in the same electrophoresis lane. In the first step, the enzymatic procedure specifically stains cholesterol on the gel. In the second step, HDL proteins are stained, and in both cases, the HDL subclasses distribution is determined by optical densitometry. This new method has two main advantages: it could be quantitative, and the HDL subclasses can be estimated also by protein for every sample in the same electrophoresis lane.
MATERIALS AND METHODS
Materials
Cholesterol esterase, cholesterol oxidase, and peroxidase were purchased from MP Biomedicals (Selem, OH). Sodium cholate, Triton 100×, thiazolyl blue teratozolium bromide (MTT), phenazine methosulfate (PMS), carboxymethylcellulose 5-cholesten-3-one, cholesteryl palmitate, and cholesterol were from Sigma-Aldrich (St. Louis, MO). Noble agar was purchased from Becton Dickinson (Franklin Lakes, NJ).
Study subjects
One hundred and twenty individuals between 18 and 85 years of age were recruited in the department of Endocrinology and the Outpatients service of the National Institute of Cardiology, Mexico. Volunteers without personal or family history of type 1 or 2 diabetes, pancreatitis, high blood pressure, angina pectoris, and CHD were accepted. All subjects had a fasting glucose of <110 mg/dl, a body mass index (BMI) of < 32 kg/m2, total cholesterol (TC) of < 220 mg/dl, triglycerides (TGs) of < 200 mg/dl, smoked fewer than five cigarettes per day, and had normal hepatic, thyroid, and renal functions, as assessed by routine laboratory analyses. All the participants gave their informed consent to take part in the study that was approved by the Ethics Committee of the National Institute of Cardiology “Ignacio Chávez”, Mexico.
Laboratory assessment
All patients were instructed to avoid strenuous exercise and to eat a light dinner the day before blood drawings were performed. Blood samples were obtained in EDTA tubes after 12 h overnight fasting from an antecubital vein after subjects had been seated for 15 min. Samples were centrifuged at 4°C, plasma was separated and analyzed or frozen at −80°C until analysis. For lipoprotein isolation and plasma lipid profile (TC, TGs, HDL-C, and LDL-C), samples were processed within 2 h after collection. Plasma glucose, TC, and TGs were determined by commercially available enzymatic methods. The phosphotungstic acid-Mg2+ precipitation procedure of apolipoprotein (apo) B-containing lipoproteins was used before quantifying HDL-C. Quality control of lipid measurements was assessed through standardization to the Center for Disease Control and Prevention (Atlanta, GA). LDL-C was estimated using the Friedewald equation modified by De Long (19). Serum levels of all lipids were determined within 48 h after drawing blood samples.
Isolation of HDL
HDLs were separated by ultracentrifugation in a Beckman optima TLX table centrifuge at 110,000 rpm in 3.2 ml polycarbonate tubes as described previously (20). Briefly, total apo B-containing lipoproteins (density < 1.063 mg/dl) were obtained after 2.16 h, whereas total HDL (1.063 < density < 1.21 g/ml) took 2.5 h. Under these conditions, 80 to 85% of total plasma apo A-I was recovered from the HDL fraction without apo B-contamination. HDLs were dialyzed against 0.09 M Tris/0.08 M boric acid/3 mM EDTA (TBE) buffer, pH 8.4.
Enzymatic staining of cholesterol on polyacrylamide gel
HDLs were separated by their hydrodynamic diameter in an 8 × 10 × 0.15 cm nondenaturing 3–30% gradient polyacrylamide gel electrophoresis, using TBE during 24 h at 170 V as previously described (3, 4, 17). Twenty five micrograms of HDL protein sample, corresponding approximately to 10 µg of cholesterol, were deposited per well.
Gels were stained for cholesterol using an enzymatic mixture of cholesterol esterase, cholesterol oxidase, and peroxidase at a final concentration of 0.075 U/ml, 0.05 U/ml, and 0.25 U/ml, respectively, in a 150 mM NaCl, 8.6 mM Na2HPO4, 1.4 mM NaH2PO4 buffer (PBS), pH 7.4. The reaction mixture also included 3 mM sodium cholate, 0.1% Triton 100×, 0.4 mM MTT, and 0.6 mM PMS. In a first series of experiments, we directly submerged the electrophoresis gels in the reaction mixture. In order to enhance the cholesterol staining, we further added Noble agar at 1.0% or carboxymethylcellulose at 1.4% as viscosant agents to the reaction mixture. For the Noble agar, 100 mg were heated in 10 ml the buffer reaction until dissolution, cooled to about 40°C, and then the enzymes were added. The final concentration of enzymes was as described above. Electrophoresis gels were kept in contact with the reaction mixture containing the viscosant agent during 1 h at 37°C in the dark. At the end of the incubation time, the reaction mixture was removed and the gels were gently washed in PBS to eliminate any remaining residue of agar or carboxymethylcellulose. Electrophoresis gels were then scanned in a GS-670 BioRad densitometer (scan 1), distained with methanol:acetic acid:water 5:2:13, and further restained for proteins with Coomassie R-250. Afterwards, gels were again scanned (scan 2). The relative proportions of each HDL subclass determined by protein were estimated by optical densitometry analysis of scan 2, using as reference globular proteins (thyroglobulin, 17 nm; ferritin, 12.2 nm; lactate deshydrogenase, 8.2 nm; and albumin, 7.1 nm; high-molecular weight calibration kit, Amersham Pharmacia Biotech, Buckimghamshire, UK) that were run in the same gel (3, 4, 17). Relative proportion of each HDL subclass is expressed as the percentage of the total HDL area under the curve, integrated from 7.94 to 13.59 nm.
The relative proportion of HDL subclasses by their cholesterol content was determined in scan 1 using the migration distances of the reference globular proteins obtained from the scan 2. Considering that the area under the curve in the densitogram represents 100% of the cholesterol in the HDL, the cholesterol plasma concentration of each HDL subclass was estimated as follows: HDLn-C = (% HDLn determined by cholesterol × HDL-C)/100 where n represents the HDL subclass, and the HDL-C is the HDL cholesterol plasma concentration. For classification of the HDL subclasses, we considered the following size intervals: HDL3c, 7.94–8.45 nm; HDL3b, 8.45–8.98 nm; HDL3a, 8.98–9.94 nm; HDL2a, 9.94–10.58 nm; and HDL2b, 10.58–13.59 nm (4).
Analysis of the enzymatic reaction homogeneity
In order to determine whether the enzymatic reaction is homogeneous along the polyacrylamide gradient gel, we used HDL labeled with [3H]cholesterol as we have previously described (4). By this procedure, 90% of the radioactive cholesterol within the HDL was esterified, whereas the remaining 10% remained as free cholesterol. Radiolabeled HDLs were then separated by electrophoresis as described above; as a consequence, the radioactive label was distributed along the whole sample in the electrophoresis lane. After the migration time, we monitored the conversion of cholesteryl esters and free cholesterol to cholestenone in the sections of the gel corresponding to the HDL subclasses. For this purpose, polyacrylamide gels were stained for cholesterol using the enzymatic mixture and scanned as described above. The sections corresponding to the different HDL subclasses were cut, lipids were extracted with chloroform:methanol 2:1, the organic solvent was evaporated under nitrogen stream, and the sample was resuspended in 75 µL of toluene. Cholestenone, cholesteryl esters, and free cholesterol were separated by thin layer chromatography in silica gel plates (Sigma Aldrich) using petroleum ether:ethylic ether:acetic acid 90:10:5 as mobile phase. Spots were identified using cold 5-cholesten-3-one (Rf=0.07), cholesteryl palmitate (Rf=0.71) and cholesterol (Rf=0.20) as standards, scratched from the plate, and counted for radioactivity in a liquid scintillation analyzer (TRI-CARB 2200CA, Packard). The rate of conversion of cholesterol to cholestenone was estimated by dividing the counted radioactivity of the cholestenone spot by the addition of the radioactivity corresponding to cholestenone, cholesteryl esters, and free cholesterol. The results were expressed as % of conversion.
Statistical analysis
Central tendency and dispersion measurements were estimated by conventional methods. Comparisons between multiple groups were assessed by ANOVA test. Normal distribution of the variables was evaluated by the Kolmogorov-Smirnoff test. The significance of the differences among parameters between men and women was tested by the Student's t-test for normally distributed variables. TGs values were logarithmically transformed for parametric statistical analysis. Comparisons of nonnormally distributed variables were performed by Mann-Whitney U and Wilcoxon tests for independent groups and paired variables, respectively. Partial correlations adjusted by age and gender were performed and statistical significance was set at P < 0.05. Unless otherwise indicated, values are expressed as mean ± SD for variables with normal distribution and as median and interquartile interval for nonnormally distributed variables. Statistical analysis was performed using SPSS V 11 software.
RESULTS
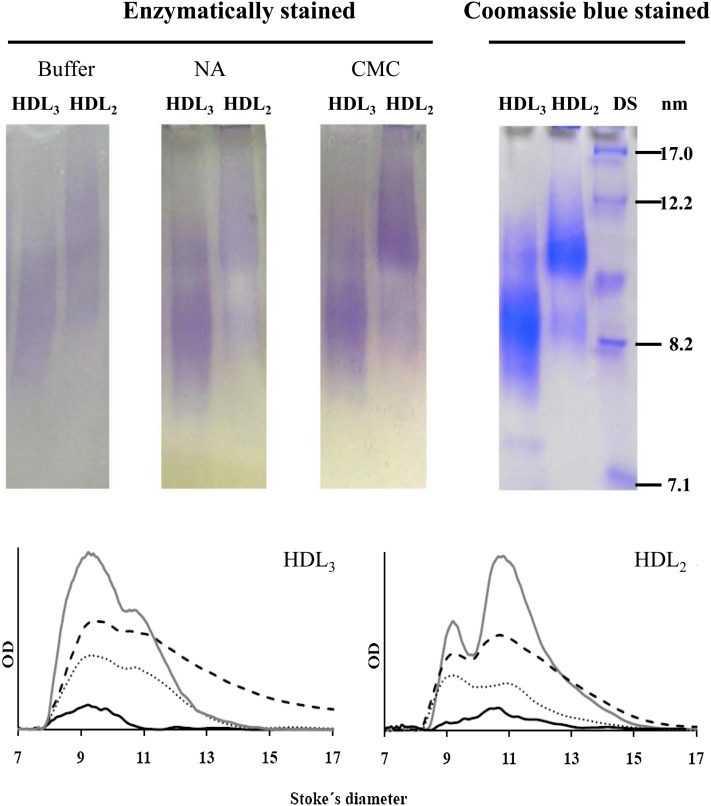
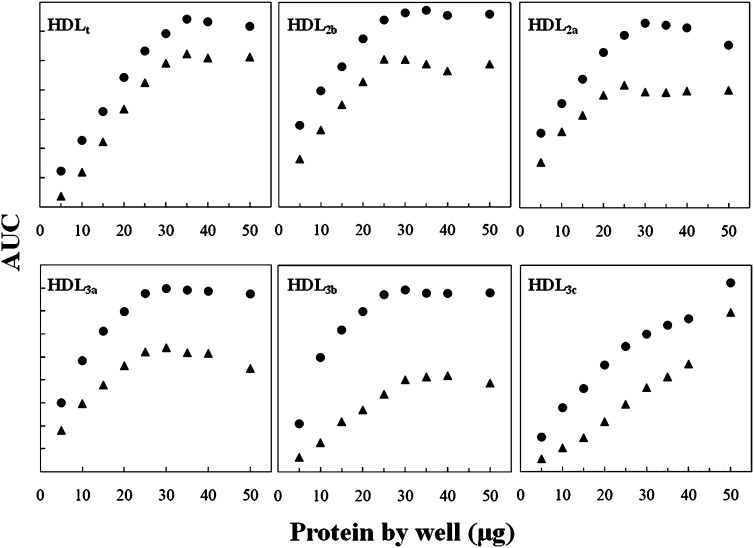
For the assessment of the optimal viscosant agent and to test the homogeneity of the enzymatic reaction along the gel, we obtained a pool of plasma from five volunteers. HDLs were isolated from this pool by ultracentrifugation and further separated on the basis of their hydrodynamic diameter by nondenaturating electrophoresis in a 3–30% PAGE, as indicated in the Methods section. The enzymatic method for the assessment of the HDL subclasses required a gel-phase reagent to enhance the intensity of the cholesterol staining, because a part of the formed products were washed away by the buffer and the mechanical agitation needed during incubation in an aqueous solution (Fig. 1). Both carboxymethylcellulose and Noble agar enhanced the intensity of the staining signal as compared with the enzymatic mixture in buffer without viscosant agent as it can be observed in the corresponding densitograms (Fig. 1). We finally chose carboxymethylcellulose at 1.4%, because the staining intensity is better than that obtained with Noble agar. Moreover, carboxymethylcellulose is soluble in the buffer at room temperature, avoiding heating-cooling processes that are necessary for agar solubilization. The best cholesterol staining with the lower background was obtained between 60 to 75 min of incubation (Supplementary Fig. I). This new procedure to stain cholesterol is linear for total HDL between 5 and 30 µg of protein deposited by well in the electrophoresis gel (Fig. 2). For HDL subfractions, the method is linear from 5 to 25 µg of protein for HDL 2b, 2a, and 3a, from 5 to 30 µg of protein for HDL3b, and up to 40 µg of protein for HDL3c (Fig. 2).
Fig. 1.
Electrophoretic scans of HDL2 and HDL3 (upper images) isolated from a pool of plasma by ultracentrifugation, further separated by PAGE, and enzymatically stained for cholesterol (left). The results obtained with the enzymatic mixture in buffer without any viscosant agent, with Nobel agar (NA) and with carboxymethylcellulose (CMC) are shown from left to right. HDL2 and HDL3 enzymatically stained using CMC are also shown after Coomassie blue staining (right), including the globular proteins used as diameter standards (DS). Densitograms for HDL2 and HDL3 (lower graphs) correspond to the enzymatic mixture in buffer (solid black line), using Noble agar (dotted line), CMC (dashed line), and stained with Coomassie blue (solid gray line). For enzymatic staining, samples were incubated 1 h at 37°C. OD, optical density.
Fig. 2.
Linearity tests for enzymatic staining of cholesterol on electrophoresis gels. Total HDL (HDLt) were run in polyacrylamide 3-30% gradient gel electrophoresis, stained for cholesterol (black triangles) and further for protein (black circles). Plots represent area under the curve (AUC) obtained by densitometric analysis for HDLt and HDL subclasses (for details, see Methods section) versus protein deposited by well. For comparison purposes, protein AUC ranges were adjusted.
To rule out the possible influence of polyacrylamide gradient on the enzymatic reaction, i.e., the lower the polyacrylamide concentration, the higher conversion of cholesterol to cholestenone, we determined the % of conversion of substrates to products during 1 h using HDL labeled with [3H]cholesterol as described in the Methods section. The mean percentage of cholesterol conversion to cholestenone was 58.9 ± 7.9% for the whole HDL (n = 5). Concerning the HDL subclasses, the mean percentage of conversion was 55.7 ± 6.1, 51.4 ± 5.1, 49.0 ± 8.6, 52.2 ± 12.8, and 56.0 ± 5.2% for HDL2b, 2a, 3a, 3b, and 3c, respectively; there was not any significant difference among these values (ANOVA test P = 0.606).
For the assessment of the intra- and inter-assay coefficient, we obtained a pool of plasma from five healthy volunteers. HDLs were isolated and separated on the basis of their hydrodynamic diameter by nondenaturating electrophoresis in a 3–30% PAGE. Cholesterol and protein were sequentially stained, densitograms were obtained, and analyzed as mentioned above. Samples were run in duplicate and the reported values are the mean of the two determinations. Under these conditions, four samples and the corresponding duplicate of the reference globular proteins were run per each electrophoresis gel. The intra-assay coefficient of variation (CV) of the method was 6.9% as assessed after 32 repetitions of the same sample in eight independent gels. The mean inter-assay CV was 4.3% as determined with the analysis of the same sample in 25 independent electrophoresis gels.
Once the conditions for this new method were established, we determined HDL subclasses distribution in 120 subjects without clinical history of CHD. Table 1 shows the lipid profile and clinical characteristics of the subjects included in the study. For comparison purposes, data of women and men were analyzed separately.
TABLE 1.
Demographic data and lipid profile of the individuals included in the study
| All Subjects | Men | Women | pa | |
|---|---|---|---|---|
| n | 120 | 60 | 60 | |
| Age (years) | 33.4 ± 16.0 | 29.2 ± 12.0 | 36.5 ± 17.9 | 0.012 |
| BMI | 25.1 ± 4.8 | 25.2 ± 4.4 | 25.0 ± 4.2 | 0.844 |
| SBP | 111.6 ± 16.1 | 113.4 ± 14.6 | 110.1 ± 16.9 | 0.320 |
| DBP | 71.7 ± 11.6 | 71.1 ± 11.1 | 72.2 ± 13.7 | 0.659 |
| Triglycerides (mg/dl) | 108 [71–147] | 102.0 [70.4–134.0] | 109.5 [71.7–170.5] | 0.193 |
| Total cholesterol (mg/dl) | 169.2 ± 42.9 | 157.4 ± 35.6 | 178.5 ± 46.1 | 0.009 |
| LDL-cholesterol (mg/dl) | 99.8 ± 34.4 | 95.1 ± 29.7 | 103.4 ± 37.5 | 0.205 |
| HDL-cholesterol (mg/dl) | 47.3 ± 14.5 | 42.9 ± 13.1 | 50.6 ± 14.8 | 0.006 |
Data represent mean ± SD for normally distributed variables or median [interquartile interval] for nonnormally distributed variables.
Student's t-test or Mann-Whitney U for variables with normal or nonnormal distribution variables, respectively.
Concerning the analysis of HDL subclasses, the relative proportion of HDL2a and HDL3c determined by cholesterol did not have a normal distribution. In contrast, the relative proportions of all HDL subclasses determined by protein were normally distributed (Table 2). In agreement with previous observations (7, 18, 21, 22), the relative proportions of HDL subclasses were different as a function of the HDL component used for their quantification (Supplementary Fig. IIA); the HDL size distribution shifted toward larger particles when determined by cholesterol as compared with the quantification by protein content (Supplementary Fig. IIA). The most important differences were observed in HDL2b and HDL3c; when subclasses were assessed by cholesterol, HDL2b were about 60% more abundant, whereas HDL3c were about 35% lower than the relative proportion obtained by protein content (Table 2).
TABLE 2.
Relative proportions, cholesterol-to-protein ratio, and cholesterol plasma concentrations of HDL subclasses
| All Subjects | Men | Women | pa | |
|---|---|---|---|---|
| HDL subclasses by cholesterol (%) | ||||
| HDL2b | 46.2 ± 11.2 | 43.2 ± 11.5 | 49.7 ± 9.6 | 0.002 |
| HDL2a | 13.9 [13.0-15.7] | 14.1 [12.6-16.1] | 14.1[13.3-15.4] | 0.501 |
| HDL3a | 22.5 ± 4.7 | 23.3 ± 4.5 | 21.6 ± 4.9 | 0.069 |
| HDL3b | 9.2 ± 3.3 | 9.7 ± 3.9 | 8.3 ± 2.6 | 0.028 |
| HDL3c | 6.3 [3.3-9.3] | 7.8 [4.4-11.1] | 5.5 [2.6-7.5] | 0.007 |
| HDL subclasses by protein (%) | ||||
| HDL2b | 28.7 ± 8.6 | 25.8 ± 8.7 | 30.5 ± 7.8 | 0.005 |
| HDL2a | 14.4 ± 4.39 | 13.5 ± 4.4 | 15.2 ± 4.4 | 0.050 |
| HDL3a | 28.9 ± 6.5 | 29.6 ± 8.4 | 28.7 ± 4.8 | 0.489 |
| HDL3b | 14.1 ± 3.3 | 14.8 ± 3.3 | 13.7 ± 3.3 | 0.096 |
| HDL3c | 13.9 ± 8.0 | 16.3 ± 9.5 | 11.9 ± 6.3 | 0.006 |
| Cholesterol-to-protein ratio | ||||
| HDL2b | 1.74 ± 0.69 | 1.88 ± 0.91 | 1.71 ± 0.44 | 0.216 |
| HDL2a | 1.02 [0.90-1.09] | 1.07 [0.89-1.19] | 0.99 [0.88-1.07] | 0.036 |
| HDL3a | 0.78 ± 0.15 | 0.80 ± 0.17 | 0.75 ± 0.13 | 0.117 |
| HDL3b | 0.61 [0.53-0.72] | 0.62 [0.49-0.72] | 0.59 [0.52-0.70] | 0.736 |
| HDL3c | 0.46 [0.36-0.73] | 0.45 [0.36-0.68] | 0.46 [0.35-0.71] | 0.606 |
| HDL subclasses -cholesterol plasma concentrations (mg/dl) | ||||
| HDL2b | 19.9 ± 4.8 | 18.6 ± 4.9 | 21.4 ± 4.1 | 0.002 |
| HDL2a | 6.9 ± 2.5 | 6.2 ± 2.4 | 7.3 ± 2.4 | 0.016 |
| HDL3a | 9.7 ± 2.0 | 10.0 ± 1.94 | 9.3 ± 2.2 | 0.069 |
| HDL3b | 3.9 ± 1.4 | 4.2 ± 1.7 | 3.6 ± 1.1 | 0.028 |
| HDL3c | 2.7 [1.4-4.0] | 3.3 [1.9-4.7] | 2.3 [1.1-2.4] | 0.007 |
Data represent mean ± SD for normally distributed variables or median [interquartile interval] for nonnormally distributed variables.
Men versus women Student's t-test or Mann-Whitney U for variables with normal or nonnormal distribution variables, respectively.
Total HDL2 (expressed as a percentage of the total HDL) was considered to be the summatory of HDL2b and HDL2a, whereas total HDL3 was the summatory of HDL3a, HDL3b, and HDL3c relative proportions (Supplementary Fig. IIB). Using this estimation, when subclasses were determined by cholesterol content, HDL2 represented the most abundant fraction of HDL (62.2% [55.4–67.5]), and HDL3 was the least abundant fraction of total HDL (39.3 ± 11.5%]). In contrast, when they were determined by protein, the HDL3 was the most abundant fraction (56.9 ± 9.8%) whereas HDL2 was the least abundant fraction (43.0 ± 9.8%) of HDL (Supplementary Fig. IIB).
HDL2b, 3b, and 3c subclasses were significantly different between men and women when gels were stained by cholesterol, whereas HDL2b, 2a, and 3c reached a significant difference when they were stained by protein (Table 2). HDL2a, 3a, and 3c correlated with the age of the subjects when they were determined by protein (Table 3). As a consequence, when we compared the relative proportions of HDL3a determined by protein and corrected by age, they reached a significant difference between men and women (P = 0.001). In contrast, only HDL2a and HDL3c showed a significant correlation with age when they were determined by cholesterol content (Table 3).
TABLE 3.
Correlation analyses between age and HDL subclasses (%)
| Determined by Cholesterol |
Determined by Protein |
|||
|---|---|---|---|---|
| Correlation | p | Correlation | p | |
| HDL subclasses (%) | ||||
| HDL2b | 0.042 | 0.679a | −0.176a | 0.071 |
| HDL2a | 0.228 | 0.022b | 0.489a | 0.000 |
| HDL3a | 0.135 | 0.179a | 0.316a | 0.001 |
| HDL3b | −0.046 | 0.644a | 0.027a | 0.781 |
| HDL3c | −0.301 | 0.002b | −0.354a | 0.000 |
Pearson's coefficient.
Spearman's rho.
We further calculated the cholesterol-to-protein (C/P) ratio as an indicator of HDL composition for each HDL subclass. As expected, HDL2b had the highest C/P ratio, and this value decreased gradually along the HDL subclasses up to the HDL3c, which had the lowest ratio (Table 2). When we compared the C/P ratio between men and women, we observed that only the HDL2a subclass differed significantly; men had a slightly higher (about 8%) content of protein than women in this HDL fraction (Table 2). However, the C/P ratios for the other HDL subclasses were comparable between men and women and they were not age dependent (data not shown).
The relative proportions of HDL subclasses determined by cholesterol staining were transformed to plasma concentrations, as indicated in the Methods section, and the results are shown in Table 2. Women had higher plasma cholesterol concentrations from large HDL2b and 2a particles, whereas men had significantly higher HDL3b and 3c cholesterol plasma levels.
We additionally investigated for possible statistical associations between HDL subclasses determined by cholesterol, HDL subclasses-cholesterol plasma concentrations, and C/P ratios with blood pressure, BMI, and waist circumference. HDL relative proportions, HDL subclasses cholesterol plasma levels, and the C/P ratio had different association patterns with the analyzed risk factors (Table 4). The correlations between the waist circumference and the relative proportion of HDL2b, 3a, and 3b were conserved when they were transformed to the corresponding cholesterol plasma concentrations. There were only three correlations observed between the C/P ratios and the CHD risk factors included in the analysis. Interestingly, the glucose plasma levels did not correlate with any relative proportion of HDL subclass determined by protein, whereas glucose correlated with HDL2a expressed as cholesterol plasma concentrations (r = 0.328, P = 0.001).
TABLE 4.
Correlation analysis between the relative proportions of HDL subclasses determined by cholesterol content, HDL subclasses cholesterol plasma concentrations, cholesterol-to-protein of HDL subclasses, and some nonlipidic coronary risk factors
| SBP |
DBP |
BMI |
Waist Circumference |
|||||
|---|---|---|---|---|---|---|---|---|
| CC | p† | CC | p† | CC | p† | CC | p† | |
| Relative proportion | ||||||||
| HDL2b | −0.238 | 0.023 | −0.211 | 0.043 | −0.325 | 0.021 | −0.338 | 0.007 |
| HDL2a* | NS | 0.216 | 0.039 | NS | NS | |||
| HDL3a | 0.250 | 0.016 | 0.326 | 0.002 | NS | 0.268 | 0.034 | |
| HDL3b | NS | NS | 0.319 | 0.024 | 0.398 | 0.001 | ||
| HDL3c* | NS | NS | 0.261 | 0.039 | NS | |||
| Plasma Concentration | ||||||||
| HDL2b | −0.240 | 0.020 | −0.210 | 0.0040 | −0.261 | 0.038 | −0.4021 | 0.004 |
| HDL2a | NS | NS | −0.468 | 0.001 | −0.461 | 0.001 | ||
| HDL3a | 0.249 | 0.015 | 0.351 | 0.003 | NS | 0.428 | 0.002 | |
| HDL3b | NS | NS | 0.301 | 0.016 | 0.417 | 0.003 | ||
| HDL3c* | NS | NS | 0.260 | 0.040 | NS | |||
| Cholesterol-to-protein ratio | ||||||||
| HDL2b | NS | NS | NS | NS | ||||
| HDL2a* | NS | NS | NS | NS | ||||
| HDL3a | NS | NS | NS | 0.279 | 0.027 | |||
| HDL3b* | NS | NS | NS | NS | ||||
| HDL3c* | 0.333 | 0.001 | 0.320 | 0.002 | NS | NS | ||
Only the correlation coefficients (CC) statistically significant (P < 0.05) are shown. NS, non statistically significant (P > 0.05). SBP, systolic blood pressure. DBP, diastolic blood pressure.
Data logarithmically transformed for analysis.
The analysis was corrected by age and gender.
DISCUSSION
In the present study, we developed a new method for the specific staining of cholesterol on a polyacrylamide gel. The specificity of the enzymes guarantee that only cholesterol was detected by this method; when cholesterol oxidase or peroxidase was omitted from the reaction mixture, there was not any detectable band on the gel (results not shown). By this procedure, we were able to perform a densitometric analysis for the semiquantitative determination of HDL subclasses. Densitometric analysis of Coomassie blue-stained gels has been extensively used for the quantification of the relative distribution of HDL subclasses (3, 4, 15–17, 20, 23). In this context, the enzymatic method developed in the present study allowed cholesterol and protein staining on the same electrophoresis lane.
Because the content of polyacrylamide increases along the gel, it was possible that the substrates could be easily reached by the enzymes at low polyacrylamide concentrations as compared with the more concentrated sections of the gel. In order to rule out this possibility, we measured the conversion of cholesteryl esters to cholestenone at 1 h of incubation using a radioactive label. The radioactivity of cholesteryl esters, free cholesterol, and cholestenone was measured in the section of the gel that corresponded to the five HDL subclasses. Our results demonstrated that there was not any significant difference in the percentage of substrates converted to products along the electrophoresis gel. Therefore, we concluded that this enzymatic method is homogeneous along the polyacrylamide gel gradient and none of the HDL subclasses are over- or sub-estimated.
As has been observed in previous studies using other methodologies, the relative proportion of HDL subclasses determined by cholesterol is notably different from those determined by protein (7, 18, 21, 22). On the basis of our results, the existing controversy regarding whether the small HDL or the large HDL is the most antiatherogenic fraction (6, 11–13, 24, 25) could be related to the method that has been used as they may not be comparable. Furthermore, this study suggests that both protein and cholesterol should be used for the quantification of HDL subclasses because they potentially offer different information. The main disadvantage of the protein staining by Coomassie blue is that the method cannot be quantitative; to our knowledge, there is no procedure that selectively assesses HDL-total protein in plasma. The protein fraction, particularly apo AI of HDL, has been used for the quantification of HDL subclasses in two-dimensional electrophoresis (10). Other components of HDL such as phospholipids and triglycerides may also be used in the quantification of subclasses. Because HDL subclasses differ in lipid composition, the HDL size distribution is expected to be different as a function of the lipidic component used for enzymatic staining. Moreover, HDL subclasses plasma lipid concentrations may provide more relevant information about atherosclerosis risk and about HDL metabolism. The validity of these suggestions should be demonstrated in future studies.
The simultaneous determination of cholesterol and protein allows for the estimation of the C/P ratio of the HDL subclasses. This ratio may be considered as indicative of HDL composition (26); even though we did not directly demonstrate the validity of this statement, the fact that the C/P ratio gradually decreased from HDL2b to HDL3c is congruent with the notion that large HDLs have a higher content of lipids, whereas small particles are protein-rich (13, 26).
To explore the potential clinical value of the different parameters generated in this study, we performed a correlation analysis between HDL subclasses and some variables associated to the cardiovascular disease risk. Our results clearly showed that the HDL relative proportions, HDL subclasses cholesterol plasma levels, and the C/P ratio had different association patterns with the analyzed risk factors; on the basis of these observations, we suggest that the new parameters derived from this study extend the possibilities to better assess the CHD risk. Hence, our study justifies future research on the potential clinical usefulness of these new parameters.
Our results also demonstrated that HDL2a, 3a, and 3c subclasses were correlated with age when they were determined by protein, whereas HDL3a subclass did not correlate when it was determined by cholesterol. These results suggest that HDL3a subclass changes its composition with increasing age. In contrast, HDL2a and 3c correlated with age when they were determined by either cholesterol or protein, suggesting that these subfractions remained of constant composition over time. Therefore, the potential clinical application of HDL subclasses could be higher when both components, protein and cholesterol, are used for their quantification.
Until now, only NMR and selective precipitation have offered quantitative results for HDL subclasses (5, 8). In this context, another advantage of our new enzymatic method for cholesterol staining on electrophoresis gel is the possibility to generate quantitative results for HDL subclasses. The polyacrylamide gradient gel for the estimation of HDL subclasses has been described as a semiquantitative method (17), and the relative proportions obtained by this method are interdependent, i.e., when one HDL subclass increases, the others decrease (15, 17, 23). This interdependence could be considered one of the most important limitations of the quantification of HDL subclasses by PAGE. Therefore, the possibility of transforming the semiquantitative data to plasma cholesterol concentrations of HDL subclasses offsets that limitation. For this reason, although men had a relative proportion of HDL2a comparable to that of women [14.1 (12.6–16.1) vs. 14.1 (13.3–15.4) %, respectively, P = 0.501] the plasma cholesterol concentrations of this HDL subclass differ by gender (6.2 ± 2.4 vs. 7.3 ± 2.4 mg/dl, for men and women respectively, P = 0.016). Therefore, quantitative analysis expressed as plasma concentrations offers more accurate information about HDL subclasses than that provided by the relative proportions expressed as a percentage of total HDL.
In summary, we described an enzymatic procedure to specifically stain cholesterol on electrophoresis gels. By combining this procedure and protein staining with Coomassie blue, we demonstrated that the relative proportions of HDL subclasses differ as a function of the HDL component used for quantification. The C/P ratio for each HDL subclass as well as their cholesterol plasma concentration may also be estimated by our enzymatic method. The potential clinical applications of this methodology should be tested in future studies.
Supplementary Material
Footnotes
Abbreviations:
- apo
- apolipoprotein
- BMI
- body mass index
- CHD
- coronary heart disease
- C/T
- cholesterol-to-protein ratio
- CV
- coefficient of variation
- HDL-C
- high-density lipoprotein-cholesterol
- LDL-C
- low-density lipoprotein-cholesterol
- MTT
- thiazolyl blue teratozolium bromide
- PMS
- phenazine methosulphate
- TBE
- Tris-borate buffer
- TC
- total cholesterol
- TG
- triglycerides
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Assmann G., Schulte H., von Eckardstein A., Huang Y. 1996. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk: the PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 124: S11–S20. [DOI] [PubMed] [Google Scholar]
- 2.Feig J. E., Shamir R., Fisher E. A. 2008. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr. Drug Targets. 9: 196–203. [DOI] [PubMed] [Google Scholar]
- 3.Blanche P. J., Gong E. L., Forte T. M., Nichols A. V. 1981. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim. Biophys. Acta. 665: 408–419. [DOI] [PubMed] [Google Scholar]
- 4.Huesca-Gómez C., Carreón-Torres E., Nepomuceno-Mejía T., Sánchez-Solorio M., Galicia-Hidalgo M., Mejía A. M., Montaño L. F., Franco M., Posadas-Romero C., Pérez-Méndez O. 2004. Contribution of cholesteryl ester transfer protein and lecithin:cholesterol acyltranferase to HDL size distribution. Endocr. Res. 30: 403–415. [DOI] [PubMed] [Google Scholar]
- 5.Gidez L. I., Miller G. J., Burstein M., Slagle S., Eder H. A. 1982. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J. Lipid Res. 23: 1206–1223. [PubMed] [Google Scholar]
- 6.Lamarche B., Moorjani S., Cantin B. 1997. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Arterioscler. Thromb. Vasc. Biol. 17: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni K. R. 2006. Cholesterol profile measurement by vertical auto profile method. Clin. Lab. Med. 26: 787–802. [DOI] [PubMed] [Google Scholar]
- 8.Otvos J. D., Jeyarajah E. J., Bennett D. W. 1991. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin. Chem. 37: 377–386. [PubMed] [Google Scholar]
- 9.Elkhalil L., Majd Z., Bakir R., Perez-Mendez O., Castro G., Poulain P., Lacroix B., Duhal N., Fruchart J. C., Luc G. 1997. Fish-eye disease: structural and in vivo metabolic abnormalities of high-density lipoproteins. Metabolism. 46: 474–483. [DOI] [PubMed] [Google Scholar]
- 10.Asztalos B. F., Lefevre M., Foster T. A., Tulley R., Windhauser M., Wong L., Roheim P. S. 1997. Normolipidemic subjects with low HDL cholesterol levels have altered HDL subpopulations. Arterioscler. Thromb. Vasc. Biol. 17: 1885–1893. [DOI] [PubMed] [Google Scholar]
- 11.Harchaoui K., Arsenault B. J., Franssen R., Després J. P., Hovingh G. K., Stroes E. S., Otvos J. D., Wareham N. J., Kastelein J. J., Khaw K. T., et al. 2009. High-density lipoprotein particle size and concentration and coronary risk. Ann. Intern. Med. 150: 84–93. [DOI] [PubMed] [Google Scholar]
- 12.Castro G. R., Fielding C. J. 1988. Early incorporation of cell-derived cholesterol into pre-beta-migrating high density lipoprotein. Biochemistry. 12: 25–29. [DOI] [PubMed] [Google Scholar]
- 13.Kontush A., Chantepie S., Chapman M. J. 2003. Small dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 14.Deakin S., Leviev I., Gomaraschi M., Calabresi L., Franceschini G., James R. W. 2002. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J. Biol. Chem. 277: 4301–4308. [DOI] [PubMed] [Google Scholar]
- 15.Carreón-Torres E., Juárez-Meavepeña M., Cardoso-Saldaña G., Gómez C. H., Franco M., Fievet C., Luc G., Juárez-Oropeza M. A., Pérez-Méndez O. 2005. Pioglitazone increases the fractional catabolic and production rates of high-density lipoproteins apo AI in the New Zealand White Rabbit. Atherosclerosis. 181: 233–240. [DOI] [PubMed] [Google Scholar]
- 16.Huesca C., Luc G., Duhal N., Pérez-Méndez O. 2004. Ciprofibrate increases synthesis and catabolism of HDL apo AI and AII in patients with hypertriglyceridemia. Atherosclerosis. 5(suppl): 64. [Google Scholar]
- 17.Warnick G. R., McNamara J. R., Boggess C. N., Clendenen F., Williams P. T., Landolt C. C. 2006. Polyacrylamide gradient gel electrophoresis of lipoprotein subclasses. Clin. Lab. Med. 26: 803–846. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni K. R., Marcovina S. M., Krauss R. M., Garber D. W., Glasscock A. M., Segrest J. P. 1997. Quantification of HDL2 and HDL3 cholesterol by the Vertical Auto Profile-II (VAP-II) methodology. J. Lipid Res. 38: 2353–2364. [PubMed] [Google Scholar]
- 19.DeLong D. M., DeLong E. R., Wood P. D., Lippel K., Rifkind B. M. 1986. A comparison of methods for the estimation of plasma low- and very low density lipoprotein cholesterol. The Lipid Research Clinics Prevalence Study. JAMA. 256: 2372–2377. [PubMed] [Google Scholar]
- 20.Huesca-Gómez C., Franco M., Luc G., Montaño L. F., Massó F., Posadas-Romero C., Pérez-Méndez O. 2002. Chronic hypothyroidism induces abnormal structure of high-density lipoproteins and impaired kinetics of apolipoprotein A-I in the rat. Metabolism. 51: 443–450. [DOI] [PubMed] [Google Scholar]
- 21.Li Z., McNamara J. R., Ordovas J. M., Schaefer E. J. 1994. Analysis of high density lipoproteins by a modified gradient gel electrophoresis method. J. Lipid Res. 35: 1698–1711. [PubMed] [Google Scholar]
- 22.Warnick G. R. 2008. High-density lipoproteins: the neglected stepchildren whose importance as a risk factor continues to be defined. Clin. Lab. Med. 54: 923–924. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Méndez O., Torres-Tamayo M., Posadas-Romero C., Vidaure Garcés V., Carreón-Torres E., Mendoza-Pérez E., Medina Urrutia A., Huesca-Gómez C., Zamora-González J., Aguilar-Herrera B. 2007. Abnormal HDL subclasses distribution in overweight children with insulin resistance or type 2 diabetes mellitus. Clin. Chim. Acta. 376: 17–22. [DOI] [PubMed] [Google Scholar]
- 24.Linsel-Nitschke P., Jansen H., Aherrarhou Z., Belz S., Mayer B., Lieb W., Huber F., Kremer W., Kalbitzer H. R., Erdmann J., et al. 2009. Macrophage cholesterol efflux correlates with lipoprotein subclass distribution and risk of obstructive coronary artery disease in patients undergoing coronary angiography. Lipids Health Dis. 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otvos J. D., Collins D., Freedman D. S., Shalaurova I., Schaefer E. J., McNamara J. R., Bloomfield H. E., Robins S. J. 2006. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 28: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 26.Rye K. A., Hime N. J., Barter P. J. 1995. The influence of cholesteryl ester transfer protein on the composition, size, and structure of spherical, reconstituted high density lipoproteins. J. Biol. Chem. 270: 189–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.