Abstract
Aging is a biological process that affects most cells, organisms and species. Human aging is associated with increased susceptibility to a variety of chronic diseases, including cardiovascular disease, Type 2 diabetes, neurological diseases and cancer. Despite the remarkable progress made during the last two decades, our understanding of the biology of aging remains incomplete. Telomere biology has recently emerged as an important player in the aging and disease process.
Keywords: aging, Alzheimer's disease, atherosclerosis, heart failure, longevity, Parkinson's disease, telomerase, telomere
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AD, Alzheimer's disease; CHF, chronic heart failure; CRP, C-reactive protein; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LDL, low-density lipoprotein; PBMC, peripheral blood mononuclear cell; PD, Parkinson's disease; SHR, spontaneous hypertensive rats; T1D, Type 1 diabetes; T2D, Type 2 diabetes; TERC, telomerase RNA component; TERRA, telomeric repeat-containing RNA; TERT, telomerase reverse transcriptase; TRF, telomeric repeat-binding factor
INTRODUCTION
The 2009 Nobel Prize in Physiology or Medicine was awarded jointly to Dr Elizabeth H. Blackburn, Dr Carol W. Greider and Dr Jack W. Szostak for their pioneer research on telomeres and the enzyme that forms them, telomerase. Telomeres are specialized structures located at the terminal ends of chromosomes and play a critical role in maintaining the integrity of the genome. In proliferating cells lacking functional telomerase, telomeres progressively shorten during every mitotic division, and the cells eventually undergo senescence. Therefore, telomere length shortening has been recognized as not only a marker of biological aging but also a mechanism with important functional consequences. However, telomere shortening is not the only factor that dictates cell fates. The presence of telomerase itself, a buffer against telomere shortening, is another critical factor. Not only is telomere biology involved in the biological process of aging, but it has been also postulated to play a role in multiple aging-associated diseases and conditions. In the present review, we will discuss the role of telomere biology in CVD (cardiovascular disease) and neurodegenerative diseases. The role of telomere biology in cancer has been extensively discussed in several recent reviews [1–4].
THE TELOMERE COMPLEX
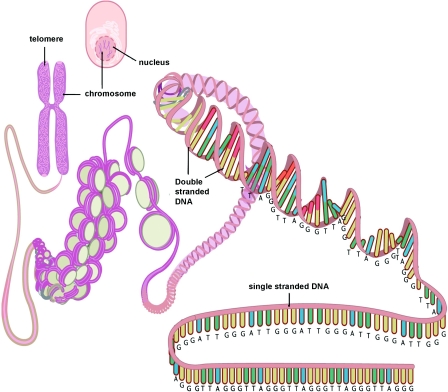
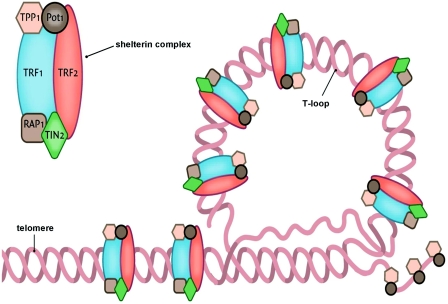
Telomeres are specialized DNA structures located at the terminal ends of the chromosomes [5]. Their primary function is to maintain genome stability. Telomeres consist of non-coding double-stranded repeats of guanine-rich tandem DNA sequences (TTAGGG) that are extended 9–15 kb in humans and end in a 50–300 nucleotide 3′ single guanine strand overhang [6]. This overhang can fold back and invade the double-stranded telomere helix, forming a large ‘T-loop’. The T-loop facilitates the formation of a high-order structure mediating the end-capping [7] (Figure 1). The stability of the T-loop is largely dependent on the integrity of the associated telomere-specific proteins, also called the shelterin complex [8] (Figure 2). The proteins involved in the shelterin complex include TRF (telomeric repeat-binding factor) 1 and TRF2, which bind to the double-stranded segment of telomeric DNA [9,10]. POT1 (protein protection of telomeres 1) binds directly to the single-stranded telomeric DNA and interacts directly with TPP1 (tripeptidyl peptidase 1) [11]. Rap1 (repressor activator protein 1) binds TRF2 [12], and TIN2 (TRF1-interacting nuclear factor 2) is a central component of the complex interacting with TRF1, TRF2 and TPP1 [13,14]. The telomere complex plays a critical role by protecting the chromosomes from recognition by the DNA damage-repair system as DNA ‘breaks’ and activation of the p53 or p16INK4a pathway, eventually leading to cellular senescence or apoptosis [15].
Figure 1. Simplified diagram depicting the structure of the telomere and its location on the chromosome and in the cell.
This Figure was reproduced from Huzen, J., van Veldhuisen, D.J., van Gilst, W.H. and van der Harst, P. (2008), Telomeres and biological ageing in cardiovascular disease, Ned. Tijdschr. Geneeskd., vol. 152, pp. 1265–1270, with permission from the Nederlands Tijdschrift voor Geneeskunde.
Figure 2. Simplified scheme depicting the terminal end of the telomere concealing the terminal single-stranded part with help of the shelterin complex.
This Figure was reproduced from Huzen, J., van Veldhuisen, D.J., van Gilst, W.H. and van der Harst, P. (2008), Telomeres and biological ageing in cardiovascular disease, Ned. Tijdschr. Geneeskd., vol. 152, pp. 1265–1270, with permission from the Nederlands Tijdschrift voor Geneeskunde.
Owing to the inability of DNA polymerase to fully replicate the 3′ end of the DNA strand, i.e. the ‘end-replication problem’, the telomeres naturally lose approx. 30–150 bp with each cell division [16]. Furthermore, telomere erosion can occur due to environmental factors, especially ROS (reactive oxygen species). Eventually, telomeres will reach a critical short length resulting in a decreased ability to recruit shelterin proteins and to form the protective internal nucleotide loops. This impairment will lead to the activation of the p53 or p16INK4a pathway and result in cellular senescence or apoptosis [3]. On average, cells are estimated to reach this ‘Hayflick limit’ after approx. 50 population doublings [17]. It is important to realize that not only the shortened telomeres, but also disruption of the associated shelterin proteins, can lead to the inability to protect the integrity of the genome.
THE TELOMERASE COMPLEX
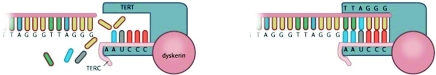
Telomere shortening is not the only factor that dictates cell fates. The presence of telomerase itself is another critical factor [18]. Telomerase not only functions to maintain telomere length [19], but also preserves healthy cell function and long-term immune function [18,20]. When telomerase is active, a ‘mitotic clock’ model does not apply; by stabilizing telomeres, telomerase effectively moves back the hands of any such ‘clock’. Active telomerase helps even very short telomeres to be functional [18]. Human telomerase consists of a RNA component [TERC (telomerase RNA component)] that serves as a template and a catalytic subunit of telomerase [TERT (telomerase reverse transcriptase)] that is required for the synthesis of new telomeric DNA repeats (Figure 3). The 5′ end of TERC contains the template region and the 3′ end of TERC contains two binding sites for additional telomerase-associated proteins. Together with NHP2, NOP10 and GAR1, dyskerin binds to an H/ACA box, which is essential for telomerase assembly and stability [21]. TCAB1 (telomerase Cajal body protein 1) interacts with dyskerin, which is crucial for facilitating telomerase trafficking to the Cajal body (CAB box) and for telomere maintenance [22].
Figure 3. Schematic overview of telomerase.
Active telomerase is composed from two RNA complexes (TERC; only one depicted) and two telomere reverse transcriptases (TERC; one depicted) stabilized by dyskerin. This Figure was reproduced from Huzen, J., van Veldhuisen, D.J., van Gilst, W.H. and van der Harst, P. (2008), Telomeres and biological ageing in cardiovascular disease, Ned. Tijdschr. Geneeskd., vol. 152, pp. 1265–1270, with permission from the Nederlands Tijdschrift voor Geneeskunde.
In addition to the widely appreciated telomere maintenance function, new roles of telomerase have recently been suggested. Loss of TERT does not alter short-term telomere integrity but, instead, affects the overall configuration of chromatin and impairs the DNA damage response [23]. In addition, TERT can promote proliferation of resting stem cells [24]. Moreover, telomerase directly modulates Wnt/β-catenin signalling by serving as a cofactor in a β-catenin transcriptional complex [25]. Wnt/β-catenin signalling plays a crucial role not only during embryogenesis, but also in normal adult tissue genesis. Emerging evidence suggests that Wnt signalling is involved in cardiac differentiation and development [26], angiogenesis, cardiac hypertrophy, cardiac failure and aging [27].
TELOMERE LENGTH DETERMINANTS
Telomere length is highly variable among individuals at the same age and to a large extent genetically determined, with heritability estimates ranging from 40 to 80%. Genome-wide association studies have identified associated loci on chromosome 18q12.2 [28], 3q26 (near TERC) [29] and 10q24.33 [30].
Telomere lengths are highly synchronized among the DNA samples from white blood cells, umbilical artery and skin within individual newborns, but exhibited a high variability among newborns [31]. In addition, telomere lengths become more heterogeneous among different organ tissues in the elderly [32]. Despite no differences in telomere length between African American and Caucasian or male and female newborns [31], African American adults have longer telomeres than their Caucasian counterparts [33], and adult males generally have shorter telomeres than females [34]. A recent study conducted in 667 Caucasian and African American adolescents showed that the ethnicity and sex differences in telomere length have already emerged during adolescence [35]. Women also exhibit a significantly lower rate of age-dependent telomere attrition as compared with men [36], possibly due to the stimulating properties of oestrogen on telomerase. A remarkable association between the levels of oestrogen and telomerase activity has been shown under physiological conditions [37]. Studies in vitro show that oestrogen rapidly up-regulates telomerase gene expression and activity [38].
Telomere length is not only genetically determined, but also shaped by environmental factors. Owing to the high content of guanine residues, telomeres are highly sensitive to damage by oxidative stress. The contribution to the telomere loss by oxidative DNA damage is believed to be greater than by the ‘end-replication problem’ [39]. Prolonged oxidative damage also dramatically decreases telomerase activity and accelerates telomere shortening in vascular smooth muscle cells and endothelial cells [40]. Conversely, addition of antioxidants decelerates telomere shortening in cultured cells and prolongs telomerase activity [41]. Systemic oxidative stress assessed by urinary 8-epiprostaglandin F2α is associated with shorter leucocyte telomeres in hypertensive men from the Framingham Heart Study [42]. A higher level of oxidized LDL (low-density lipoprotein) is associated with shorter leucocyte telomere length and increased stiffness of the carotid artery [43].
Inflammation is also thought to contribute to telomere attrition in cells of the immune system by promoting leucocyte turnover. An increased production of certain cytokines has been shown to adversely affect telomerase activity and telomere length [44]. CRP (C-reactive protein), an index of inflammation, is inversely correlated with leucocyte telomeres in premenopausal but not postmenopausal women [45]. Shorter telomeres have been related to higher IL-6 (interleukin-6) and CRP in haemodialysis patients [46] and in men [47].
Psychological and life stress have been shown to be significantly associated with higher levels of oxidative stress, lower telomerase activity and shorter leucocyte telomere lengths. Decreased perceived mental health is associated with shorter leucocyte telomeres in patients with CHF (chronic heart failure) [48]. Most strikingly, childhood mistreatment, adverse life events, chronic and serious illness are associated with shorter telomeres later in life [49]. Smoking is typically marked by an increased oxidative stress and inflammation in concert with shortened telomere length [43]. Female smokers tend to have shorter telomeres, and smoking reduces telomere length in a dose-dependent manner [50]. Physical activity has been positively associated with telomere length in adults [51] and in adolescents [35], suggesting an anti-aging benefit. Increased dietary intake of marine ω−3 fatty acids is associated with prolonged survival in patients with coronary heart disease. Farzaneh-Far et al. [52] recently showed that individuals in the lowest quartile of DHA+EPA (docosahexaenoic acid+eicosapentaenoic acid) experienced the fastest telomere shortening, whereas those in the highest quartile experienced the slowest telomere shortening over 5 years. Each S.D. increase in DHA+EPA levels was associated with a 32% reduction in the odds of telomere shortening. Other lifestyle factors such as socioeconomic status also have an impact on telomere length [53].
REGULATION OF TELOMERASE FUNCTION
Telomerase function is also regulated by genetic, epigenetic and environmental factors. Although most genes involved in telomere biology are highly conserved between species and have limited genetic diversity in humans [54], several polymorphisms in the TERT promoter region and genes coding for telomerase-associated proteins have been shown to regulate telomerase gene expression and activity level [55–58]. It is becoming increasingly apparent that epigenetic modifications including CpG methylation, histone methylation and acetylation is important for TERT transcriptional regulation [59–61].
Chronic life stress is linked to shorter telomeres and has been associated with reduced telomerase activity [62]. Oxidized LDL and TNF-α (tumour necrosis factor-α) were inversely correlated to telomerase activity in haemodialysis patients [63]. Numerous in vitro studies demonstrate that oxidative stress and inflammation reduce telomerase activity and shorten telomere length in cultured endothelial progenitor cells, human-derived fibroblasts and neoplastic cells [64–67]. Lifestyle factors are also important regulators of telomerase function. Exercise increased aortic telomerase activity and telomere-stabilizing proteins in mice. Additionally, exercise down-regulated vascular cell-cycle inhibitors and apoptosis regulators, and this down-regulation was completely absent in TERT−/− mice. Young and middle-aged athletes were also characterized by a profound up-regulation of telomerase activity and telomere proteins as well as down-regulation of pro-apoptotic proteins compared with untrained individuals [68]. Ornish et al. [69] conducted a 3-month comprehensive lifestyle intervention (including nutrition and physical activity) in 24 low-risk prostate cancer patients and showed that, by the end of 3 months, PBMC (peripheral blood mononuclear cell) telomerase activity had increased by 29%. That study is important because it shows that PBMC telomerase activity is not only measurable, but also might be boosted by healthy lifestyle behaviours.
Regulation of telomerase activity is not only controlled at the level of TERT transcription, but also at the post-transcriptional level [70]. Phosphorylation and nuclear translocation both play an important role in the post-transcriptional regulation of telomerase activity [71]. Several pathways are involved in TERT post-transcriptional regulation such as the PI3K (phosphoinositide 3-kinase)/Akt signal transduction pathway. Resveratrol, a drug compound, possesses diverse biochemical and physiological actions, including cardioprotective properties and has been shown to increase telomerase activity through increased Akt phosphorylation [72]. ACEIs (angiotensin-converting enzyme inhibitors) promote endothelial cell survival and prolong their lifespan partly through increased telomerase function by Akt phosphorylation [73].
Recently, the notion that telomeres are transcriptionally silent has been challenged. TERRA (telomeric repeat-containing RNA) is a new addition to the telomeric ribonucleoprotein complex [74–76]. TERRA is a heterogeneous non-coding RNA that consists of a combination of subtelomeric and telomeric region that can form an intermolecule G-quadruplex structure with telomeric DNA repeats. TERRA is transcribed in a centromere to telomere direction, indicating that the transcriptional start site lies within the subtelomeric sequence [74–76]. In mammals, TERRA ranges between 100 and 9 kb, is expressed in most tissues, and is regulated by the nonsense-mediated RNA decay machinery [77,78]. TERRA displays a strong inverse correlation with telomerase activity [76,79]. Emerging evidence suggests that TERRA plays important roles in the regulation of telomerase, maintaining higher-order telomeric chromatin structure and mediating several crucial functions of the telomeres [74,77].
TELOMERE BIOLOGY IN STEM CELLS
Stem cells regenerate our body. Telomeres and telomerase are main components of the stem cell ‘ignition’ mechanism, providing a way to restrain cancer and delay aging [80]. Stem cells and progenitor cells play an important role in maintaining tissue homoeostasis by replenishing senescent or apoptotic cells and repairing damage that occurs throughout life. Exhaustion of the stem cell or progenitor cell pool is a significant risk factor in the aging process [81]. Stem cells are able to divide past the Hayflick limit due to the presence of telomerase. Normal tissue stem cells show progressive telomere shortening with age, and telomerase is highly regulated. Conversely, telomere length is maintained in cancer cells, and telomerase is continuously expressed [82]. Stem cell populations in various compartments of the body maintain telomeres of different lengths, with the longest telomeres observed in the testis, skin, small intestine, cornea and brain. Male germ cells are exceptional because their telomeres actually get longer with aging presumably, in part, due to high telomerase activity. Additionally, less differentiated stem cells maintain longer telomeres than more differentiated cells [83]. Haematopoietic stem cells give rise to the various types of blood cells. Although they express telomerase, it is insufficient to maintain telomere length, and they undergo a functional decline with age corresponding to decreased regenerative capacity. Indeed, the age of the donor in bone marrow transplantation is significant for recipient survival [84]. Telomerase activity is increased in haematopoietic stem cells upon immune stimulation allowing for rapid clonal expansion in response to antigen. Human embryonic stem cells display greater telomere stability than adult stem cells, possibly due to increased DNA maintenance or decreased oxygen radicals [85].
TELOMERE BIOLOGY AND HUMAN AGING-ASSOCIATED DISEASES
The role of dysfunctional telomere biology in cancer is well established. Several anti-cancer drugs and cancer vaccines that target the telomerase are in Phase III clinical trials. By contrast, the role of telomere biology in the development of aging-related diseases, including CVD, is only partly understood, despite the progress made during the last decade.
CVD
CVD is the leading cause of death worldwide. The cardiovascular system is a vital organ system mostly affected by the aging process. Unfortunately, aetiologies of CVD remain largely unknown, which hampers prevention and treatment efforts. Although genetic and environmental factors are clearly responsible, common genetic variants identified by genome-wide association studies, so far, confer relatively small increments in risk and explain only a small proportion of familial clustering. Rare variants, telomere dysfunction and epigenetic effects are most likely to account for the missing heritability [86,87]. Indeed, evidence for an association between circulating leucocyte telomere length and CVD is rapidly accumulating [88].
Hypertension
One in three U.S. adults suffers from hypertension. Data from animal and human studies suggest that telomere dysfunction and hypertension may be causally linked. TERT expression and telomerase activity were increased in the aortae of SHR (spontaneous hypertensive rats) at ages preceding the establishment of hypertension [89]. Moreover, TERC−/− mice show increased ET-1 (endothelin-1) production and develop hypertension [90]. Endothelial progenitor cells from hypertensive patients and from SHR exhibit reduce telomerase activity and accelerate senescence [91]. A study of 49 adult twin pairs found that telomere length was inversely associated with pulse pressure. Both telomere length and pulse pressure are highly familial [92]. Men with shorter telomere length were more likely to exhibit higher pulse pressure and pulse wave velocity [93]. Pulse pressure is a clinical marker of large artery stiffness that increases with chronological age and is a predictor of cardiovascular mortality and morbidity. In pre-hypertension and established hypertension, the RAS (renin–angiotensin system) is often activated. Telomere length was found to be shorter in individuals with a higher renin/aldosterone ratio, especially in participants with hypertension in the Framingham Heart Study [94]. Moreover, shorter telomere length was associated with hypertension, increased insulin resistance and oxidative stress in the offspring cohort of the Framingham Heart Study [42]. Furthermore, normotensive individuals with shorter telomeres were more likely to develop hypertension, and hypertensive subjects with shorter telomeres were more susceptible to develop atherosclerosis [95].
Atherosclerosis
Atherosclerosis is also an aging-related systemic disease with early origins. A tight interplay between cell senescence and atherosclerosis has been suggested by in vitro and in vivo studies. Senescent-positive endothelial cells can be found in almost any atherosclerotic plaque, and endothelial dysfunction is recognized as one of the earliest events of atherosclerosis [96]. The endothelial and smooth muscle cells in the vessel, which are most susceptible to develop atherosclerosis, are highly proliferative and highly subjected to stress by increased mean arterial pressure, cholesterol and oxidative stress [81]. In complicated plaques, senescence of various cell types, including vascular smooth muscle cells and macrophages, favours both plaque rupture and atherothrombosis [97]. Cell senescence induced by telomere shortening contributes to the development of atherosclerosis. The pioneer clinical study, conducted by Samani et al. [98] in 2001, showed that leucocyte telomere length was 303 bp shorter in patients with severe coronary artery disease than that in age-matched controls, which is equivalent to 8.6 years of aging. Later, large-scale studies confirmed these findings. A retrospective survey of 203 subjects with a premature myocardial infarction before the age of 50 and 180 controls showed that age- and sex-adjusted mean telomere length of cases was 300 bp shorter than that of controls and on average equivalent to controls 11.3 years older [99]. Farzaneh-Far et al. [100] measured telomere length of 780 patients with stable coronary artery disease in the Heart and Soul Study; patients in the lowest quartile of telomere length remained at significantly increased risk of death compared with those in the highest quartile and were also at significantly increased risk of hospitalization for heart failure, suggesting a prognostic value of short telomeres. In the West of Scotland Primary Prevention Study of 1542 participants, subjects in the middle and the lowest tertiles of telomere length were more at risk of developing a coronary heart disease event than were those in the highest tertile; moreover, statin treatment was more beneficial to patients with short telomeres [101]. The Bruneck Study, a population-based prospective study, recently unraveled a highly significant association between short telomeres and cardiovascular risk, independently of age, sex and standard risk factors. Remarkably, telomere length was strongly associated with advanced, but not early, atherosclerosis [102]. In addition, leucocyte telomere length is negatively associated with the presence of coronary atherosclerosis in a low-risk middle-aged cohort free of previously diagnosed CVD [103]. Importantly, healthy lifestyle behaviours might attenuate the association between shorter telomere length and coronary atherosclerosis [104].
Heart failure
Heart failure is a common, costly, disabling and potentially deadly condition. It is associated with a high health expenditure of more than $35 billion annually in the U.S.A. Advanced age is one of the major risk factors for the development of CHF (chronic heart failure), and CHF is characterized by increased myocyte apoptosis and telomere erosion [105]. Data from animal studies suggest a role of telomere dysfunction in myocyte apoptosis and CHF. Telomere shortening in G2 and G5 TERC−/− mice was coupled with attenuation in cardiac myocyte proliferation, increased apoptosis and cardiac myocyte hypertrophy. Eventually, left ventricular failure and pathological cardiac remodelling developed in later generations of TERC−/− mice with critically short telomeres [106].
Patients with CHF have 40% reduced telomeres compared with age- and gender-matched controls, and the degree of telomere shortening is associated with the severity of disease [107]. Moreover, shorter telomeres are associated with decreased renal function, worse outcome in patients with CHF, possibly due to dropout of functional nephrons [81,108]. One S.D. longer telomeres are associated with a 5% higher left ventricular ejection fraction, and telomere length accounts for 12% of the observed variability in ejection fraction in subjects of 85 years and older [109]. In aged diseased hearts characterized by moderate hypertrophy and dilation, cell death markedly increased and occurred only in cells expressing a p16INK4a pathway that had significant telomere shortening [110]. Additionally, shorter telomeres increase the risk of having anaemia associated with poor prognosis in CHF patients [111]. In a longitudinal study of 890 patients with New York Heart Association functional class II to IV heart failure, patients with shorter telomeres were at an increased risk (1.79-fold) of reaching the primary end point (time to death or hospitalization for heart failure) 18 months later. Short telomeres might predict the occurrence of death or hospitalization in patients with chronic heart failure [112].
Diabetes
T2D (Type 2 diabetes), another aging-related disorder, is caused by a combination of peripheral insulin resistance and β-cell dysfunction. Recent evidence, however, suggests that T2D is additionally characterized by impaired β-cell regeneration and reduced β-cell mass [113,114]. Shortened telomeres have been previously associated with diabetes in several small-scale studies, which has been recently confirmed by two large studies [115,116]. Zee et al. [115] studied 432 Caucasian T2D cases and 424 controls; telomeres were shorter in cases than in controls, and shorter telomeres were significantly associated with T2D (adjusted odds ratio=1.748). Salpea et al. [116] studied 569 Caucasian, 103 South Asian and 70 Afro-Caribbean T2D patients and 448 healthy controls; shorter telomeres were associated with the presence of T2D and this could be partially attributed to the high oxidative stress in these patients. Interestingly, short telomeres are independent predictors of progression of diabetic nephropathy in patients with T1D (Type 1 diabetes), an early-onset disease, although telomere length was not different in T1D patients compared with healthy controls [117]. A longitudinal study with 11 years follow-up showed that short telomeres also predicted all-cause mortality in T1D patients [118]. Additionally, both T1D and T2D patients demonstrate shorter telomeres in their arterial walls. Telomere shortening in both mononuclear cells and arterial cells can be reduced by controlling blood sugar levels in both types of diabetes mellitus. Moreover, the mononuclear telomere attrition was completely prevented by adequately glycaemic control in T2D [119]. Pre-diabetes patients with impaired glucose tolerance also display shorter telomeres than healthy controls, and diabetic patients with atherosclerotic plaques display shorter telomeres than diabetic patients without atherosclerosis [120]. Obesity and insulin resistance are also associated with leucocyte telomere length among adult Arabs [121]. Recently, a direct causal link between impaired telomerase activity and impaired insulin secretion as well as glucose intolerance was reported. Young adult TERC−/− mice exhibited impaired glucose tolerance, which was caused by impaired glucose-stimulated insulin secretion from pancreatic islets. The impaired insulin secretion capacity was due to reduced islet size, which was linked to an impaired replication capacity of insulin-producing β-cells in TERC−/− mice [122].
Telomerase dysfunction and CVD
Despite the evidence of telomere shortening in the development of CVD, little is known about the role of telomerase dysfunction in the development of CVD. Existing evidence is mostly from animal research. For example, TERT transgenic mice exhibited a reduced myocardial infarct area after coronary artery ligation, and exogenous TERT expression in cardiac myocytes promoted survival [123]. In addition, TERT transgenic mice showed significant resistance to ischaemic brain injury [124]. Conversely, TERT−/− mice are more susceptible to oxidative stress and to the development of stroke [125]. Werner et al. [126] showed that exercise increased telomerase activity and TERT and TRF2 expression in wild-type mice, but not in TERT−/− mice. This suggests that, in the absence of telomerase, up-regulation of telomere-stabilizing proteins is challenged, and cardiac apoptosis is more severe [127]. Similarly, TERC−/− mice are also well studied. Perez-Rivero et al. [90] reported a direct link between telomerase activity and hypertension. TERC−/− mice exhibited higher ECE (endothelin-converting enzyme) expression, thus increased ET-1 (endothelin-1) production and developed hypertension. Moreover, endothelial progenitor cells from hypertensive patients and from SHR exhibit reduced telomerase activity and accelerated senescence [91]. Late generation TERC−/− mice had diminished angiogenesis potential compared with wild-type mice [128] and suffered from severe left ventricular failure, cardiac structure change and myocyte hypertrophy [106].
Despite the promising data from the mouse models, most findings have not been translated back to human studies [127]. One of the challenges faced by large-scale human epidemiological studies is the requirement of freshly processed PBMCs, which prevents the usage of many existing cohorts. There are only a few small-scale human studies available. The pioneering work conducted by Epel et al. [129] showed that low leucocyte telomerase activity was associated with the major risk factors for CVD, including smoking, poor lipid profile, high systolic blood pressure, fasting glucose and greater abdominal adiposity, in 62 healthy premenopausal women, whereas no association was found between telomere length and CVD risk factors. Therefore they proposed that low leucocyte telomerase constitutes an early marker of CVD risk, possibly preceding shortened telomeres. In addition, this relationship could result, in part, from chronic stress arousal. A couple of other small-scale studies have also showed that telomerase activity of PBMCs was decreased in rheumatoid arthritis patients [130] and haemodialysis patients [131].
AD (Alzheimer's disease)
AD, the major cause of dementia in the elderly, is an aging-associated progressive neurodegenerative disorder. Telomere dysfunction emerged as a potential contributor to the pathogenesis of AD. AD patients have significantly shorter leucocyte telomere length than age-matched controls [132–134]. Older females with Down's syndrome and AD-type dementia had shorter T-cell telomeres than did age-matched controls [135]. Additionally, telomere length in leucocyte and buccal cells were shorter in the younger and older AD patients compared with the old control group. Surprisingly, telomere length within the brain hippocampal tissue was found to be 49% longer in the brain from AD patients compared with normal control tissue. The underlying mechanisms of these interesting phenomena remain to be elucidated [134]. On the other hand, a couple of studies were unable to confirm the above association [136,137]. Lukens et al. [136] found that leucocyte and cerebellum telomere lengths were directly correlated in AD patients. However, cerebellum telomere length was not significantly different between AD patients and age-matched controls, possibly due to insufficient power.
Zhang et al. [138] investigated the telomerase activity of phytohaemagglutinin-activated lymphocytes from AD patients. Telomerase activity in AD patients was significantly elevated compared with healthy controls and vascular dementia patients, possibly as a response to the telomere erosion accompanying the disease. In addition, the telomerase activity of lymphocytes was significantly correlated with the degree of dementia in AD patients, suggesting an accelerated telomere dysfunction in lymphocytes and impaired immune function of AD patients.
PD (Parkinson's disease)
PD is another common neurodegenerative disorder in the elderly characterized by a progressive degeneration of dopaminergic neurons. Both inflammation and oxidative stress contribute to the aetiology of PD. Fewer studies have examined the relationship between telomere length and PD. Wang et al. [139] showed that men with shorter telomeres had a lower risk for PD, which contrasts with the observations in other aging-related diseases. In a study of 28 Japanese male PD patients, leucocyte telomere length in PD patients was not different from those in controls. However, mean telomere length shorter than 5 kb was only found in PD patients, suggesting that telomere shortening is accelerated in PD patients [140]. Additionally, Maede et al. [141] investigated the age-associated alterations of subtelomeric methylation in Japanese PD patients. Short telomeres with hypomethylated subtelomeres increased with aging in the healthy controls, but did not change in the PD patients.
Frailty
At the clinical level, the majority of the aging population spends a variable period before death in a state of declining function, described as the frailty syndrome, representing an excess of deficits over assets in a dynamic state of balance, covering physical, functional, psychological, nutritional and social domains [142,143]. The frailty index is a measure of frailty [144,145]. Few data are available on telomere shortening and frailty, although an association may be expected, since telomere shortening is associated with many diseases and lifestyle that predispose to frailty. In a large sample of Chinese men and women aged 65 years and over, women were frailer than men, but had longer telomere length. In men only, there was a positive association between frailty index and mortality. However, there was no correlation between telomere length and frailty index in either sex [143]. In the National Long Term Care Survey study, leucocyte telomere length is predictive of disability of older individuals in the U.S.A. population [146].
Longevity
Lifespan is determined by processes that are multifactorial and complex. Human longevity is at least partly heritable, the genetic component being approx. 30% [147]. Mutations of Caenorhabditis elegans clk-2 gene, a homologue of yeast Tel2p, are associated with longer telomere length and an extended lifespan [148]. In a cancer-resistant mouse model, mice constitutively expressing the TERT had longer telomeres and extended lifespan [149]. In humans, telomere length positively correlates with years of healthy life [150]. Sequence analysis of TERT and TERC showed overexpression of synonymous and intronic mutations among Ashkenazi centenarians relative to controls [151]. In addition, variations in human telomerase gene are associated with better maintenance of telomere length; longer telomeres are associated with protection from age-related diseases, better cognitive function, and lipid profiles, thus may confer healthy aging and exceptional longevity [151]. Importantly, a common TERT haplotype associated with longer telomere length is found in Ashkenazi centenarians and their offspring, indicating that TERT may function as a genetic agent in lifespan determination [151]. Within-pair analyses of Swedish twins demonstrated that telomere length at advanced age is a biomarker that predicts survival beyond the impact of early familial environment and genetic factors [152]. Similar results were reported from the elderly Danish twin cohort. The co-twin with the shorter telomeres died first, suggesting that telomere length is not only a biomarker of aging, but also a determinant of lifespan [153]. By contrast, some studies reported no apparent relationship between telomere length and survival in elderly people or centenarians [154,155].
CONCLUSIONS AND FUTURE PERSPECTIVES
Most human studies in telomere biology and aging-related diseases are cross-sectional in nature. Large-scale and well-designed studies are lacking. Nonetheless, emerging evidence demonstrates that telomere biology is involved in the pathophysiology of aging and human diseases. Whether telomere biology is causally involved in the development of human diseases awaits further research from large, prospective and longitudinal as well as interventional studies. The answer to this question may provide new prevention and treatment opportunities to combat chronic aging-related diseases. The telomere/telomerase system could be a promising target, offering possibilities to increase the viability of the cell for therapeutic purposes. Animal studies demonstrated that several antihypertensive and lipid-lowering drugs also increase telomerase activity [156–158]. For example, ACEIs are safe, common and long-used drugs whose use could be expanded beyond the treatment of hypertension and into anti-aging [159]. Finally, more research or clinical trials are needed to assess the effects of lifestyle intervention and other medications on the telomere/telomerase system. On the basis of the promising in vitro and in vivo results, it is to be hoped that telomerase-based prevention and therapeutics will be utilized in the future to combat aging-related disease including CVD.
FUNDING
Our own work was supported by Netherlands Heart Foundation [grant numbers 2006B140, 2006T003); the Innovational Research Incentives Scheme Program of the Netherlands Organization for Scientific Research (NWO VENI) [grant number 916.76.170 (to P.v.d.H.); the Interuniversitair Cardiologisch Instituut Nederland (ICIN); and the National Heart, Lung, and Blood Institute [grant number HL85817].
References
- 1.Artandi S. E., DePinho R. A. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesare A. J., Reddel R. R. Alternative lengthening of telomeres: models, mechanisms and implications. Nat. Rev. Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 3.Blasco M. A. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 4.Perona R., Machado-Pinilla R., Manguan C., Carrillo J. Telomerase deficiency and cancer susceptibility syndromes. Clin. Transl. Oncol. 2009;11:711–714. doi: 10.1007/s12094-009-0432-9. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 6.Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 8.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 9.Chong L., van Steensel B., Broccoli D., ErdjumentBromage H., Hanish J., Tempst P., de Lange T. A human telomeric protein. Science. 1995;270:1663–1667. doi: 10.1126/science.270.5242.1663. [DOI] [PubMed] [Google Scholar]
- 10.Broccoli D., Smogorzewska A., Chong L., de Lange T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 1997;17:231–235. doi: 10.1038/ng1097-231. [DOI] [PubMed] [Google Scholar]
- 11.Wang F., Podell E. R., Zaug A. J., Yang Y., Baciu P., Cech T. R., Lei M. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 12.Li B., Oestreich S., de Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–483. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim S. H., Kaminker P., Campisi J. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 1999;23:405–412. doi: 10.1038/70508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connor M. S., Safari A., Xin H., Liu D., Songyang Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11874–11879. doi: 10.1073/pnas.0605303103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d'Adda di Fagagna F., Reaper P. M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N. P., Jackson S. P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 16.Blackburn E. H. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 17.Hayflick L., Moorhead P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 18.Blackburn E. H. Telomere states and cell fates. Nature. 2002;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 19.Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 20.Weng N. P., Granger L., Hodes R. J. Telomere lengthening and telomerase activation during human B cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10827–10832. doi: 10.1073/pnas.94.20.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S. B., Graham M. E., Lovrecz G. O., Bache N., Robinson P. J., Reddel R. R. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 22.Venteicher A. S., Abreu E. B., Meng Z., McCann K. E., Terns R. M., Veenstra T. D., Terns M. P., Artandi S. E. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masutomi K., Possemato R., Wong J. M., Currier J. L., Tothova Z., Manola J. B., Ganesan S., Lansdorp P. M., Collins K., Hahn W. C. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8222–8227. doi: 10.1073/pnas.0503095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarin K. Y., Cheung P., Gilison D., Lee E., Tennen R. I., Wang E., Artandi M. K., Oro A. E., Artandi S. E. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J. I., Venteicher A. S., Hong J. Y., Choi J., Jun S., Shkreli M., Chang W., Meng Z., Cheung P., Ji H., et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gessert S., Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ. Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 27.Rao T. P., Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ. Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 28.Mangino M., Richards J. B., Soranzo N., Zhai G., Aviv A., Valdes A. M., Samani N. J., Deloukas P., Spector T. D. A genome-wide association study identifies a novel locus on chromosome 18q12.2 influencing white cell telomere length. J. Med. Genet. 2009;46:451–454. doi: 10.1136/jmg.2008.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Codd V., Mangino M., van der Harst P., Braund P. S., Kaiser M., Beveridge A. J., Rafelt S., Moore J., Nelson C., Soranzo N., et al. Common variants near TERC are associated with mean telomere length. Nat. Genet. 2010;42:197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy D., Neuhausen S. L., Hunt S. C., Kimura M., Hwang S. J., Chen W., Bis J. C., Fitzpatrick A. L., Smith E., Johnson A. D., et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9293–9298. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuda K., Bardeguez A., Gardner J. P., Rodriguez P., Ganesh V., Kimura M., Skurnick J., Awad G., Aviv A. Telomere length in the newborn. Pediatr. Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Butler M. G., Tilburt J., DeVries A., Muralidhar B., Aue G., Hedges L., Atkinson J., Schwartz H. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet. Cytogenet. 1998;105:138–144. doi: 10.1016/s0165-4608(98)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt S. C., Chen W., Gardner J. P., Kimura M., Srinivasan S. R., Eckfeldt J. H., Berenson G. S., Aviv A. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benetos A., Okuda K., Lajemi M., Kimura M., Thomas F., Skurnick J., Labat C., Bean K., Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 35.Zhu H., Wang X., Gutin B., Davis C. L., Keeton D., Thomas J., Stallmann-Jorgensen I., Mooken G., Bundy V., Snieder H., et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. J. Pediatr. 2010 doi: 10.1016/j.jpeds.2010.08.007. doi:10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bekaert S., De Meyer T., Rietzschel E. R., De Buyzere M. L., De Bacquer D., Langlois M., Segers P., Cooman L., Van Damme P., Cassiman P., et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama Y., Takahashi Y., Morishita S., Hashimoto M., Niwa K., Tamaya T. Telomerase activity in the human endometrium throughout the menstrual cycle. Mol. Hum. Reprod. 1998;4:173–177. doi: 10.1093/molehr/4.2.173. [DOI] [PubMed] [Google Scholar]
- 38.Misiti S., Nanni S., Fontemaggi G., Cong Y. S., Wen J., Hirte H. W., Piaggio G., Sacchi A., Pontecorvi A., Bacchetti S., Farsetti A. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol. Cell. Biol. 2000;20:3764–3771. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 40.Matthews C., Gorenne I., Scott S., Figg N., Kirkpatrick P., Ritchie A., Goddard M., Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ. Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 41.Haendeler J., Hoffmann J., Diehl J. F., Vasa M., Spyridopoulos I., Zeiher A. M., Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 42.Demissie S., Levy D., Benjamin E. J., Cupples L. A., Gardner J. P., Herbert A., Kimura M., Larson M. G., Meigs J. B., Keaney J. F., Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 43.Nawrot T. S., Staessen J. A., Holvoet P., Struijker-Boudier H. A., Schiffers P., Van Bortel L. M., Fagard R. H., Gardner J. P., Kimura M., Aviv A. Telomere length and its associations with oxidized-LDL, carotid artery distensibility and smoking. Front. Biosci. 2010;2:1164–1168. doi: 10.2741/e176. [DOI] [PubMed] [Google Scholar]
- 44.Xu D., Erickson S., Szeps M., Gruber A., Sangfelt O., Einhorn S., Pisa P., Grander D. Interferon α down-regulates telomerase reverse transcriptase and telomerase activity in human malignant and nonmalignant hematopoietic cells. Blood. 2000;96:4313–4318. [PubMed] [Google Scholar]
- 45.Aviv A., Valdes A., Gardner J. P., Swaminathan R., Kimura M., Spector T. D. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J. Clin. Endocrinol. Metab. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 46.Carrero J. J., Stenvinkel P., Fellstrom B., Qureshi A. R., Lamb K., Heimburger O., Barany P., Radhakrishnan K., Lindholm B., Soveri I., et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J. Intern. Med. 2008;263:302–312. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 47.Fitzpatrick A. L., Kronmal R. A., Gardner J. P., Psaty B. M., Jenny N. S., Tracy R. P., Walston J., Kimura M., Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am. J. Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 48.Huzen J., van der Harst P., de Boer R. A., Lesman-Leegte I., Voors A. A., van Gilst W. H., Samani N. J., Jaarsma T., van Veldhuisen D. J. Telomere length and psychological well-being in patients with chronic heart failure. Age Ageing. 2010;39:223–227. doi: 10.1093/ageing/afp256. [DOI] [PubMed] [Google Scholar]
- 49.Tyrka A. R., Price L. H., Kao H. T., Porton B., Marsella S. A., Carpenter L. L. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol. Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valdes A. M., Andrew T., Gardner J. P., Kimura M., Oelsner E., Cherkas L. F., Aviv A., Spector T. D. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 51.Cherkas L. F., Hunkin J. L., Kato B. S., Richards J. B., Gardner J. P., Surdulescu G. L., Kimura M., Lu X., Spector T. D., Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 52.Farzaneh-Far R., Lin J., Epel E. S., Harris W. S., Blackburn E. H., Whooley M. A. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA, J. Am. Med. Assoc. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherkas L. F., Aviv A., Valdes A. M., Hunkin J. L., Gardner J. P., Surdulescu G. L., Kimura M., Spector T. D. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 54.Savage S. A., Stewart B. J., Eckert A., Kiley M., Liao J. S., Chanock S. J. Genetic variation, nucleotide diversity, and linkage disequilibrium in seven telomere stability genes suggest that these genes may be under constraint. Hum. Mutat. 2005;26:343–350. doi: 10.1002/humu.20226. [DOI] [PubMed] [Google Scholar]
- 55.Cluett C., Melzer D. Human genetic variations: beacons on the pathways to successful ageing. Mech. Ageing Dev. 2009;130:553–563. doi: 10.1016/j.mad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Hsu C. P., Hsu N. Y., Lee L. W., Ko J. L. Ets2 binding site single nucleotide polymorphism at the hTERT gene promoter—effect on telomerase expression and telomere length maintenance in non-small cell lung cancer. Eur. J. Cancer. 2006;42:1466–1474. doi: 10.1016/j.ejca.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Savage S. A., Chanock S. J., Lissowska J., Brinton L. A., Richesson D., Peplonska B., Bardin-Mikolajczak A., Zatonski W., Szeszenia-Dabrowska N., Garcia-Closas M. Genetic variation in five genes important in telomere biology and risk for breast cancer. Br. J. Cancer. 2007;97:832–836. doi: 10.1038/sj.bjc.6603934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsubara Y., Murata M., Yoshida T., Watanabe K., Saito I., Miyaki K., Omae K., Ikeda Y. Telomere length of normal leukocytes is affected by a functional polymorphism of hTERT. Biochem. Biophys. Res. Commun. 2006;341:128–131. doi: 10.1016/j.bbrc.2005.12.163. [DOI] [PubMed] [Google Scholar]
- 59.Kyo S., Takakura M., Fujiwara T., Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumari A., Srinivasan R., Vasishta R. K., Wig J. D. Positive regulation of human telomerase reverse transcriptase gene expression and telomerase activity by DNA methylation in pancreatic cancer. Ann. Surg. Oncol. 2009;16:1051–1059. doi: 10.1245/s10434-009-0333-8. [DOI] [PubMed] [Google Scholar]
- 61.Iliopoulos D., Satra M., Drakaki A., Poultsides G. A., Tsezou A. Epigenetic regulation of hTERT promoter in hepatocellular carcinomas. Int. J. Oncol. 2009;34:391–399. [PubMed] [Google Scholar]
- 62.Epel E. S., Blackburn E. H., Lin J., Dhabhar F. S., Adler N. E., Morrow J. D., Cawthon R. M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsirpanlis G., Chatzipanagiotou S., Boufidou F., Kordinas V., Zoga M., Alevyzaki F., Stamatelou K., Frangou E., Savva L., Nicolaou C. Serum oxidized low-density lipoprotein is inversely correlated to telomerase activity in peripheral blood mononuclear cells of haemodialysis patients. Nephrology. 2006;11:506–509. doi: 10.1111/j.1440-1797.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 64.Makpol S., Abidin A. Z., Sairin K., Mazlan M., Top G. M., Ngah W. Z. γ-Tocotrienol prevents oxidative stress-induced telomere shortening in human fibroblasts derived from different aged individuals. Oxid. Med. Cell Longev. 2010;3:35–43. doi: 10.4161/oxim.3.1.9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scalera F., Closs E. I., Flick E., Martens-Lobenhoffer J., Boissel J. P., Lendeckel U., Heimburg A., Bode-Boger S. M. Paradoxical effect of L-arginine: acceleration of endothelial cell senescence. Biochem. Biophys. Res. Commun. 2009;386:650–655. doi: 10.1016/j.bbrc.2009.06.091. [DOI] [PubMed] [Google Scholar]
- 66.Dixit D., Sharma V., Ghosh S., Koul N., Mishra P. K., Sen E. Manumycin inhibits STAT3, telomerase activity, and growth of glioma cells by elevating intracellular reactive oxygen species generation. Free Radical Biol. Med. 2009;47:364–374. doi: 10.1016/j.freeradbiomed.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 67.Li H., Xu D., Li J., Berndt M. C., Liu J. P. Transforming growth factor β suppresses human telomerase reverse transcriptase (hTERT) by Smad3 interactions with c-Myc and the hTERT gene. J. Biol. Chem. 2006;281:25588–25600. doi: 10.1074/jbc.M602381200. [DOI] [PubMed] [Google Scholar]
- 68.Werner C., Furster T., Widmann T., Poss J., Roggia C., Hanhoun M., Scharhag J., Buchner N., Meyer T., Kindermann W., et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 69.Ornish D., Lin J., Daubenmier J., Weidner G., Epel E., Kemp C., Magbanua M. J., Marlin R., Yglecias L., Carroll P. R., Blackburn E. H. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 70.Liu K., Schoonmaker M. M., Levine B. L., June C. H., Hodes R. J., Weng N. P. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5147–5152. doi: 10.1073/pnas.96.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu K., Hodes R. J., Weng N. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J. Immunol. 2001;166:4826–4830. doi: 10.4049/jimmunol.166.8.4826. [DOI] [PubMed] [Google Scholar]
- 72.Xia L., Wang X. X., Hu X. S., Guo X. G., Shang Y. P., Chen H. J., Zeng C. L., Zhang F. R., Chen J. Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008;155:387–394. doi: 10.1038/bjp.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donnini S., Terzuoli E., Ziche M., Morbidelli L. Sulfhydryl angiotensin-converting enzyme inhibitor promotes endothelial cell survival through nitric-oxide synthase, fibroblast growth factor-2, and telomerase cross-talk. J. Pharmacol. Exp. Ther. 2010;332:776–784. doi: 10.1124/jpet.109.159178. [DOI] [PubMed] [Google Scholar]
- 74.Azzalin C. M., Reichenbach P., Khoriauli L., Giulotto E., Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 75.Luke B., Panza A., Redon S., Iglesias N., Li Z., Lingner J. The Rat1p 5′ to 3′ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol. Cell. 2008;32:465–477. doi: 10.1016/j.molcel.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 76.Schoeftner S., Blasco M. A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008;10:228–236. doi: 10.1038/ncb1685. [DOI] [PubMed] [Google Scholar]
- 77.Luke B., Lingner J. TERRA: telomeric repeat-containing RNA. EMBO J. 2009;28:2503–2510. doi: 10.1038/emboj.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Azzalin C. M., Lingner J. Telomeres: the silence is broken. Cell Cycle. 2008;7:1161–1165. doi: 10.4161/cc.7.9.5836. [DOI] [PubMed] [Google Scholar]
- 79.Ng L. J., Cropley J. E., Pickett H. A., Reddel R. R., Suter C. M. Telomerase activity is associated with an increase in DNA methylation at the proximal subtelomere and a reduction in telomeric transcription. Nucleic Acids Res. 2009;37:1152–1159. doi: 10.1093/nar/gkn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flores I., Blasco M. A. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584:3826–3830. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 81.Oeseburg H., de Boer R. A., van Gilst W. H., van der Harst P. Telomere biology in healthy aging and disease. Pflugers Arch. 2010;459:259–268. doi: 10.1007/s00424-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shay J. W., Wright W. E. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010 doi: 10.1016/j.febslet.2010.05.026. May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flores I., Benetti R., Blasco M. A. Telomerase regulation and stem cell behaviour. Curr. Opin. Cell Biol. 2006;18:254–260. doi: 10.1016/j.ceb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Geiger H., Rudolph K. L. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30:360–365. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Allen N. D., Baird D. M. Telomere length maintenance in stem cell populations. Biochim. Biophys. Acta. 2009;1792:324–328. doi: 10.1016/j.bbadis.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Manolio T. A., Collins F. S., Cox N. J., Goldstein D. B., Hindorff L. A., Hunter D. J., McCarthy M. I., Ramos E. M., Cardon L. R., Chakravarti A., et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Humphries S. E., Drenos F., Ken-Dror G., Talmud P. J. Coronary heart disease risk prediction in the era of genome-wide association studies: current status and what the future holds. Circulation. 2010;121:2235–2248. doi: 10.1161/CIRCULATIONAHA.109.914192. [DOI] [PubMed] [Google Scholar]
- 88.Samani N. J., van der Harst P. Biological ageing and cardiovascular disease. Heart. 2008;94:537–539. doi: 10.1136/hrt.2007.136010. [DOI] [PubMed] [Google Scholar]
- 89.Cao Y., Li H., Mu F. T., Ebisui O., Funder J. W., Liu J. P. Telomerase activation causes vascular smooth muscle cell proliferation in genetic hypertension. FASEB J. 2002;16:96–98. doi: 10.1096/cj.01-0447fje. [DOI] [PubMed] [Google Scholar]
- 90.Perez-Rivero G., Ruiz-Torres M. P., Rivas-Elena J. V., Jerkic M., Diez-Marques M. L., Lopez-Novoa J. M., Blasco M. A., Rodriguez-Puyol D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114:309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. [DOI] [PubMed] [Google Scholar]
- 91.Imanishi T., Moriwaki C., Hano T., Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J. Hypertens. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 92.Jeanclos E., Schork N. J., Kyvik K. O., Kimura M., Skurnick J. H., Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 93.Benetos A., Okuda K., Lajemi M., Kimura M., Thomas F., Skurnick J., Labat C., Bean K., Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 94.Vasan R. S., Demissie S., Kimura M., Cupples L. A., Rifai N., White C., Wang T. J., Gardner J. P., Cao X., Benjamin E. J., et al. Association of leukocyte telomere length with circulating biomarkers of the renin–angiotensin–aldosterone system: the Framingham Heart Study. Circulation. 2008;117:1138–1144. doi: 10.1161/CIRCULATIONAHA.107.731794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Z., Huang X., Jiang H., Zhang Y., Liu H., Qin C., Eisner G. M., Jose P. A., Rudolph L., Ju Z. Short telomeres and prognosis of hypertension in a Chinese population. Hypertension. 2009;53:639–645. doi: 10.1161/HYPERTENSIONAHA.108.123752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asselbergs F. W., van der Harst P., Jessurun G. A., Tio R. A., van Gilst W. H. Clinical impact of vasomotor function assessment and the role of ACE-inhibitors and statins. Vasc. Pharmacol. 2005;42:125–140. doi: 10.1016/j.vph.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 97.Comi P., Chiaramonte R., Maier J. A. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp. Cell Res. 1995;219:304–308. doi: 10.1006/excr.1995.1232. [DOI] [PubMed] [Google Scholar]
- 98.Samani N. J., Boultby R., Butler R., Thompson J. R., Goodall A. H. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 99.Brouilette S., Singh R. K., Thompson J. R., Goodall A. H., Samani N. J. White cell telomere length and risk of premature myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 100.Farzaneh-Far R., Cawthon R. M., Na B., Browner W. S., Schiller N. B., Whooley M. A. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler. Thromb. Vasc. Biol. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brouilette S. W., Moore J. S., McMahon A. D., Thompson J. R., Ford I., Shepherd J., Packard C. J., Samani N. J. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 102.Willeit P., Willeit J., Brandstatter A., Ehrlenbach S., Mayr A., Gasperi A., Weger S., Oberhollenzer F., Reindl M., Kronenberg F., Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 103.Mainous A. G., III, Codd V., Diaz V. A., Schoepf U. J., Everett C. J., Player M. S., Samani N. J. Leukocyte telomere length and coronary artery calcification. Atherosclerosis. 2010;210:262–267. doi: 10.1016/j.atherosclerosis.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 104.Diaz V. A., Mainous A. G., III, Everett C. J., Schoepf U. J., Codd V., Samanii N. J. Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. Am. J. Cardiol. 2010;106:659–663. doi: 10.1016/j.amjcard.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 105.Wong L. S., de Boer R. A., Samani N. J., van Veldhuisen D. J., van der Harst P. Telomere biology in heart failure. Eur. J. Heart Failure. 2008;10:1049–1056. doi: 10.1016/j.ejheart.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 106.Leri A., Franco S., Zacheo A., Barlucchi L., Chimenti S., Limana F., Nadal-Ginard B., Kajstura J., Anversa P., Blasco M. A. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van der Harst P., van der Steege G., de Boer R. A., Voors A. A., Hall A. S., Mulder M. J., van Gilst W. H., van Veldhuisen D. J. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 108.van der Harst P., Wong L. S., de Boer R. A., Brouilette S. W., van der Steege G., Voors A. A., Hall A. S., Samani N. J., Wikstrand J., van Gilst W. H., van Veldhuisen D. J. Possible association between telomere length and renal dysfunction in patients with chronic heart failure. Am. J. Cardiol. 2008;102:207–210. doi: 10.1016/j.amjcard.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 109.Collerton J., Martin-Ruiz C., Kenny A., Barrass K., von Zglinicki T., Kirkwood T., Keavney B. Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ study. Eur. Heart J. 2007;28:172–176. doi: 10.1093/eurheartj/ehl437. [DOI] [PubMed] [Google Scholar]
- 110.Chimenti C., Kajstura J., Torella D., Urbanek K., Heleniak H., Colussi C., Di Meglio F., Nadal-Ginard B., Frustaci A., Leri A., et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ. Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 111.Wong L. S., Huzen J., van der Harst P., de Boer R. A., Codd V., Westenbrink B. D., Benus G. F., Voors A. A., van Gilst W. H., Samani N. J., et al. Anaemia is associated with shorter leucocyte telomere length in patients with chronic heart failure. Eur. J. Heart Failure. 2010;12:348–353. doi: 10.1093/eurjhf/hfq007. [DOI] [PubMed] [Google Scholar]
- 112.van der Harst P., de Boer R. A., Samani N. J., Wong L. S., Huzen J., Codd V., Hillege H. L., Voors A. A., van Gilst W. H., Jaarsma T., van Veldhuisen D. J. Telomere length and outcome in heart failure. Ann. Med. 2010;42:36–44. doi: 10.3109/07853890903321567. [DOI] [PubMed] [Google Scholar]
- 113.Rhodes C. J. Type 2 diabetes: a matter of β-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 114.Georgia S., Bhushan A. β Cell replication is the primary mechanism for maintaining postnatal β cell mass. J. Clin. Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zee R. Y., Castonguay A. J., Barton N. S., Germer S., Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl. Res. 2010;155:166–169. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 116.Salpea K. D., Talmud P. J., Cooper J. A., Maubaret C. G., Stephens J. W., Abelak K., Humphries S. E. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209:42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fyhrquist F., Tiitu A., Saijonmaa O., Forsblom C., Groop P. H. Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J. Intern. Med. 2010;267:278–286. doi: 10.1111/j.1365-2796.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 118.Astrup A. S., Tarnow L., Jorsal A., Lajer M., Nzietchueng R., Benetos A., Rossing P., Parving H. H. Telomere length predicts all-cause mortality in patients with type 1 diabetes. Diabetologia. 2010;53:45–48. doi: 10.1007/s00125-009-1542-1. [DOI] [PubMed] [Google Scholar]
- 119.Uziel O., Singer J. A., Danicek V., Sahar G., Berkov E., Luchansky M., Fraser A., Ram R., Lahav M. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Exp. Gerontol. 42:971–978. doi: 10.1016/j.exger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 120.Adaikalakoteswari A., Balasubramanyam M., Ravikumar R., Deepa R., Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis. 2007;195:83–89. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Al-Attas O. S., Al-Daghri N. M., Alokail M. S., Alfadda A., Bamakhramah A., Sabico S., Pritlove D., Harte A., Tripathi G., McTernan P. G., et al. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: the influence of circulating adiponectin. Eur. J. Endocrinol. 2010;163:601–607. doi: 10.1530/EJE-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuhlow D., Florian S., von Figura G., Weimer S., Schulz N., Petzke K. J., Zarse K., Pfeiffer A. F., Rudolph K. L., Ristow M. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging. 2010;2:650–658. doi: 10.18632/aging.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh H., Taffet G. E., Youker K. A., Entman M. L., Overbeek P. A., Michael L. H., Schneider M. D. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kang H. J., Choi Y. S., Hong S. B., Kim K. W., Woo R. S., Won S. J., Kim E. J., Jeon H. K., Jo S. Y., Kim T. K., et al. Ectopic expression of the catalytic subunit of telomerase protects against brain injury resulting from ischemia and NMDA-induced neurotoxicity. J. Neurosci. 2004;24:1280–1287. doi: 10.1523/JNEUROSCI.4082-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang B., Chen L., Swartz K. R., Bruemmer D., Eum S. Y., Huang W., Seelbach M., Choi Y. J., Hennig B., Toborek M. Deficiency of telomerase activity aggravates the blood–brain barrier disruption and neuroinflammatory responses in a model of experimental stroke. J. Neurosci. Res. 2010;88:2859–2868. doi: 10.1002/jnr.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Werner C., Hanhoun M., Widmann T., Kazakov A., Semenov A., Poss J., Bauersachs J., Thum T., Pfreundschuh M., Muller P., et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J. Am. Coll. Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 127.Wong L. S., Oeseburg H., de Boer R. A., van Gilst W. H., van Veldhuisen D. J., van der Hars P. Telomere biology in cardiovascular disease: the TERC−/− mouse as a model for heart failure and ageing. Cardiovasc. Res. 2009;81:244–252. doi: 10.1093/cvr/cvn337. [DOI] [PubMed] [Google Scholar]
- 128.Franco S., Segura I., Riese H. H., Blasco M. A. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 2002;62:552–559. [PubMed] [Google Scholar]
- 129.Epel E. S., Lin J., Wilhelm F. H., Wolkowitz O. M., Cawthon R., Adler N. E., Dolbier C., Mendes W. B., Blackburn E. H. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 130.Fujii H., Shao L., Colmegna I., Goronzy J. J., Weyand C. M. Telomerase insufficiency in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsirpanlis G., Chatzipanagiotou S., Boufidou F., Kordinas V., Alevyzaki F., Zoga M., Kyritsis I., Stamatelou K., Triantafyllis G., Nicolaou C. Telomerase activity is decreased in peripheral blood mononuclear cells of hemodialysis patients. Am. J. Nephrol. 2006;26:91–96. doi: 10.1159/000092031. [DOI] [PubMed] [Google Scholar]
- 132.Panossian L. A., Porter V. R., Valenzuela H. F., Zhu X., Reback E., Masterman D., Cummings J. L., Effros R. B. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol. Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 133.Honig L. S., Schupf N., Lee J. H., Tang M. X., Mayeux R. Shorter telomeres are associated with mortality in those with APOEϵ4 and dementia. Ann. Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- 134.Thomas P., O'Callaghan N. J., Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mech. Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Jenkins E. C., Velinov M. T., Ye L., Gu H., Li S., Jenkins E. C., Jr, Brooks S. S., Pang D., Devenny D. A., Zigman W. B., et al. Telomere shortening in T lymphocytes of older individuals with Down syndrome and dementia. Neurobiol. Aging. 2006;27:941–945. doi: 10.1016/j.neurobiolaging.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 136.Lukens J. N., Van Deerlin V., Clark C. M., Xie S. X., Johnson F. B. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer's disease. Alzheimers Dement. 2009;5:463–469. doi: 10.1016/j.jalz.2009.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lof-Ohlin Z. M., Hagnelius N. O., Nilsson T. K. Relative telomere length in patients with late-onset Alzheimer's dementia or vascular dementia. NeuroReport. 2008;19:1199–1202. doi: 10.1097/WNR.0b013e3283089220. [DOI] [PubMed] [Google Scholar]
- 138.Zhang J., Kong Q., Zhang Z., Ge P., Ba D., He W. Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cogn. Behav. Neurol. 2003;16:170–176. doi: 10.1097/00146965-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 139.Wang H., Chen H., Gao X., McGrath M., Deer D., De Vivo I., Schwarzschild M. A., Ascherio A. Telomere length and risk of Parkinson's disease. Mov. Disord. 2008;23:302–305. doi: 10.1002/mds.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guan J. Z., Maeda T., Sugano M., Oyama J., Higuchi Y., Suzuki T., Makino N. A percentage analysis of the telomere length in Parkinson's disease patients. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:467–473. doi: 10.1093/gerona/63.5.467. [DOI] [PubMed] [Google Scholar]
- 141.Maeda T., Guan J. Z., Oyama J., Higuchi Y., Makino N. Aging-associated alteration of subtelomeric methylation in Parkinson's disease. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:949–955. doi: 10.1093/gerona/glp070. [DOI] [PubMed] [Google Scholar]
- 142.Fulop T., Larbi A., Witkowski J. M., McElhaney J., Loeb M., Mitnitski A., Pawelec G. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 143.Woo J., Tang N. L., Suen E., Leung J. C., Leung P. C. Telomeres and frailty. Mech. Ageing Dev. 2008;129:642–648. doi: 10.1016/j.mad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 144.Markle-Reid M., Browne G. Conceptualizations of frailty in relation to older adults. J. Adv. Nurs. 2003;44:58–68. doi: 10.1046/j.1365-2648.2003.02767.x. [DOI] [PubMed] [Google Scholar]
- 145.Woo J., Goggins W., Sham A., Ho S. C. Public health significance of the frailty index. Disabil. Rehabil. 2006;28:515–521. doi: 10.1080/09638280500215867. [DOI] [PubMed] [Google Scholar]
- 146.Risques R. A., Arbeev K. G., Yashin A. I., Ukraintseva S. V., Martin G. M., Rabinovitch P. S., Oshima J. Leukocyte telomere length is associated with disability in older U.S. population. J. Am. Geriatr. Soc. 2010;58:1289–1298. doi: 10.1111/j.1532-5415.2010.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lescai F., Marchegiani F., Franceschi C. PON1 is a longevity gene: results of a meta-analysis. Ageing Res. Rev. 2009;8:277–284. doi: 10.1016/j.arr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 148.Park M. C., Park D., Lee E. K., Park T., Lee J. Genomic analysis of the telomeric length effect on organismic lifespan in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2010;396:382–387. doi: 10.1016/j.bbrc.2010.04.101. [DOI] [PubMed] [Google Scholar]
- 149.Tomas-Loba A., Flores I., Fernandez-Marcos P. J., Cayuela M. L., Maraver A., Tejera A., Borras C., Matheu A., Klatt P., Flores J. M., et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 150.Njajou O. T., Hsueh W. C., Blackburn E. H., Newman A. B., Wu S. H., Li R., Simonsick E. M., Harris T. M., Cummings S. R., Cawthon R. M. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Atzmon G., Cho M., Cawthon R. M., Budagov T., Katz M., Yang X., Siegel G., Bergman A., Huffman D. M., Schechter C. B., et al. Evolution in health and medicine Sackler colloquium: genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl. Acad. Sci. U.S.A. 2010;107(Suppl. 1):1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bakaysa S. L., Mucci L. A., Slagboom P. E., Boomsma D. I., McClearn G. E., Johansson B., Pedersen N. L. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 153.Kimura M., Hjelmborg J. V., Gardner J. P., Bathum L., Brimacombe M., Lu X., Christiansen L., Vaupel J. W., Aviv A., Christensen K. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am. J. Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bischoff C., Petersen H. C., Graakjaer J., Andersen-Ranberg K., Vaupel J. W., Bohr V. A., Kolvraa S., Christensen K. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- 155.Martin-Ruiz C. M., Gussekloo J., van Heemst D., von Zglinicki T., Westendorp R. G. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 156.Kobayashi K., Imanishi T., Akasaka T. Endothelial progenitor cell differentiation and senescence in an angiotensin II-infusion rat model. Hypertens. Res. 2006;29:449–455. doi: 10.1291/hypres.29.449. [DOI] [PubMed] [Google Scholar]
- 157.Spyridopoulos I., Haendeler J., Urbich C., Brummendorf T. H., Oh H., Schneider M. D., Zeiher A. M., Dimmeler S. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation. 2004;110:3136–3142. doi: 10.1161/01.CIR.0000142866.50300.EB. [DOI] [PubMed] [Google Scholar]
- 158.Mahmoudi M., Gorenne I., Mercer J., Figg N., Littlewood T., Bennett M. Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ. Res. 2008;103:717–725. doi: 10.1161/CIRCRESAHA.108.182899. [DOI] [PubMed] [Google Scholar]
- 159.Marques F. Z., Markus M. A., Morris B. J. The molecular basis of longevity, and clinical implications. Maturitas. 2010;65:87–91. doi: 10.1016/j.maturitas.2009.12.008. [DOI] [PubMed] [Google Scholar]