Abstract
New effective strategies and new highly effective neuroprotective agents are being searched for the therapy of human stroke and cerebral ischemia. The compound SMe1EC2 is a new derivative of stobadine, with enhanced antioxidant properties compared to the maternal drug. Carvedilol, a non-selective beta-blocker, possesses besides its cardioprotective and vasculoprotective properties also an antioxidant effect. We compared the effect of carvedilol and SMe1EC2, antioxidants with a similar chemical structure, in two experimental models of oxidative stress in young and adult rat brain tissue. SMe1EC2 was found to improve the resistance of hippocampal neurons to ischemia in vitro in young and even in 18-month-old rats and inhibited formation of protein carbonyl groups induced by the Fe2+/ascorbic acid pro-oxidative system in brain cortex homogenates of young rats. Carvedilol exerted a protective effect only in the hippocampus of 2-month-old rats and that at the concentration 10-times higher than did SMe1EC2. The inhibitory effect of carvedilol on protein carbonyl formation induced by the pro-oxidative system was not proved in the cortex of either young or adult rats. An increased baseline level of the content of protein carbonyl groups in the adult versus young rat brain cortex confirmed age-related changes in neuronal tissue and may be due to increased production of reactive oxygen species and low antioxidant defense mechanisms in the adult rat brain. The results revealed the new pyridoindole SMe1EC2 to be more effective than carvedilol in neuroprotection of rat brain tissue in both experimental models involving oxidative stress.
Keywords: brain cortex, protein carbonyls, hippocampus, population spike, antioxidants
Introduction
Major interest is currently focused on the development of new effective strategies and new highly effective agents for the pharmacological therapy of human stroke and cerebral ischemia.
The new substance 2-ethoxycarbonyl-8-methoxy-2,3,4,4a,5,9b-hexahydro-1H-pyrido-[4,3b]indolinium chloride with the code SMe1EC2 (m.w. 312.79 Da, chemical purity < 99%), a derivative of stobadine (STO), (both synthetized in the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, Slovak Republic) was recently found to have a higher antioxidant capability than the parent compound STO with established neuroprotective and cardioprotective properties (Horáková & Štolc, 1998, Štolc et al., 2006). A toxicological and teratological study of SMe1EC2 showed its low toxicity and no embryotoxic and teratogenic effects on developing rats (Štolc et al., 2008, Ujházy et al., 2008).
Carvedilol, a non-selective beta-blocker with alpha-blocker properties, currently used to treat hypertension, heart failure and coronary artery diseases, has besides its cardioprotective and vasculoprotective properties also antioxidant effects. The antioxidant properties of carvedilol, and its relation to mitochondrial oxidative phosphorylation, calcium homeostasis and energy production, make this drug a unique beta-blocker, reinforcing its advantageous use in cardiac pathologies associated with enhanced cellular oxidative stress. Carvedilol administered subcutaneously directly after transient forebrain ischemia protected a population of neurons in the hippocampal CA1 area in gerbils (Strosznajder et al., 2005). Thus carvedilol raises high expectations also in the therapy of ischemia.
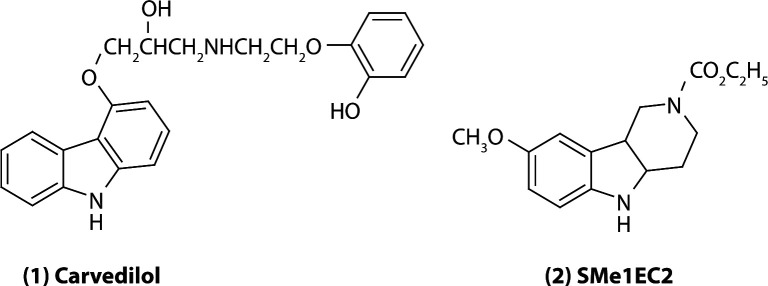
Both compounds tested, carvedilol and SMe1EC2, have a tri-cyclic basal skeleton bridged by the NH group. This represents the active site of the molecule responsible for interaction with free radicals (Figure 1). The antioxidant activity of both molecules is caused by the ability of the NH group to scavenge radicals by abstraction of the hydrogen from the NH group and by subsequent formation of a more stable N radical. Both compounds have an electron-donor group on the aromatic skeleton, which increases the stability of the N radical and thus its viability.
Figure 1.
Chemical formula of (1) carvedilol and (2) SMe1EC2.
In two experimental models involving oxidative stress on rat brain tissues, we studied the effect of the new pyridoindole SMe1EC2, derived from the neuroprotective and cardioprotective drug STO, and of carvedilol, a beta adrenoreceptor antagonist with potent antioxidant properties. We focused on 1) comparison of the effect of carvedilol and SMe1EC2 on the resistance of the rat hippocampus exposed to model ischemia in vitro (transient glucose/oxygen deprivation followed by reoxygenation), and on 2) comparison of their effect on protein carbonyl formation induced by the Fe2+/ascorbic acid pro-oxidative system in the rat brain cortex, both in young and adult rats.
Methods
Animals
Male Wistar rats aged 2, 10 and 18 months (weight 216 ± 8 g; 450 ± 8 g and 497 ± 7 g, respectively) (n=20, n=22 and n=15, respectively) from the breeding station Dobrá Voda (Slovak Republic, reg. No. SK CH 4004) were used in electrophysiological and biochemical experiments. The rats had free access to water and food pellets and were kept on a 12/12 h light/dark cycle. All procedures involving animals were performed in compliance with the Principles of Laboratory Animal Care issued by the Ethical Committee of the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences and by the State Veterinary and Food Administration of the Slovak Republic.
Drugs
The pyridoindole derivative SMe1EC2 was synthetized in the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, Slovak Republic.
Carvedilol, (±)-1-(Carbazol-4-yloxy)-3-[[2-(o-methoxyphenoxy)ethyl]amino]-2-propanol was obtained from La Roche (Mannheim, Germany). Stock solution of carvedilol in the concentration of 1 mmol/l was prepared by dissolution in wine acid and distilled water, heated up to 37 °C and sonicated three times for 5 min.
Oxygen/glucose deprivation and field action potential in rat hippocampal slices
Rat hippocampal slices (400 µm) were prepared by a conventional technique described in detail earlier (Vlkolinský & Štolc, 1999, Gáspárová et al., 2006). Bipolar wire electrodes were used to stimulate Schäffer collaterals evoking trans-synaptically activity in the CA1 area. Field action potential (FAP) was registered in the CA1 pyramidal cell layer by a glass microelectrode and stored in the computer for further analysis. Oxygen/glucose deprivation was obtained by replacement of the gas mixture containing O2 with the gas mixture with N2 by switching the valves and by replacement of the artificial cerebrospinal fluid (ACSF) equilibrated with oxygen to oxygen-free ACSF, with glucose diminished from 10 to 4 mmol/l. Oxygen/glucose deprivation elicited a decrease of FAP with its subsequent decay. Hippocampal slices were exposed to transient 6-min hypoxia/hypoglycemia followed by 20-min reoxygenation. Recovery of FAP after hypoxia/hypoglycemia was monitored during the 20-min reoxygenation, while population spike (PS) amplitude was measured in a later analysis. Each drug tested was present in the superfusing ACSF throughout the whole experiment: 30 min before oxygen/glucose deprivation, during 6-min hypoxia/hypoglycemia and during 20-min reoxygenation.
Pro-oxidative system of Fe2+/ascorbic acid and protein carbonyl formation in rat brain cortex
Protein carbonyl formation was determined by the method of Levine and coworkers (1990), modified by Blackburn and coworkers (1999) where 2,4-dinitrophenylhydrazine reacts with the protein carbonyl group and protein hydrazon is generated, which is detected spectrophotometrically with absorbance maximum at 360–370 nm. Proteins were detected spectrophotometrically in the same sample at 280 nm. Homogenate of the rat brain cortex (10%) was used. The pro-oxidative system was comprised of FeSO4 (0.1 mmol/l) and ascorbic acid (0.5 mMmol/l).
Results
Effect of SMe1EC2 and carvedilol during model ischemia in vitro
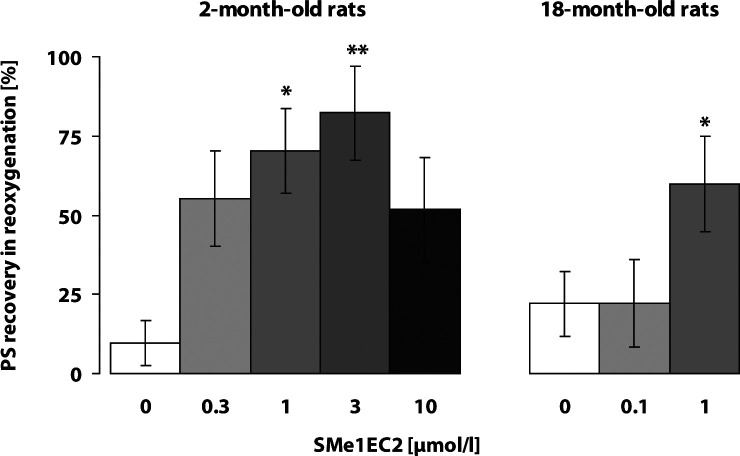
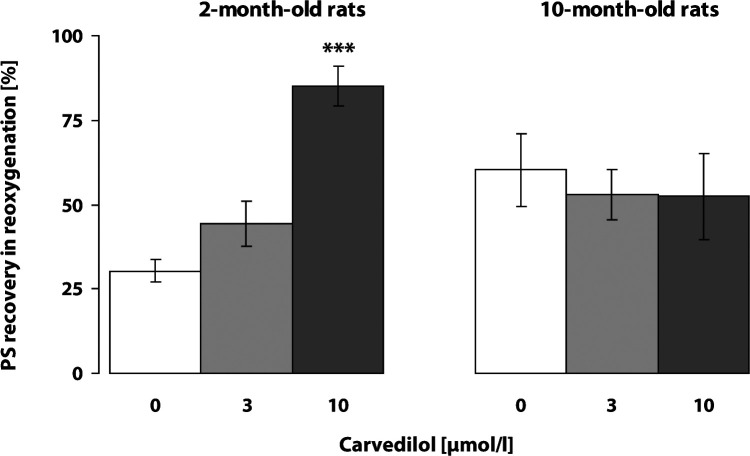
Transient 6-min ischemia in vitro (hypoxia with hypoglycemia) elicited a decrease and failure of electrically evoked response recorded in the pyramidal neurons of the CA1 area in the rat hippocampus. Untreated control slices showed low recovery of electrically evoked responses at the end of 20-min reoxygenation. SMe1EC2 (1 and 3 µmol/l) significantly improved recovery of the PS magnitude at the end of reoxygenation in the hippocampus of the young 2-month-old rats and established a neuroprotective effect even in the18-month-old rats (1 µmol/l) (Figure 2). Carvedilol, in the concentration of 10 µmol/l, significantly improved the resistance of hippocampal CA1 neurons to transient ischemia in vitro, yet only in young rats, while in 10-month-old rats it had no protective effect at any concentration tested (3 and 10 µmol/l) (Figure 3).
Figure 2.
Effect of SMe1EC2 on resistance of the CA1 neurons in hippocampus exposed to transient ischemia in vitro: 6-min hypoxia with hypoglycemia followed by 20-min reoxygenation. Ten to 12 slices from 6–8 rats were used in each experimental group. Cessation of field action potential and PS amplitude recovery were monitored. Significant difference in the PS amplitude recovery between untreated and treated slices at the end of 20-min reoxygenation was calculated by Student' t-test,*p<0.05, **p<0.01.
Figure 3.
Effect of carvedilol on resistance of CA1 neurons in hippocampus exposed to transient ischemia in vitro: 6-min hypoxia with hypoglycemia followed by 20-min reoxygenation. Nine to 11 slices from 6–8 rats were used in each experimental group. Cessation of field action potential and PS amplitude recovery were monitored. Significant difference in the PS amplitude recovery between untreated and treated slices at the end of 20-min reoxygenation was calculated by Student' t-test, ***p<0.001.
Effect of SMe1EC2 and carvedilol in Fe2+/ascorbic acid system
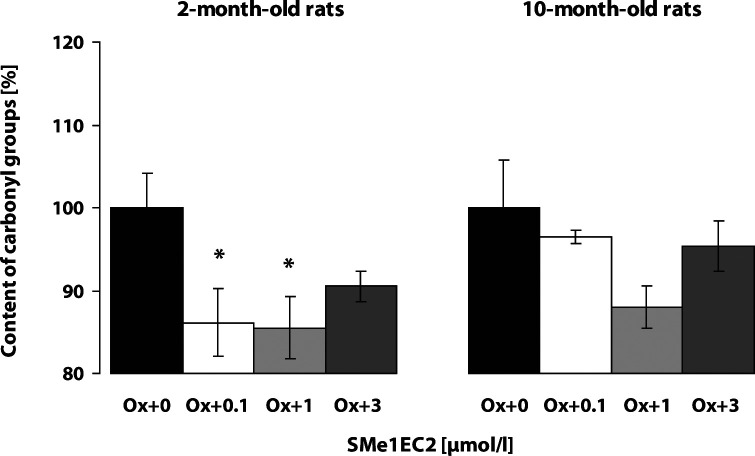
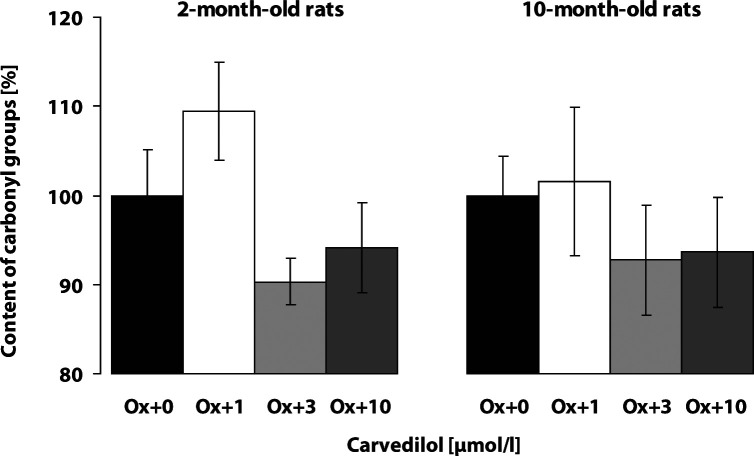
The baseline level of protein carbonyl groups in the cortex of control rats was significantly higher in the adult 10-month-old rats (2.12 ± 0.14 nmol/mg prot., n=20) as compared to the young 2-month-old rats (1.72 ± 0.12 nmol/mg prot., n=22; p<0.05). The Fe2+/ascorbic acid system induced marked oxidative modification of proteins in both the young and adult rat cortex homogenates, resulting in an increase of the content of protein carbonyl groups (p<0.001). The increase of protein carbonyl formation was of similar intensity in the young rat cortex as in the adult one (187.76 ± 6.20%; n=14 and 178.33 ± 8.24%; n=14, respectively). The compound SMe1EC2 protected brain cortex tissue against the oxidative damage induced by the Fe2+/ascorbic acid pro-oxidative system in 2-month-old rats even in a very low concentration (0.1 µmol/l) and showed the best effect in the concentration of 1 µmol/l (Figure 4). Carvedilol did not significantly suppress the Fe2+/ascorbic acid system induced carbonyl formation in the cortex of either young or adult rats (Figure 5).
Figure 4.
Effect of SMe1EC2 on Fe2+/ascorbic acid induced protein carbonyl formation in brain cortex. Rat brain cortex homogenates (n=5–6 samples in each group) from 5–6 rats were used. The content of protein carbonyl groups was determined spectrophotometrically. The content of carbonyls in the presence of the pro-oxidative system without the drug tested was considered 100% (Ox+0). Inhibitory effect of the antioxidant tested was monitored (Ox+0.1; Ox+1; Ox+3 µmol/l of SMe1EC2). Significant difference in protein carbonyl content between the untreated oxidation modified brain cortex homogenates and the treated oxidation modified homogenates was calculated by Student' t-test, *p<0.05.
Figure 5.
Effect of carvedilol on Fe2+/ascorbic acid induced protein carbonyl formation in brain cortex. Rat brain cortex homogenates (n=5–6 samples in each group) from 5–6 rats were used. The content of protein carbonyl groups was determined spectrophotometrically. The content of carbonyls in the presence of the pro-oxidative system without the drug tested was considered 100% (Ox+0). Effect of the drug tested on protein carbonyl content was monitored (Ox+1; Ox+3; Ox+10 µmol/l of carvedilol).
Discussion
To date many natural and synthetic compounds have been established possessing antioxidant properties. Antioxidants and radical scavengers may protect the nervous system against toxic effects of increased levels of reactive oxygen species and free oxygen radicals and thus attract many researchers to study their effect concerning brain protection.
Carvedilol is a multiple-action antihypertensive agent with a potential for cardiovascular protection beyond the normalization of high blood pressure. It has alpha1- and beta-adrenergic receptor blocking action, calcium channel blocking action, suppressive effect on cardiac necrosis and neuroprotective activities in animal models of brain ischemia and infarction (Ruffolo et al., 1990, Rabasseda 1998, Strosznajder et al., 2005). Carvedilol exerts an additional neuroprotective activity as a Na+ channel modulator and glutamate release inhibitor (Lysko et al., 1994). Recently, carvedilol was found to inhibit mitochondrial permeability transition, mitochondrial swelling, oxidation of thiol groups, and to protect mitochondria against oxidative damage induced by the xanthine oxidase/hypoxanthine pro-oxidant system (Oliviera et al., 2004, Oliviera et al. 2005, Carreira et al., 2006). Chronic administration of carvedilol resulted in an improvement of memory retention (evaluated in the Morris water maze task paradigms) and in attenuation of oxidative damage in the streptozotocin induced model of dementia in rats (Prakash & Kumar, 2009). Carvedilol may have a potential in the treatment of neurodegenerative diseases.
The idea of comparing the effect of these two compounds tested was based on their similar chemical structure and their antioxidant properties, proved in several different approaches. At concentrations above 1 µmol/l, carvedilol was found to be a calcium channel antagonist (Ruffolo et al., 1990). We tested it in the concentrations of 1, 3 and 10 µmol/l. In our experiments, the reported antioxidant and neuroprotective action of carvedilol was proved only in the hippocampal slices of young 2-month-old rats exposed to transient hypoxia with hypoglycemia followed by reoxygenation.
The compound SMe1EC2, with an antioxidant and neuroprotective effect established previously (Štolc et al., 2006; Štolc et al., 2008; Gáspárová et al., 2009; 2010), improved the resistance of hippocampal neurons exposed to transient ischemia in vitro in young and even in 18-month-old rats and significantly reduced the formation of protein carbonyl groups induced by the pro-oxidative system in young rat cortex homogenates. However, no inhibitory effect of SMe1EC2 on protein carbonyl formation was found in adult 10-month-old rats. This finding might be supported by results where the protective effect of some antioxidants, e.g. vitamin E (Sumien et al., 2004; Sung et al., 2004), melatonin (von Gall & Weaver, 2008), garlic extract (Brunetti et al., 2009), and beta-blockers (Gleibus and Lippa, 2007) was not proved either in adult and aged experimental animals or in elderly people. The increased native baseline level of the content of protein carbonyls in adult rats and a consequent further increase of carbonyls due to exposure to a pro-oxidative system, may be the reason for the failed protective effect of each antioxidant tested in adult 10-month-old rats. The increased baseline level of the content of protein carbonyl groups in the brain cortex of 10-month-old versus 2-month-old rats found in our experiments confirmed age-related changes in neuronal tissue. Our results are in good agreement with findings, for example of Balu and co-workers (2005), reporting age-associated increase in protein oxidation and reactive oxygen species production in the cerebral cortex and hippocampus of aged rats. The determined higher level of oxidatively modified proteins in native rat cortex homogenates of 10-month-old rats compared to young ones could be due to a lower baseline level of antioxidants, which was reported in the neuronal antioxidant system of adult and aged animals compared to young ones (Desole et al., 1993; Squier, 2001). The assumption has been voiced that oxidative stress is an early event of chronic brain diseases and antioxidant therapy may be beneficial only if given at this stage of the disease process (Sung et al., 2004).
Fe (II) is a potential pro-oxidant and can induce cellular oxidative stress. Ascorbic acid is a powerful physiological antioxidant and, in the presence of free Fe (II), it can exhibit pro-oxidant effects in vitro. We found that exposure of brain cortex homogenates to this pro-oxidative system induced an increase in protein carbonyl formation of a range comparable in young and adult rats. Thus in the pro-oxidative system tested, no age-dependent difference was found as to the vulnerability of brain tissue to oxidative stress. To date the relation between oxidant status and antioxidant defense mechanisms in aged animals of different species, organs or sexes has been investigated extensively, yet the results found have frequently been contradictory. Further studies are needed to elucidate these mechanisms and to determine the main signal pathways responsible for changes associated with aging.
Conclusion
The compound SMe1EC2 showed a significant neuroprotective effect in both experimental models involving oxidative stress and it was effective in lower concentrations than carvedilol. On the basis of previous results obtained with SMe1EC2, along with the findings reported here, we suggest that the new pyridoindole derivative SMe1EC2 is a new prospective and promising compound which might find a beneficial use in the treatment of neuronal impairment, such as stroke and brain ischemia.
Acknowledgement
This work was supported by the grants VEGA 2/0093/08 and APVV 51-017905, Bratislava, Slovak Republic. The authors thank to Mrs. Júlia Poláková and Mrs. Soňa Zacharová for technical assistance.
REFERENCES
- Balu M, Sangeetha P, Murali G, Panneerselvam C. Age-related oxidative protein damages in central nervous system of rats: modulatory role of grape seed extract. Int J Dev Neurosci. 2005;23:501–507. doi: 10.1016/j.ijdevneu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Doe WF, Buffinton GD. Protein carbonyl formation on mucosal proteins in vitro and in dextran sulfate-induced colitis. Free Radic Biol Med. 1999;27:262–270. doi: 10.1016/s0891-5849(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Brunetti L, Menghini L, Orlando G, Recinella L, Leone S, Epifano F, Lazzarin F, Chiavaroli A, Ferrante C, Vacca M. Antioxidant effects of garlic in young and aged rat brain in vitro. J Med Food. 2009;12:1166–1169. doi: 10.1089/jmf.2008.0176. [DOI] [PubMed] [Google Scholar]
- Carreira RS, Monteiro P, Goncalves LM, Providencia LA. Carvedilol: just another beta-blocker or a powerful cardioprotector? Cardiovasc Hematol Disord Drug Targets. 2006;6:257–266. doi: 10.2174/187152906779010746. [DOI] [PubMed] [Google Scholar]
- Desole MS, Esposito G, Enrico P, Miele M, Fresu L, De Natale G, Miele E, Grella G. Effects of ageing on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxic effects on striatum and brainstem in the rat. Neurosci Lett. 1993;159:143–146. doi: 10.1016/0304-3940(93)90819-7. [DOI] [PubMed] [Google Scholar]
- Gáspárová Z, Štolc S, Šnirc V. In vitro physiological evidence of enhanced neuroprotective and antioxidant action of 2,3-dihydromelatonin: a melatonin analogue. Pharmacol Res. 2006;53:22–27. doi: 10.1016/j.phrs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Gáspárová Z, Janega P, Babál P, Šnirc V, Štolc S, Mach M, Ujházy E. Effect of the new pyridoindole antioxidant SMe1EC2 on functional deficits and oedema formation in rat hippocampus exposed to ischaemia in vitro . Neuro Endocrinol Lett. 2009;30:574–581. [PubMed] [Google Scholar]
- Gáspárová Z, Šnirc V, Štolc S, Dubovický M, Mach M, Ujházy E. Maternal treatment of rats with the new pyridoindole antioxidant during pregnancy and lactation resulting in improved offspring hippocampal resistance to ischemia in vitro . NeuroEndocrinol Lett. 2010;31:348–352. [PubMed] [Google Scholar]
- Gleibus G, Lippa CF. The influence of beta-blockers on delayed memory function in people with cognitive function impairment. Am J Alzheimers Dis Other Demen. 2007;22:57–61. doi: 10.1177/1533317506295889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horáková L, Štolc S. Antioxidant and pharmacodynamic effect of pyridoindole stobadine. Gen Pharmacol. 1998;30:627–638. doi: 10.1016/s0306-3623(97)00300-5. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Lysko PG, Webb CL, Feuerstein G. Neuroprotective effects of carvedilol, a new antihypertensive, as a Na+ channel modulator and glutamate transport inhibitor. Neurosci Lett. 1994;171:77–80. doi: 10.1016/0304-3940(94)90609-2. [DOI] [PubMed] [Google Scholar]
- Oliviera PJ, Esteves T, Rolo AP, Palmeira CM, Moreno AJ. Carvedilol inhibits the mitochondrial permeability transition by an antioxidant mechanism. Cardivasc Toxicol. 2004;4:11–20. doi: 10.1385/ct:4:1:11. [DOI] [PubMed] [Google Scholar]
- Oliviera PJ, Goncalves L, Monteiro P, Providencia LA, Moreno AJ. Are the antioxidant properties of carvedilol important for the protection of cardiac mitochondria? Curr Vasc Pharmacol. 2005;3:147–158. doi: 10.2174/1570161053586903. [DOI] [PubMed] [Google Scholar]
- Prakash AK, Kumar A. Effect of chronic treatment of carvedilol on oxidative stress in an intracerebroventricular streptozotocin induced model of dementia in rats. J Pharm Pharmacol. 2009;61:1665–1672. doi: 10.1211/jpp/61.12.0012. [DOI] [PubMed] [Google Scholar]
- Rabasseda X. Carvedilol: an effextive antihypertensive drug with antiischemic/antioxidant cardioprotective properties. Drugs Today (Barc) 1998;34:905–926. doi: 10.1358/dot.1998.34.11.487475. [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Gellai M, Hieble JP, Willette RN, Nichols AJ. The pharmacology of carvedilol. Eur. J Clin Pharmacol. 1990;38(2):S82–88. doi: 10.1007/BF01409471. [DOI] [PubMed] [Google Scholar]
- Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Strosznajder RP, Jesko H, Dziewulska J. Effetc of carvedilol on neuronal survival and poly(ADP-ribose) polymerase activity in hippocampus after transient forebrain ischemia. Acta Neurobiol Exp. 2005;65:137–144. doi: 10.55782/ane-2005-1546. [DOI] [PubMed] [Google Scholar]
- Sumien H, Heinrich KR, Sohal RS, Forster MJ. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med. 2004;36:1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer′s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- Štolc S, Šnirc V, Májeková M, Gáspárová Z, Gajdošíková A, Štvrtina S. Development of the new group of indole-derived neuroprotective drugs affecting oxidative stress. Cell Mol Neurobiol. 2006;26:1493–1502. doi: 10.1007/s10571-006-9037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štolc S, Šnirc V, Gajdošíková A, Gajdošík A, Gáspárová Z, Ondrejičková O, Sotníková R, Viola A, Rapta P, Jariabka P, Syneková I, Vajdová M, Zacharová S, Nemček V, Krchnárová V. New pyridoindoles with antioxidant and neuroprotective actions. In: Bauer V, editor. Trends in pharmacological research. Bratislava, Slovakia: Institute of Experimental Pharmacology; 2008. pp. 118–136. [Google Scholar]
- Ujházy E, Dubovický M, Ponechalová V, Navarová J, Brucknerová J, Šnirc V, Mach M. Prenatal developmental toxicity study of the pyridolindole antioxidant SMe1EC2 in rats. Neuro Endocrinol Lett. 2008;29:639–643. [PubMed] [Google Scholar]
- Vlkolinský R, Štolc S. Effects of stobadine, melatonin, and other antioxidants on hypoxia / reoxygenation-induced synaptic transmission failure in rat hippocampal slices. Brain Res. 1999;850:118–126. doi: 10.1016/s0006-8993(99)02110-1. [DOI] [PubMed] [Google Scholar]
- von Gall C, Weaver DR. Loss of responsiveness to melatonin in the aging mouse suprachiasmatic nucleus. Neurobiol Aging. 2008;29:464–470. doi: 10.1016/j.neurobiolaging.2006.10.015. [DOI] [PubMed] [Google Scholar]