Abstract
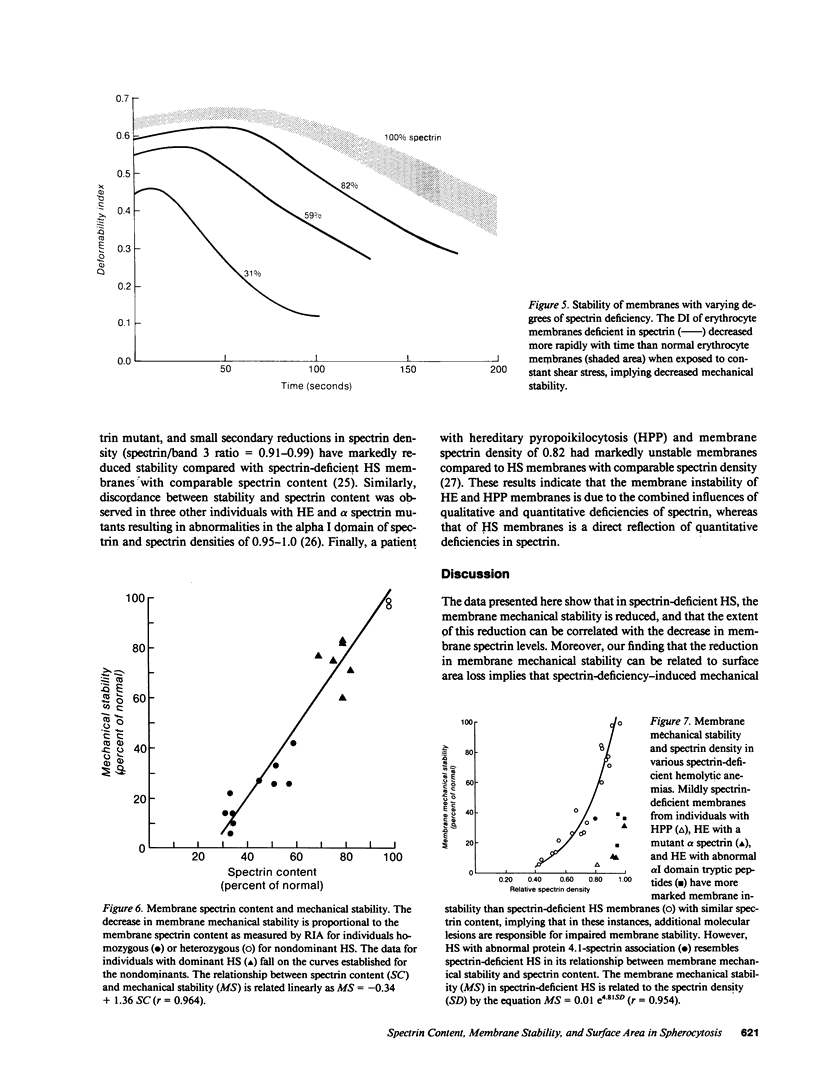
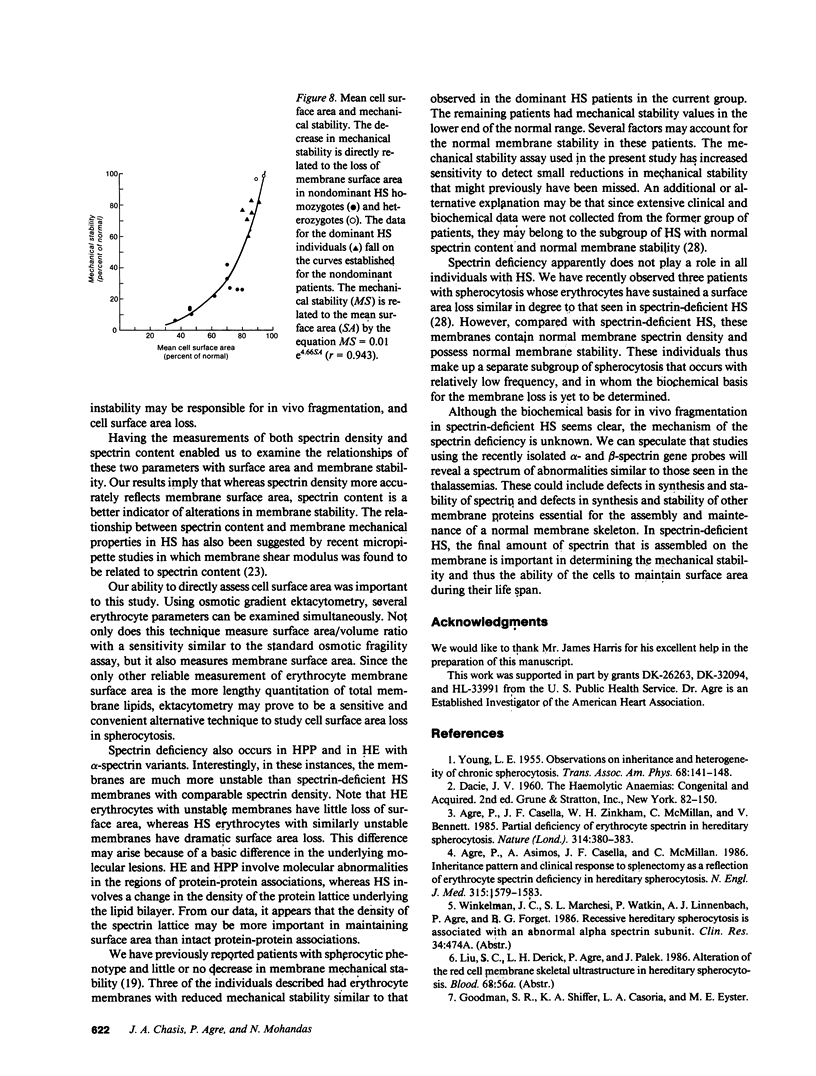
Whereas marked variations in the clinical manifestations of hereditary spherocytosis have long been recognized, we have only recently begun to define the molecular basis for this heterogeneity. An important unanswered question is whether decreased spectrin results in reduced membrane mechanical stability, and if this reduction in membrane mechanical stability can be related to in vivo surface area loss. Using the ektacytometer, we quantitated membrane surface area and stability in erythrocytes from 18 individuals with hereditary spherocytosis and deficiencies of spectrin (30-80% of normal spectrin level). Membrane mechanical stability was reduced and the magnitude of the reductions correlated with the spectrin content. Moreover, the reductions in mechanical stability correlated with in vivo loss of membrane surface area. These data indicate that decreased spectrin content results in reduced membrane mechanical stability and surface area loss in vivo. We conclude that partial deficiencies of spectrin, reductions in membrane mechanical stability, and loss of membrane surface area are directly related and are major features determining the heterogeneous clinical manifestations of hereditary spherocytosis.
Full text
PDF

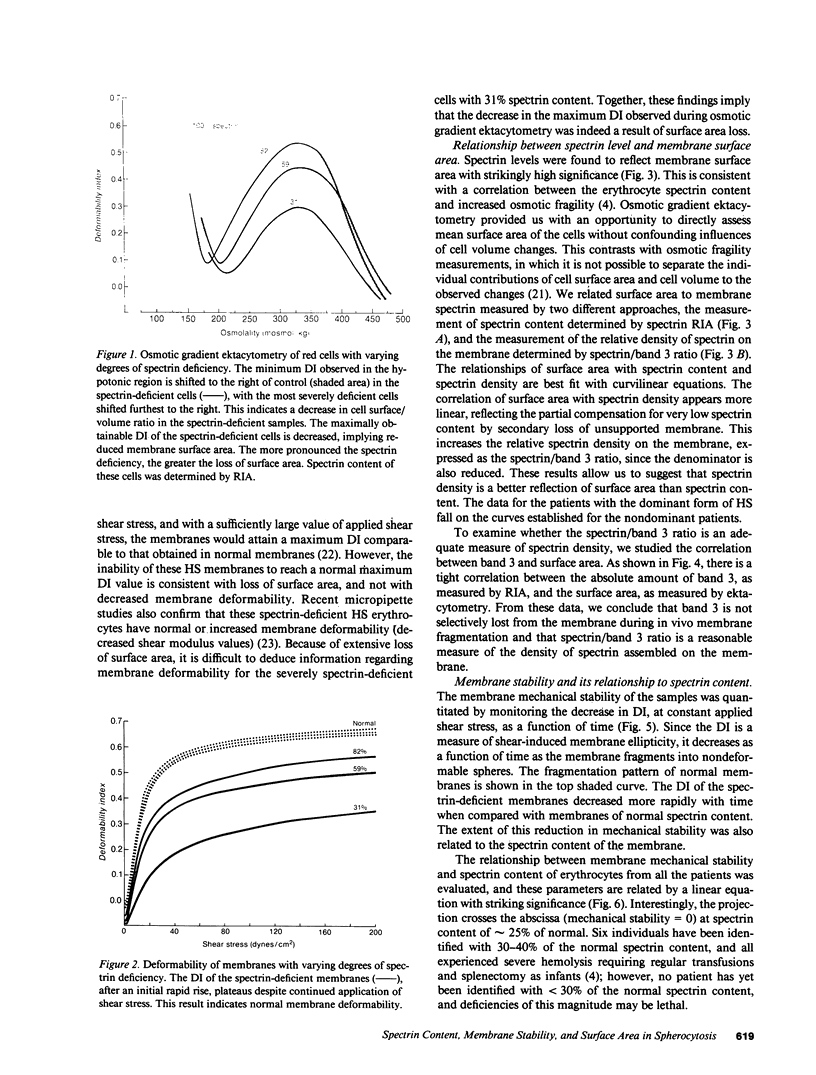
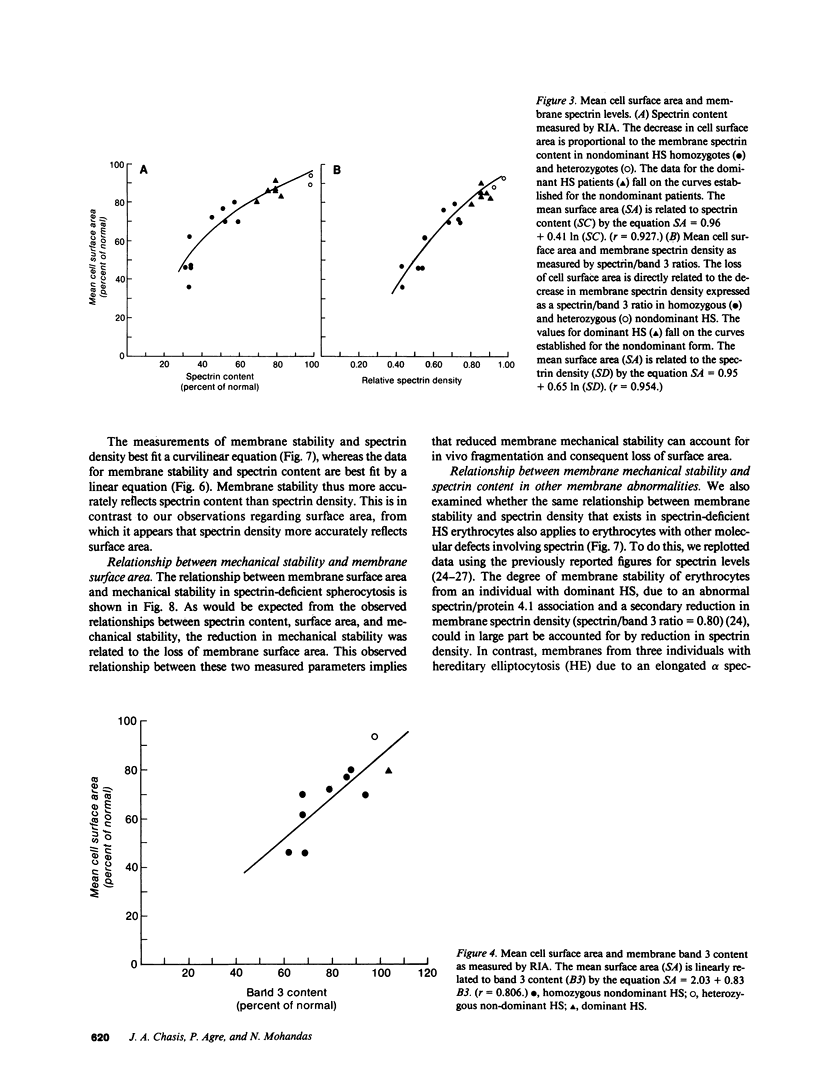

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Asimos A., Casella J. F., McMillan C. Inheritance pattern and clinical response to splenectomy as a reflection of erythrocyte spectrin deficiency in hereditary spherocytosis. N Engl J Med. 1986 Dec 18;315(25):1579–1583. doi: 10.1056/NEJM198612183152504. [DOI] [PubMed] [Google Scholar]
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Lux S. E. Hereditary spherocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):15–43. [PubMed] [Google Scholar]
- Becker P. S., Morrow J. S., Lux S. E. Abnormal oxidant sensitivity and beta-chain structure of spectrin in hereditary spherocytosis associated with defective spectrin-protein 4.1 binding. J Clin Invest. 1987 Aug;80(2):557–565. doi: 10.1172/JCI113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N., Shohet S. B. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J Clin Invest. 1985 Jun;75(6):1919–1926. doi: 10.1172/JCI111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Shohet S. B. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 1983 May;61(5):899–910. [PubMed] [Google Scholar]
- Coetzer T. L., Lawler J., Liu S. C., Prchal J. T., Gualtieri R. J., Brain M. C., Dacie J. V., Palek J. Partial ankyrin and spectrin deficiency in severe, atypical hereditary spherocytosis. N Engl J Med. 1988 Jan 28;318(4):230–234. doi: 10.1056/NEJM198801283180407. [DOI] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Heath B. P., Mohandas N., Wyatt J. L., Shohet S. B. Deformability of isolated red blood cell membranes. Biochim Biophys Acta. 1982 Oct 7;691(2):211–219. doi: 10.1016/0005-2736(82)90409-6. [DOI] [PubMed] [Google Scholar]
- Johnson R. M. The kinetics of resealing of washed erythrocyte ghosts. J Membr Biol. 1975 Jul 24;22(3-4):231–253. doi: 10.1007/BF01868173. [DOI] [PubMed] [Google Scholar]
- Knowles W. J., Morrow J. S., Speicher D. W., Zarkowsky H. S., Mohandas N., Mentzer W. C., Shohet S. B., Marchesi V. T. Molecular and functional changes in spectrin from patients with hereditary pyropoikilocytosis. J Clin Invest. 1983 Jun;71(6):1867–1877. doi: 10.1172/JCI110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P. A., Shew R. L., Iarocci T. A., Mohandas N., Hays T., Mentzer W. C. Unique alpha-spectrin mutant in a kindred with common hereditary elliptocytosis. J Clin Invest. 1987 Mar;79(3):989–996. doi: 10.1172/JCI112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzer W. C., Jr, Iarocci T. A., Mohandas N., Lane P. A., Smith B., Lazerson J., Hays T. Modulation of erythrocyte membrane mechanical stability by 2,3-diphosphoglycerate in the neonatal poikilocytosis/elliptocytosis syndrome. J Clin Invest. 1987 Mar;79(3):943–949. doi: 10.1172/JCI112905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Lie-Injo L. E., Friedman M., Mak J. W. Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood. 1984 Jun;63(6):1385–1392. [PubMed] [Google Scholar]
- Waugh R. E. Effects of inherited membrane abnormalities on the viscoelastic properties of erythrocyte membrane. Biophys J. 1987 Mar;51(3):363–369. doi: 10.1016/S0006-3495(87)83358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]
- YOUNG L. E. Observations on inheritance and heterogeneity of chronic spherocytosis. Trans Assoc Am Physicians. 1955;68:141–148. [PubMed] [Google Scholar]