Abstract
We report the first case of pulmonary tuberculosis caused by Mycobacterium bovis subsp. caprae in a captive Siberian tiger, an endangered feline. The pathogen was isolated from a tracheal aspirate obtained by bronchoscopy. This procedure provided a reliable in vivo diagnostic method in conjunction with conventional and molecular tests for the detection of mycobacteria.
Mycobacterium bovis, a member of the M. tuberculosis complex (MTBC), can cause tuberculosis in a wide range of domestic and wild animals and also in humans (1,2). Routine differentiation of M. bovis is based on a number of phenotypic characteristics and biochemical tests (2). M. bovis shows dysgonic growth on Löwenstein-Jensen (LJ) medium and has been described as negative for nitrate reduction and niacin accumulation (2). As a further criterion for the differentiation of M. bovis, intrinsic resistance to pyrazinamide (PZA) has been described (2). However, more recently, PZA-susceptible strains of M. bovis were found in Spain and Germany; these strains were also characterized by specific molecular techniques (3–5). As a consequence, M. bovis was split into two subspecies: M. bovis subsp. bovis, which showed resistance to PZA, and M. bovis subsp. caprae, which was sensitive to PZA (6,7). M. bovis subsp. caprae was initially isolated from sheep and goats in Spain (3,4,7); however, further studies confirm its infectivity in humans, cattle, and red deer (6,8). We report the unusual case of a M. bovis subsp. caprae infection in a captive Siberian tiger.
Case Report
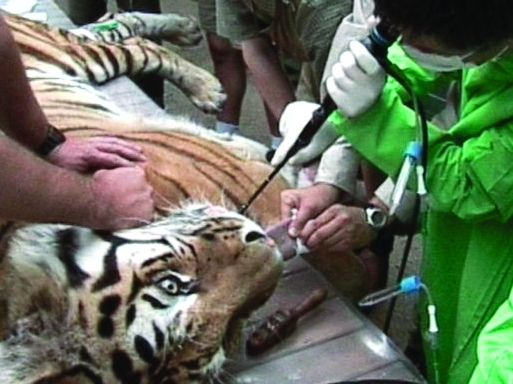
An 8-year-old male Siberian tiger at the Budapest Zoological and Botanical Garden had episodes of coughing in October 2001. Because the coughing did not stop in 6 to 7 days, an expectorant (Bisolvon; Boehringer Ingelheim Vetmed Gmbh., Ingelheim am Rhein, Germany) was given for 10 days. His condition showed a temporary improvement; however, after a few weeks, the animal started coughing again, and his appetite decreased. Amoxicillin plus clavulanic acid (Amoksiklav; Lek Animal Health, Ljubljana, Slovenia) and ketoprophen (Ketofen, Merial, Lyons, France) therapy was given for 7 days. The tiger’s condition did not show any notable improvement. In addition, in May 2002, the animal’s respiratory rate became elevated, he became dyspneic and emaciated, and his daily activity substantially decreased. Further antibacterial treatment was administered (cefatroxil, Cefa-cure; Intervet, Boxmeer, the Netherlands) during that month without clinical effect. At that point, the animal was anesthetized, and tracheoscopy was performed with a flexible 56-cm bronchoscope (Olympus B3R; Tokyo, Japan (Figure 1). The examination found a large amount of purulent mucus in the trachea. Therefore, several tracheal washings were taken for microbiologic tests by using a commercially available tracheal suction set (Medinorm Medizintechnik GmbH, Quierschied, Germany (Figure 1). A chest radiograph showed a severe and extensive bronchointerstitial pattern with cavernous lesions in both lungs.
Figure 1.
Obtaining a tracheal washing of the Siberian tiger by bronchoscopy.
Nine days after the specimens were taken, cultures for mycobacteria showed growth in the broth-based MGIT 960 system (Becton-Dickinson Microbiology Systems, Sparks, MD). The acid-fast organism that was isolated was identified as MTBC by the AccuProbe TB assay (Gen-Probe Inc., San Diego, CA).
Since the tiger had stopped eating and his condition had dramatically deteriorated, the animal was euthanized and necropsy was performed. Hematoxylin and eosin–stained histologic sections of the lung segments showed an extensive multifocal infiltration of lymphocytes, histiocytes, and some scattered multinuclear giant cells within the framework of proliferated connective tissue and collagen fibers of the cavernous lesions. Ziehl-Neelsen staining showed an intracellular accumulation of acid-fast bacteria in several alveolar macrophages and epithelioid cells.
The keepers of the tiger also underwent pulmonary radiographs and tuberculin skin testing. Their skin test results were negative, and clinical or radiologic signs of tuberculosis were not detected.
Characterization of MTBC Isolate
Colony morphology of the isolated MTBC strain showed dysgonic growth on LJ medium and microaerophilic growth on Lebek medium. The strain was susceptible to PZA (100 μg/mL) and thiophen-2-carboxylic acid hydrazide (TCH; 1 μg/mL) and negative for niacin accumulation and nitrate reduction (9–11).
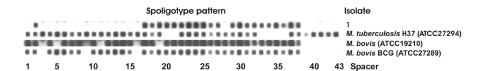
The genome of the isolate was analyzed for specific mutations in the pncA, oxyR, and gyrB genes by automated DNA sequencing, polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) technique, and spoligotyping as described previously (5,12–16). Susceptibility of the isolate to PZA was linked with a wild-type pncA sequence. In addition, the isolate contained the M. bovis–specific G-to-A mutation at position 285 in the oxyR gene and the G-to-A mutation at position 756 in the gyrB gene. However, spoligotyping showed a pattern with the absence of spacer sequences 39–43 and 3–16 (Figure 2), and T to G mutation at position 1,311 in the gyrB gene, characteristic of M. bovis subsp. caprae, could also be detected by DNA sequencing. On the basis of these phenotypic and genetic characteristics, the strain was identified as M. bovis subsp. caprae (3,6,7,17).
Figure 2.
Spoligotype patterns of the isolate obtained from the Siberian tiger: lane 1; spacer sequences 3–16 and 39–43 are absent); lane 2, control strain Mycobacterium tuberculosis H37Rv ATCC 27294; lane 3, control strain M. bovis ATCC 19210; lane 4, M. bovis BCG ATCC 27289.
Conclusions
MTBC comprises these closely related organisms: M. tuberculosis; M. africanum; M. bovis; the vaccine strain, M. bovis bacillus Calmette-Guérin; and three rarely seen members, M. microti, M. canettii, and the recently described seal bacillus, M. pinnipedii (17–20). Differentiation within MTBC is necessary for individual patient treatment (i.e., inclusion or exclusion of PZA) and for epidemiologic purposes, especially in areas of the world where tuberculosis has reached epidemic proportions or wherever the transmission of M. bovis between animals, animal products, and humans is a problem (10).
The host range of M. bovis is wide, including many animal species and humans. Carnivores such as large felines may acquire the infection through the alimentary tract by eating infected meat (4). Reports of tuberculosis in large captive or free-living felines are not common (21–26), however.
To our knowledge, this case is the first in which tuberculosis attributable to M. bovis subsp. caprae was diagnosed in a large feline. The rapid and accurate in vivo diagnosis of tuberculosis is indispensable in endangered captive animals such as the Siberian tiger, not only because of the declining population of this species but also to prevent the transmission of the disease to other animals. Although nasal or throat swabs are used most often, we found tracheal washing by bronchoscopy was easy to perform, rapid, and more adequate than swabs (provided a larger sample volume from the lower airways) for obtaining clinical specimen for mycobacterial or other microbiologic tests.
The rapid diagnosis of tuberculosis is essential for adequate antituberculosis treatment to be started as early as possible. The effectiveness of antituberculosis therapy in felines is controversial (27). However, when an endangered animal is involved, early diagnosis of the disease might help control it in time to save the animal, especially with the help of a rapid in vivo diagnostic method such as tracheal washing through bronchoscopy. Tracheal washing can also be the method of choice to bacteriologically monitor the efficacy of therapy. In this case, the poor appetite and condition of the animal did not allow survival long enough for delivery of antituberculosis treatment. The source of infection could not be conclusively identified retrospectively; infected goat meat (a usual diet of the animal) is a likely possibility because the tuberculosis-related control measures are not as strict with goats as with cattle in Hungary (annual tuberculin skin testing of goats is not mandatory, for example) (28).
This report indicates that routine differentiation within the MTBC is indispensable for understanding the epidemiology of tuberculosis and for determining the prevalence, transmission, and clinical importance of the different members of the complex.
Biography
Dr. Lantos is an assistant professor in the Department of Respiratory Medicine, School of Medicine, Semmelweis University, Budapest, Hungary, where he is also head of the bronchology unit. His research interests include the application of novel diagnostic methods for detecting mycobacterial infections and identifying mycobacteria.
Footnotes
Suggested citation for this article: Lantos Á, Niemann S, Mezősi L, Sós E, Erdélyi K, Dávid S, et al. Pulmonary tuberculosis due to Mycobacterium bovis in captive Siberian tiger. Emerg Infect Dis [serial online] 2003 Nov [date cited]. Available from: URL: http://www.cdc.gov/ncidod/EID/vol9no11/03-0297.htm
References
- 1.O’Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995;76(Suppl 1):1–46. 10.1016/0962-8479(95)90591-X [DOI] [PubMed] [Google Scholar]
- 2.Wayne LG, Kubica GP. The mycobacteria. In: Sneath PHA, Holt JG, editors. Bergey’s manual of systemic bacteriology. Vol. 2. Baltimore: Williams and Wilkins; 1986. p. 1435–57. [Google Scholar]
- 3.Aranaz A, Liebana E, Mateos A, Dominguez L, Vidal D, Domingo M, et al. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J Clin Microbiol. 1996;34:2734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez M, Samper S, Jimenez MS, van Embden JD, Marin JF, Martin C. Identification by spoligotyping of a caprine genotype in Mycobacterium bovis strains causing human tuberculosis. J Clin Microbiol. 1997;35:3328–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niemann S, Richter E, Rusch-Gerdes S. Differentiation among members of the Mycobacterium tuberculosis complex by molecular and biochemical features: evidence for two pyrazinamide-susceptible subtypes of M. bovis. J Clin Microbiol. 2000;38:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niemann S, Richter E, Rusch-Gerdes S. Biochemical and genetic evidence for the transfer of Mycobacterium tuberculosis subsp. caprae Aranaz et al. 1999 to the species Mycobacterium bovis Karlson and Lessel 1970 (approved lists 1980) as Mycobacterium bovis subsp. caprae comb. nov. Int J Syst Evol Microbiol. 2002;52:433–6. [DOI] [PubMed] [Google Scholar]
- 7.Aranaz A, Liebana E, Gomez-Mampaso E, Galan JC, Cousins D, Ortega A, et al. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int J Syst Bacteriol. 1999;3:1263–73. 10.1099/00207713-49-3-1263 [DOI] [PubMed] [Google Scholar]
- 8.Prodinger WM, Eigentler A, Allerberger F, Schonbauer M, Glawischnig W. Infection of red deer, cattle, and humans with Mycobacterium bovis subsp. caprae in western Austria. J Clin Microbiol. 2002;40:2270–2. 10.1128/JCM.40.6.2270-2272.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent PT, Kubica GP. Public health mycobacteriology. A guide for a level III laboratory. Atlanta: Center for Disease Control and Prevention; 1985. [Google Scholar]
- 10.Parsons LM, Brosch R, Cole ST, Somoskovi A, Loder A, Bretzel G, et al. Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J Clin Microbiol. 2002;40:2339–45. 10.1128/JCM.40.7.2339-2345.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebek G. Über den Nachweis des unterschiedlichen Sauerstoffoptimums des humanen and bovinen Mycobacterium tuberculosis. Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt 1 Orig 1958;173:581–7. [PubMed]
- 12.Kasai H, Ezaki T, Harayama S. Differentiation of phylogenetically related slowly growing mycobacteria by their gyrB sequences. J Clin Microbiol. 2000;38:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sreevatsan S, Escalante P, Pan X, Gillies DA II, Siddiqui S, Khalaf CN, et al. Identification of a polymorphic nucleotide in oxyR specific for Mycobacterium bovis. J Clin Microbiol. 1996;34:2007–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2:662–7. 10.1038/nm0696-662 [DOI] [PubMed] [Google Scholar]
- 15.Niemann S, Harmsen D, Rusch-Gerdes S, Richter E. Differentiation of clinical Mycobacterium tuberculosis complex isolates by gyrB DNA sequence polymorphism analysis. J Clin Microbiol. 2000;38:3231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A. 2002;99:3684–9. 10.1073/pnas.052548299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cousins D, Bastida R, Cataldi A, Quse V, Redrobe S, Dow S, et al. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int J Syst Evol Microbiol. 2003;53:1305–14. 10.1099/ijs.0.02401-0 [DOI] [PubMed] [Google Scholar]
- 19.Tsukamura M, Mizuno S, Toyama H. Taxonomic studies on the Mycobacterium tuberculosis series. Microbiol Immunol. 1985;29:285–99. [DOI] [PubMed] [Google Scholar]
- 20.van Soolingen D, Hoogenboezem T, de Haas PE, Hermans PW, Koedam MA, Teppema KS, et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Syst Bacteriol. 1997;47:1236–45. 10.1099/00207713-47-4-1236 [DOI] [PubMed] [Google Scholar]
- 21.Briones V, de Juan L, Sanchez C, Vela AI, Galka M, Montero, et al. Bovine tuberculosis and the endangered Iberian lynx. Emerg Infect Dis. 2000;6:189–91. 10.3201/eid0602.000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helman RG, Russell WC, Jenny A, Miller J, Payeur J. Diagnosis of tuberculosis in two snow leopards using polymerase chain reaction. J Vet Diagn Invest. 1998;10:89–92. [DOI] [PubMed] [Google Scholar]
- 23.Lumeij JT, Hajer R, Dik KJ, Dorrestein GM, Engel HW. Diagnosis of pulmonary Mycobacterium bovis infection in a tiger. Vet Rec. 1987;120:302–4. 10.1136/vr.120.13.302 [DOI] [PubMed] [Google Scholar]
- 24.Thorel MF, Karoui C, Varnerot A, Fleury C, Vincent V. Isolation of Mycobacterium bovis from baboons, leopards and a sea-lion. Vet Res. 1998;29:207–12. [PubMed] [Google Scholar]
- 25.De Vos V, Bengis RG, Kriek NP, Michel A, Keet DF, Raath JP, et al. The epidemiology of tuberculosis in free-ranging African buffalo (Syncerus caffer) in the Kruger National Park, South Africa. Onderstepoort J Vet Res. 2001;68:119–30. [PubMed] [Google Scholar]
- 26.Keet DF, Kriek NP, Penrith ML, Michel A, Huchzermeyer H. Tuberculosis in buffaloes (Syncerus caffer) in the Kruger National Park: spread of the disease to other species. Onderstepoort J Vet Res. 1996;63:239–44. [PubMed] [Google Scholar]
- 27.Gunn-Moore DA, Jenkins PA, Lucke VM. Feline tuberculosis: a literature review and discussion of 19 cases caused by an unusual mycobacterial variant. Vet Rec. 1996;138:53–8. 10.1136/vr.138.3.53 [DOI] [PubMed] [Google Scholar]
- 28.The 41st Decree of 1997 (May 28) of the Hungarian Ministry of Agriculture; 1997.