Abstract
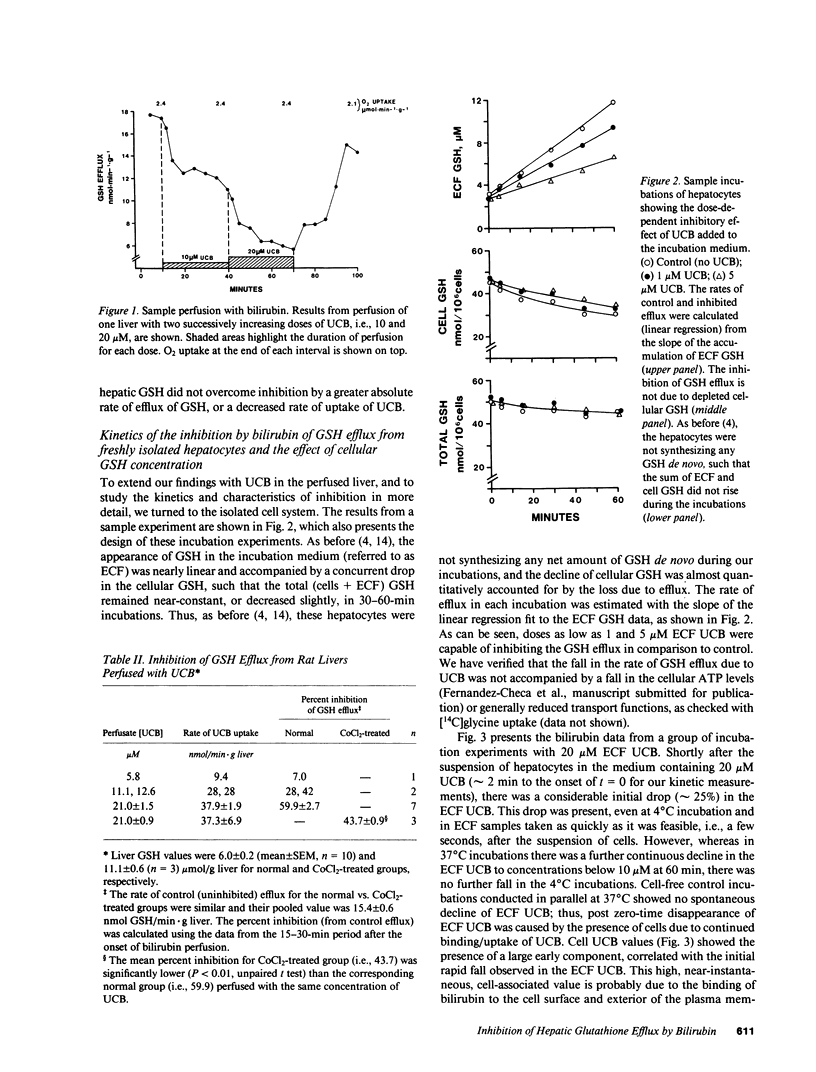
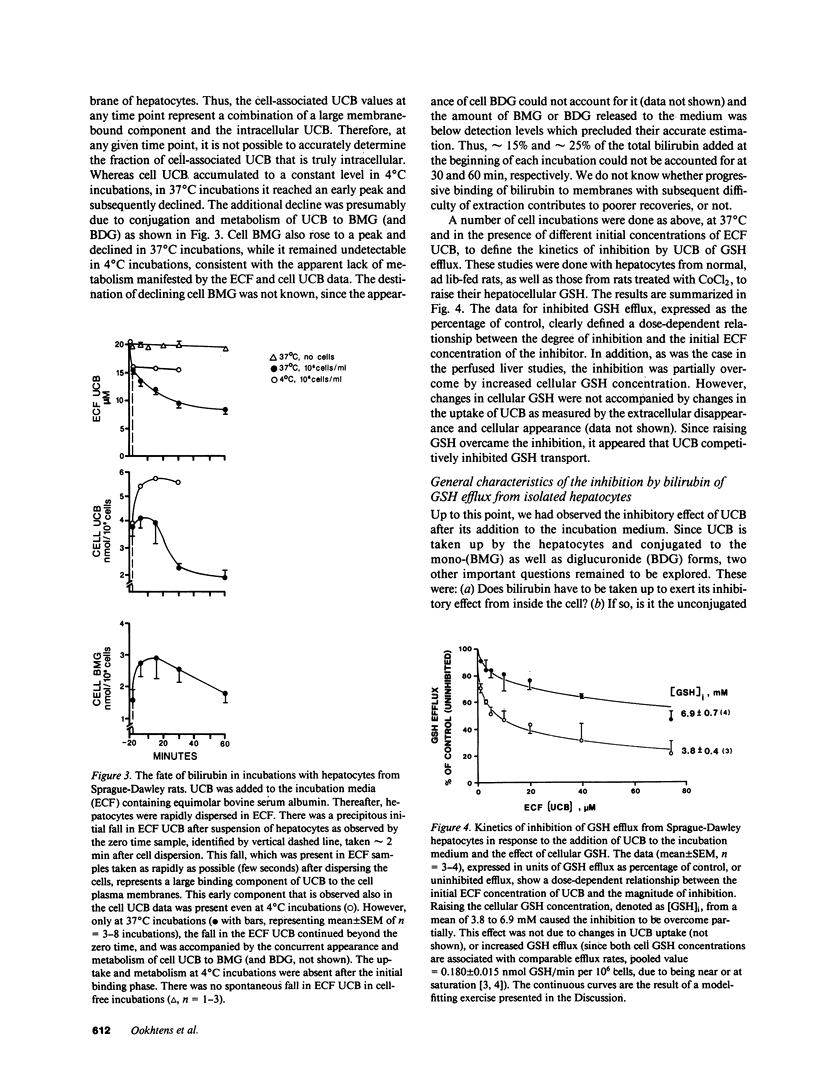
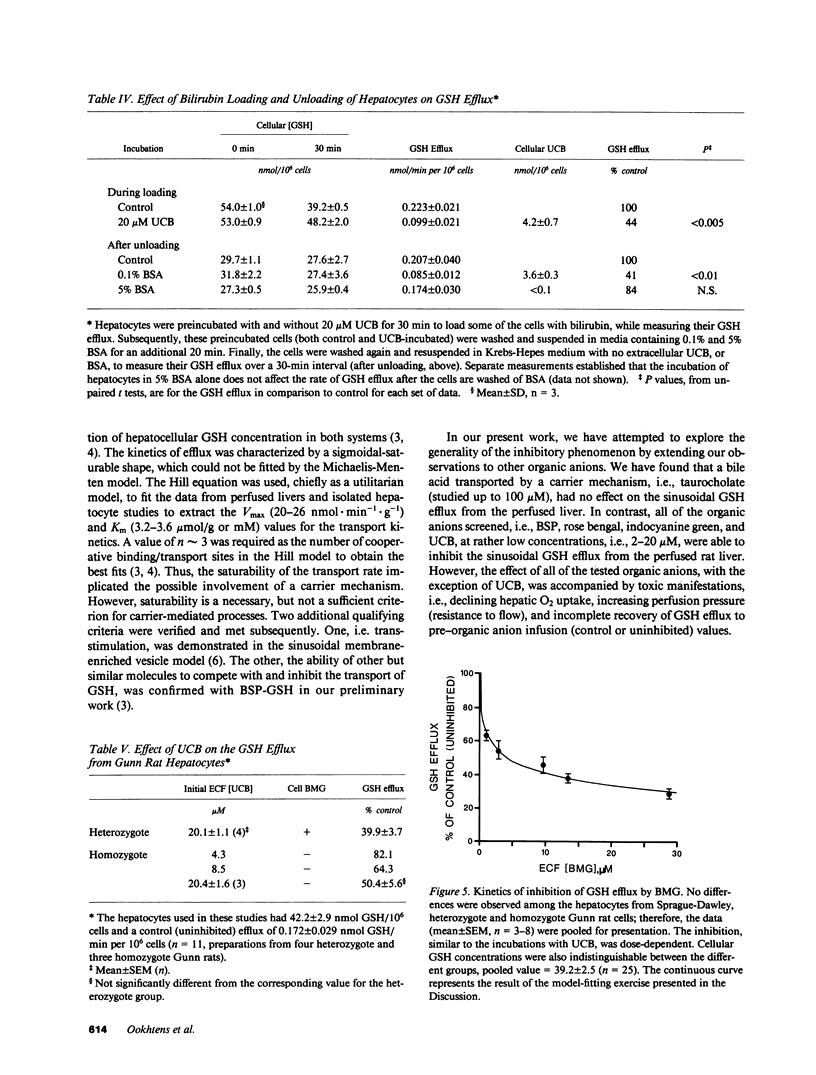
Using isolated, in situ, single-pass perfused rat livers, incubations of freshly isolated hepatocytes, and sinusoidal membrane-enriched vesicles, we and others have shown the saturability of transport (efflux) of hepatic glutathione (GSH). These observations have implicated a carrier mechanism. Our present studies were designed to provide further evidence in support of a carrier mechanism for hepatic GSH efflux by demonstrating competition by liver-specific ligands which are taken up by hepatocytes. Perfusing livers with different substances, we found that: (a) sulfobromophthalein-GSH (BSP-GSH) had a dose-dependent and fully reversible inhibitory effect on GSH efflux, while GSH alone did not have any effect; (b) taurocholate had no inhibitory effect; (c) all of the organic anions studied, i.e., BSP, rose bengal, indocyanine green, and unconjugated bilirubin (UCB), manifested potent, dose-dependent inhibitory effects, with absence of toxic effects and complete reversibility of inhibition in the case of UCB. The inhibitory effects of UCB could be overcome partially by raising (CoCl2-induced) hepatic GSH concentration. Because of the physiological importance of UCB, we conducted a detailed study of its inhibitory kinetics in the isolated hepatocyte model in the range of circulating concentrations of UCB. Studies with Cl- -free media, to inhibit the uptake of UCB by hepatocytes, showed that the inhibition of GSH efflux by UCB is apparently from inside the cell. This point was confirmed by showing that the inhibition is overcome only when bilirubin-loaded cells are cleared of bilirubin (incubation with 5% bovine serum albumin). Using Gunn rat hepatocytes and purified bilirubin mono- and diglucuronides, we found that both UCB and glucuronide forms of bilirubin inhibit GSH efflux in a dose-dependent manner. We conclude that the organic anions, although taken up by a mechanism independent of GSH, may competitively inhibit the carrier for GSH efflux from inside the hepatocyte.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aw T. Y., Ookhtens M., Kaplowitz N. Mechanism of inhibition of glutathione efflux by methionine from isolated rat hepatocytes. Am J Physiol. 1986 Sep;251(3 Pt 1):G354–G361. doi: 10.1152/ajpgi.1986.251.3.G354. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Ookhtens M., Kuhlenkamp J. F., Kaplowitz N. Trans-stimulation and driving forces for GSH transport in sinusoidal membrane vesicles from rat liver. Biochem Biophys Res Commun. 1987 Feb 27;143(1):377–382. doi: 10.1016/0006-291x(87)90676-0. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Ookhtens M., Ren C., Kaplowitz N. Kinetics of glutathione efflux from isolated rat hepatocytes. Am J Physiol. 1986 Feb;250(2 Pt 1):G236–G243. doi: 10.1152/ajpgi.1986.250.2.G236. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Jacob R., Boyer J. L. Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J Biol Chem. 1986 Jun 15;261(17):7860–7865. [PubMed] [Google Scholar]
- Gollan J., Hammaker L., Licko V., Schmid R. Bilirubin kinetics in intact rats and isolated perfused liver. Evidence for hepatic deconjugation of bilirubin glucuronides. J Clin Invest. 1981 Apr;67(4):1003–1015. doi: 10.1172/JCI110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Akerboom T. P., Sies H., Kinne R., Thao T., Arias I. M. Biliary transport of glutathione S-conjugate by rat liver canalicular membrane vesicles. J Biol Chem. 1984 Apr 25;259(8):4998–5002. [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Glutathione transport across hepatocyte plasma membranes. Analysis using isolated rat-liver sinusoidal-membrane vesicles. Eur J Biochem. 1984 Feb 1;138(3):491–495. doi: 10.1111/j.1432-1033.1984.tb07943.x. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Aw T. Y., Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Eberle D. E., Petrini J., Touloukian J., Corvasce M. C., Kuhlenkamp J. Factors influencing the efflux of hepatic glutathione into bile in rats. J Pharmacol Exp Ther. 1983 Jan;224(1):141–147. [PubMed] [Google Scholar]
- Lauterburg B. H., Adams J. D., Mitchell J. R. Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover. Hepatology. 1984 Jul-Aug;4(4):586–590. doi: 10.1002/hep.1840040402. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- OWENS C. W., BELCHER R. V. A COLORIMETRIC MICRO-METHOD FOR THE DETERMINATION OF GLUTATHIONE. Biochem J. 1965 Mar;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookhtens M., Hobdy K., Corvasce M. C., Aw T. Y., Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985 Jan;75(1):258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumgartner G., Reichen J. Kinetics of hepatic uptake of unconjugated bilirubin. Clin Sci Mol Med. 1976 Aug;51(2):169–176. doi: 10.1042/cs0510169. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Waggoner J. G., Berk P. D. Hepatic organic anion uptake in the rat. J Clin Invest. 1975 Nov;56(5):1280–1292. doi: 10.1172/JCI108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak W., Carey M. C. Reverse-phase h.p.l.c. separation, quantification and preparation of bilirubin and its conjugates from native bile. Quantitative analysis of the intact tetrapyrroles based on h.p.l.c. of their ethyl anthranilate azo derivatives. Biochem J. 1985 Feb 1;225(3):787–805. doi: 10.1042/bj2250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak W., Yuey W. Application of a rapid and efficient h.p.l.c. method to measure bilirubin and its conjugates from native bile and in model bile systems. Potential use as a tool for kinetic reactions and as an aid in diagnosis of hepatobiliary disease. Biochem J. 1986 Feb 15;234(1):101–109. doi: 10.1042/bj2340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]
- Wolkoff A. W., Samuelson A. C., Johansen K. L., Nakata R., Withers D. M., Sosiak A. Influence of Cl- on organic anion transport in short-term cultured rat hepatocytes and isolated perfused rat liver. J Clin Invest. 1987 Apr;79(4):1259–1268. doi: 10.1172/JCI112946. [DOI] [PMC free article] [PubMed] [Google Scholar]



