Abstract
Purpose
Given discrepancies between preclinical and clinical observations of mTOR inhibition in prostate cancer (PC), we sought to determine the pharmacodynamic (PD) effects of the mTOR inhibitor rapamycin in men with intermediate-high risk PC undergoing radical prostatectomy (RP).
Experimental Design
Rapamycin was given at 3 or 6 mg orally for 14 days prior to RP in men with multifocal Gleason ≥7 PC; 10 untreated control subjects were included. The primary outcome was inhibition of phosphorylation of ribosomal S6 in post-treatment RP vs. pre-treatment biopsy tumor tissue, evaluated using a Simon 2-stage design for PD efficacy.
Results
Thirty-two subjects were accrued, 20 at 3 mg, 2 at 6 mg, 10 controls. No dose-limiting toxicities (DLTs) were observed at 3 mg; however, 2/2 men enrolled at 6 mg experienced DLTs including thrombocytopenia and fever with grade 3 stomatitis. Adverse events observed at 3 mg included stomatitis, rash, ileus, and neutropenia. PD studies demonstrated tumor S6 phosphorylation inhibition in 50% of 10 evaluable rapamycin treated men with sufficient paired tissue (median 58% decline, p=0.049 vs. 2% decline in controls, p=0.75) with no significant effect on AKT activity. There was no change in Ki-67 or caspase-3 cleavage but we noted a reduction in cytoplasmic p27 staining with increased nuclear localization with rapamycin. Prostate tissue rapamycin concentrations were 3–4 fold higher than blood.
Conclusions
At 3 mg daily, rapamycin successfully and safely inhibited PC S6 phosphorylation and achieved relatively high prostate tissue concentrations. No effect on AKT phosphorylation or tumor proliferation or apoptosis was observed.
Introduction
While rapamycin analogs have provided benefit to patients with advanced renal cell carcinoma, the impact of mTOR inhibition in other solid tumors, including prostate cancer (PC), remains unclear(1–3). The rationale for mTOR inhibition is strong in advanced PC, given the high prevalence of activation of the PI3 kinase/AKT pathway due largely to loss of expression/function of the tumor suppressor PTEN, and the association of this pathway with adverse pathologic features, recurrence after radical prostatectomy (RP), and systemic treatment resistance(4–8). Preclinical studies have shown an ability of TORC1 inhibitors to revert prostatic intraepithelial neoplasia (PIN) and reduce tumor volume and growth/proliferation particularly in tumors with activated AKT or that lack PTEN(9–12). Thus, preclinical studies support the development of mTOR inhibitors in PC(11;13–15).
The clinical experience of TORC1 inhibition with rapamycin analogs in men with castrate-resistant PC, however, has been disappointing with few responses and a short time to progression(16;17), despite the common loss of PTEN observed in PC metastases (5;18;19). These observations mirror the modest activity of rapamycin analogs in other unselected cancers(1;20–23). Thus, studies that investigate mechanisms of primary resistance to TORC1 inhibition are needed. Recent findings suggest that rapamycin inhibits one form of mTOR, TORC1, but a second mTOR complex, TORC2, may remain uninhibited in a cell lineage-specific manner(24–26). While rapamycin may inhibit endothelial AKT activity and growth/survival(13;14;27), AKT activity and growth is often potentiated with rapamycin in part through inactivation of S6 kinase with resultant feedback derepression of IRS-1 activity(28;29). In addition, increased MYC activity has recently been linked to rapamycin resistance through 4EBP1 modulation(30). Thus, to achieve a significant clinical impact, TORC1 inhibition with rapamycin may require the addition of upstream inhibitors, such as insulin-like growth factor signaling or PI3 kinase signaling(28;29;31;32) or, alternatively, more effective inhibition of both TORC1 and TORC2 activity.
The pre-prostatectomy setting(33–36) represents a reasonable venue in which paired tumor tissue can be evaluated before and after exposure to a drug to address target specificity, drug delivery, physiologic effects on tumor growth and apoptosis, and correlation of biomarkers with clinical activity. Here, we sought to characterize the pharmacodynamic effects of TORC1 inhibition in men with localized PC to elucidate the anti-cancer mechanisms, if any, of rapamycin in men with primary untreated PC.
Materials and Methods
Subject Eligibility Criteria
We conducted a two-arm open label multi-dose multicenter prospective clinical trial of rapamycin in men with localized intermediate/high risk PC undergoing RP. This study was conducted through the Department of Defense Prostate Cancer Clinical Trial Consortium at Duke University, Johns Hopkins University, and the University of Michigan. Eligible treated and control men had PC clinical stages T1c-T3, no metastases, Gleason sum 7–10, multiple positive diagnostic cores, ECOG performance status 0–1, and were a candidate for RP. Men were sequentially enrolled to the 3 mg dosing cohort initially, followed by the 6 mg cohort, followed by the control cohort. Subjects were required to have adequate hepatic, renal, and bone marrow function, no allergy to rapamycins, avoid medications interfering with rapamycin metabolism, no active infection, no prior therapies for PC, and be age ≥18. This trial was approved by institutional review boards at each site and all men signed informed consent. This trial was registered at clinicaltrials.gov as NCT00311623.
Treatment and Safety Analysis
Men were treated with oral once-daily rapamycin at either 3 or 6 mg (Wyeth Pharmaceuticals, 1 and 2 mg tablets) or no treatment on days 1–14. Men were instructed to take rapamycin one hour before or two hours after food. Modified retropubic open RP was performed on day 15; the last rapamycin dose was given the morning prior to surgery. Toxicity was evaluated using NCI CTC v3.0. Lipids, PSA, CBC with differential, and hepatic/renal function were checked at baseline, day 14, and day 90 post-operatively. Dose limiting toxicity (DLT) was defined as grade 3/4 neutropenia with fever or lasting ≥7 days, platelets <100,000/mm3 or associated with bleeding, grade ≥3 non-hematologic toxicity, or irreversible grade 2 toxicity related to rapamycin.
Pathologic and Pharmacodynamic Analysis
Pre-treatment diagnostic transrectal paraffin-embedded core needle biopsies and post-treatment RP specimens were obtained on all subjects for PD analysis. Biopsies were reviewed for pathological diagnoses at each participating institution. Representative H&E sections and unstained tissue sections were then sent for central pathology review and IHC studies by one of two pathologists at the lead institution (GJN, AMD).
Immunohistochemistry (IHC)
The primary endpoint was inhibition of S6 phosphorylation in RP tumor tissue compared with pre-treatment biopsies. S6 phosphorylation at serine 240/244 was assessed by IHC as an indirect measure of S6 kinase activity using a validated anti-phospho S6 antibody. Scoring for S6 and phospho-AKT (serine 473) was performed using the H-score, a semi-quantitative measure of the percentage of cells scoring positive (0–100) multiplied by the intensity of staining (0–3). Additional secondary PD markers included Ki-67, nuclear cleaved caspase 3, p27, and PTEN. All IHC interpretations were performed and scored by two urologic pathologists blinded to treatment group and sample pairing. Discrepancies were resolved through consensus. See supplementary methods for further details of tissue processing and antibodies/controls.
Peripheral Blood Mononuclear Cell (PBMC) Pharmacodynamic Analysis
PBMCs were collected at baseline and on day 15 in the operating room within 1 hour of prostate removal in rapamycin treated subjects. Each sample was collected and processed as previously published(2); see supplementary methods for further details.
Pharmacokinetic Analysis
Whole blood was collected at baseline and day 15 within 1 hour of prostatectomy in all rapamycin-treated subjects. Snap frozen prostate tissue was evaluated for tissue rapamycin levels in the Analytical Pharmacology Core Laboratory at Johns Hopkins.(2) See supplementary methods for details.
Statistical Analysis
The minimax two-stage design of Simon was used to test a hypothesis about PD response, taken as a ≥60% inhibition in S6 kinase activity (≥60% decrease in the H-score for S6 phosphorylation in the RP tumor tissue as compared to pre-treatment biopsy tumor tissue). A reduction in S6 activity was found to correlate with potential clinical benefit in prior studies, and this benchmark was chosen empirically as indicative of a substantial decrease (37). Assuming a two-sided type I error rate of 0.05 and null PD response rate of 10%, the trial was designed to have 95% power to reject the null hypothesis if the true response rate was 40%. In the first stage, 12 patients were to be enrolled; if <2 responses were observed the trial was to be terminated. However, if ≥2 responses were observed, an additional 9 patients were to be enrolled at the second stage for a total of 21 patients. The null hypothesis is rejected if ≥4 patients responded at the end of the second stage. If ≥2/12 DLTs were observed at a given dose level, this dose was deemed unsafe for further testing in this population, and further accrual stopped; otherwise dose was escalated to 6 mg and the process repeated. Ten untreated control men were enrolled to provide estimates of variability for each PD biomarker. Descriptive graphs were developed to describe PD endpoints on paired tissue on an intra-individual basis and between treated and control subjects. Comparisons of the primary PD endpoint were conducted using the nonparametric Wilcoxon rank sum test (between groups) or Wilcoxon signed rank test (within groups), respectively. PK data were summarized using descriptive statistics. In addition, the Spearman correlation coefficients were computed for the PK and PD endpoints.
Results
From 1/2007-11/2008, 32 men were enrolled, including 20 subjects treated at 3 mg, 2 subjects at 6 mg, and 10 control subjects. Baseline characteristics are shown in the Table. Most men (77% rapamycin treated, 80% controls) enrolled in each arm had intermediate risk PC, with no observed differences across treatment groups for age, race/ethnicity, PSA, Gleason sum, or stage(38). No differences in baseline PSA values or decline in PSA over time were seen across treatment groups, and >80% of men had an undetectable PSA in each arm 90 days following RP (Table).
Table.
Baseline and surgical characteristics of the patients on study. RP=radical prostatectomy, EBL=estimated blood loss, BMI=body mass index, PSA=prostate specific antigen.
| Patient Characteristics | Rapamycin Treated Men 3 mg (n=20) |
Rapamycin Treated Men 3 mg (evaluable for primary endpoint, n=10) |
Rapamycin Treated Men 6 mg (n=2) |
Control Group (n=10) |
|---|---|---|---|---|
| Demographics | ||||
| Median Age, years (range) | 61.5 (50–81) | 59 (50–72) | 57.5 (51–64) | 64 (51–68) |
| Race: Caucasian (%), | 16 (80), | 9 (90) | 2 (100) | 10 (100) |
| African-American (%) | 4 (20) | 1 (10) | 0 (0) | 0 (0) |
| Ethnicity: Non-Hispanic (%) | 22 (100) | 10 (100) | 2 (100) | 10 (100) |
| Median BMI (kg/m2) | 27.8 | 27.4 | 28.7 | 28.0 |
| Clinical characteristics | ||||
| Biopsy Gleason sum (%): | ||||
| 6 | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| 7 | 17 (85) | 9 (90) | 1 (50) | 9 (90) |
| 8–10 | 2 (10) | 1 (10) | 1 (50) | 1 (10) |
| RP Gleason sum: | ||||
| 6 | 3 (15) | 1 (10) | 0 (0) | 3 (30) |
| 7 | 15 (75) | 8 (80) | 2 (100) | 6 (60) |
| 8–10 | 2 (10) | 1 (10) | 0 (0) | 1 (10) |
| Pre-operative clinical stage: | ||||
| T1c | 13 (65) | 5 (50) | 2 (100) | 6 (60) |
| T2a | 4 (20) | 2 (20) | 0 (0) | 0 (0) |
| T2b | 2 (10) | 2 (20) | 0 (0) | 3 (30) |
| T2c | 1 (5) | 1 (10) | 0 (0) | 1 (10) |
| Pathologic stage: | ||||
| T2 | 10 (50) | 1 (10) | 2 (100) | 6 (60) |
| T3a | 7 (35) | 6 (60) | 0 (0) | 3 (30) |
| T3b | 3 (15) | 3 (30) | 0 (0) | 0 (0) |
| TX N1 | 0 (0) | 0 (0) | 0 (0) | 1 (10) |
| Median baseline PSA, ng/dl | 5.5 | 6.5 | 3.8 | 6.7 |
| Median day 14 PSA, ng/dl | 6.6 | 8.1 | 3.9 | 8.5 |
| Any PSA decline (%) | 4/17 (27%) | 3/8 (38%) | 1/2 (50%) | 1/5 (20%) |
| Median EBL (mL) | 550 | 450 | 325 | 625 |
| Pre-operative D’Amico risk: | ||||
| Low | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Intermediate | 17 (85) | 9 (90) | 1 (50) | 8 (80) |
| High | 2 (10) | 1 (10) | 1 (50) | 2 (20) |
| Median day 90 PSA | 0 | 0 | 0 | 0 |
| % undetectable day 90 PSA | 17/20 (85%) | 8/10 (80%) | 2/2 (100%) | 4/5 (80%) |
Among control men, no adverse events were observed other than one prolonged post-operative ileus that resolved with conservative management. In the 3 mg cohort, two cases of post-operative ileus were noted which also resolved with medical management. Known toxicities associated with rapamycin were observed at 3 mg: grade 1 maculopapular rash (1), grade 1 thrombocytopenia (1), grade 1–2 neutropenia without fever (2), and grade 2 stomatitis (2) without DLTs. In the 6 mg cohort, 2/2 subjects experienced DLTs likely related to rapamycin, consisting of thrombocytopenia requiring delay in RP (platelet count of 90,000/mm3), and grade 3 stomatitis, fever, and diarrhea; no further subjects were treated at this dose level per protocol. No obvious differences were noted in wound healing complications or estimated blood loss between rapamycin-treated and control subjects (Table). Thus, 3 mg was selected as the MTD in this setting.
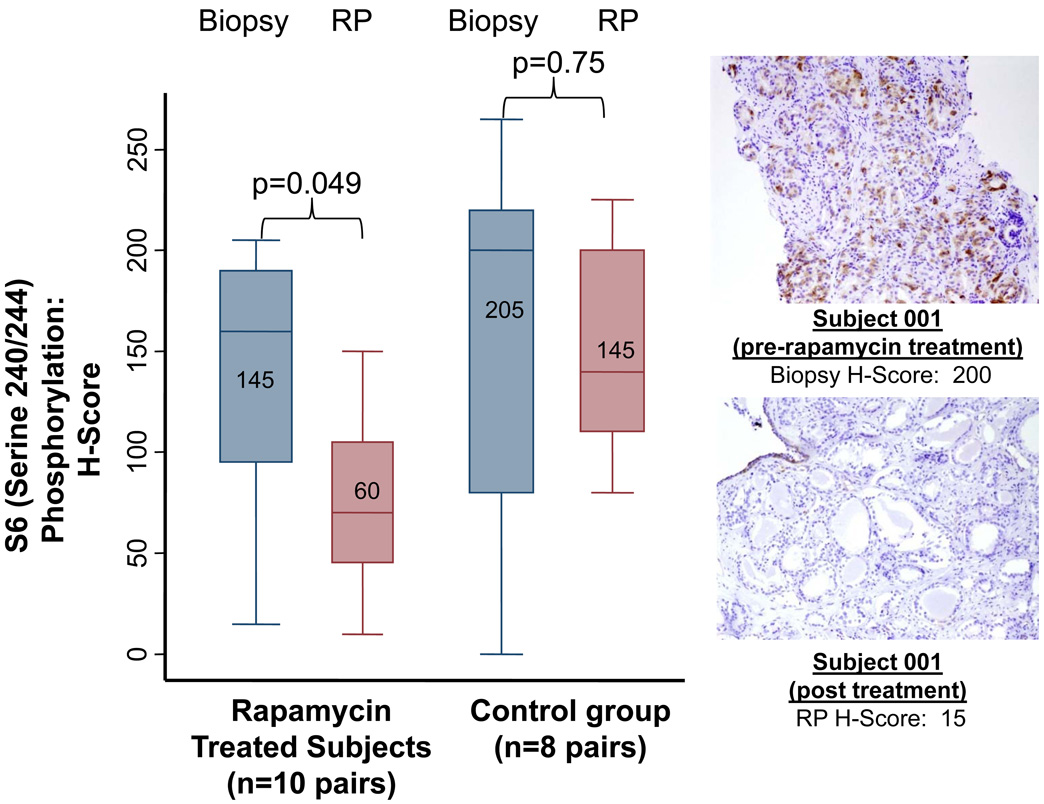
The 3 mg cohort was expanded after 2 PD responses and no DLTs were observed in the first 12 subjects. Among the 20 men treated at 3 mg, 10 men had adequate paired tissue for evaluation of PD response, largely due to lack of availability or inadequacy of either biopsy or RP (n=10) specimens. There were no significant differences in baseline characteristics in men with vs. those without adequate paired tissues. Five of ten men (50%, 95% CI = 19–81%, p<0.0001 vs. null hypothesis of 10%) achieved a ≥60% tumor S6 kinase inhibition (inhibition of S6 phosphorylation) with rapamycin treatment, thus meeting the pre-specified primary endpoint of the study. One of eight (12.5%) men in the control arm experienced a PD response (p=0.81 vs. null hypothesis). Median S6 kinase inhibition was 58% (inter-quartile range (IQR) −15 to 82%) in rapamycin treated subjects versus 2% (IQR −6 to 30%) among eight controls with adequate available paired tissue. Post-treatment reduction in S6 kinase activity was statistically significant in rapamycin treated men (Wilcoxon p=0.049) but not in control men (p=0.75, Figure 1). The median post-treatment S6 activity H-score was 70 in 13 rapamycin treated men, and 140 in 9 control men with evaluable tissue (Wilcoxon p=0.006). In addition, we found that a decrease in serum PSA between baseline and day 14 was observed with rapamycin treatment in 0/4 evaluable men who had a tumor S6 PD response (median 23% rise), while 3/4 men without a tumor S6 PD response had a PSA decline (median 7% decline, chi square p=0.028).
Figure 1.
Left: Box plots and pathologic representation of the primary PD endpoint, S6 kinase inhibition (S6 phosphorylation), in paired prostate tissues (biopsy and radical prostatectomy (RP) cancer tissue) among rapamycin treated and control subjects. Right: Example of immunohistochemical staining of subject 1 pre-treatment biopsy tissue and post-treatment RP specimen, with H-score depicted. Figures shown within boxes represent median values.
We next examined the relationship of pre-treatment PTEN and AKT status with PD efficacy. Biopsy PTEN H-scores ranged from 0–250 (median 80), while biopsy phospho-AKT H-scores ranged from 0–240 (median 80). We found a modest correlation (R2=0.83) between biopsy PTEN expression and inhibition of S6 kinase in rapamycin-treated men (n=10). For example, 2/2 (100%) treated men with PTEN-null tumors (H-Score 0–50) on biopsy had a PD response, 1/3 (33%) men with PTEN-low tumors (H-Score 50–80) had a PD response, and 1/4 (25%) men with tumors expressing moderate levels of PTEN (H-score >80) had a PD response. Only one rapamycin-treated subject had no PTEN expression detectable in the biopsy specimen; likewise only 2 subjects had no PTEN expression at the time of RP. We observed no correlation between biopsy and RP PTEN expression, despite efforts to score RP tumor samples from the same location as the pre-treatment biopsy. Of the biopsies with low PTEN expression (H Score≤100), 6/12 (50%) had AKT phosphorylation; of the biopsies with intact PTEN (H Score>100), 2/7 (29%) had AKT phosphorylation. No correlation was observed between pretreatment biopsy AKT phosphorylation or PTEN status with biopsy S6 phosphorylation or subsequent S6 inhibition.
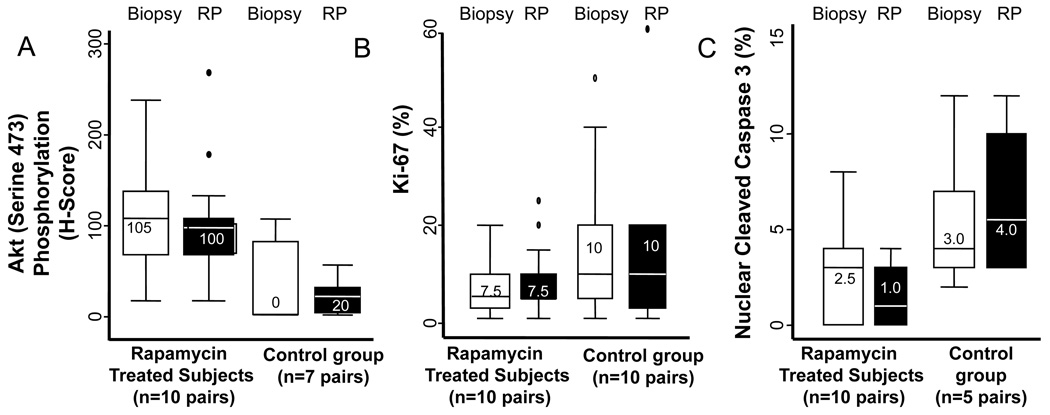
In men with evaluable paired tumor tissue (10 rapamycin treated men at 3 mg and 7 controls), we detected no difference in phosphorylated AKT induction across treatment groups (Figure 2a). Among rapamycin-treated men, any increase in AKT phosphorylation was seen in 4 men, any decrease in 4 men, and no change in 2 men. Among controls, we observed an increase in AKT phosphorylation in 3 men, a decrease in 3 men, and no change in 1 man. We observed no relationship between post-treatment AKT phosphorylation induction and biopsy PTEN status. The median post-treatment phospho-Akt H-score was 100 in 13 rapamycin treated men, and 20 in 8 control men with evaluable tissue.
Figure 2.
Box plot representations of the secondary PD endpoints as measured in paired biopsy and radical prostatectomy (RP) specimens for: A) AKT activity (phosphorylation) change, B) change in proliferation (Ki-67), and C) changes in nuclear cleaved caspase 3 products. Figures shown within boxes represent median values for each biomarker.
No changes were observed in proliferation (Ki-67 expression) in paired samples in either rapamycin treated (median 7.5% vs. 8.2%) or control subjects (17% vs. 15.1%, Figure 2b). We did not observe a difference in Ki-67 expression among rapamycin treated men who had tumor S6 inhibition as compared to those without S6 inhibition (2% vs. 0% reduction, n=10). No difference in proliferation was noted in post-treatment Ki67 levels in 13 rapamycin treated vs. 10 control men with evaluable tissue (median 5% vs. 10%). No relationship between post-treatment PSA declines and changes in tumor phospho-Akt was observed. However, a PSA decline was observed in 3/6 (50%) with a reduction in tumor Ki67 after rapamycin and in 1/7 (14%) men without a Ki67 reduction.
We next evaluated tumors for evidence of nuclear caspase 3 cleavage as a biomarker of apoptosis in paired tissues (Figure 2c). Nuclear expression of caspase-3 cleavage was overall low in most biopsy and RP specimens (median 3.0%, IQR= 2.0–6.0 in 20 biopsies; median 2.5%, IQR= 0–4.0 in 20 RP specimens) with no significant differences in caspase 3 induction observed in rapamycin treated (n=10 pairs) or control men (n=5 pairs), nor in PD responders vs. non-responders. Nuclear caspase 3 cleavage was higher in RP specimens in control men (5.5%, n=6) compared to rapamycin treated men (1%, n=14). While biopsy caspase 3 cleavage was also higher in control men (4% vs. 3%), this difference was not significant.
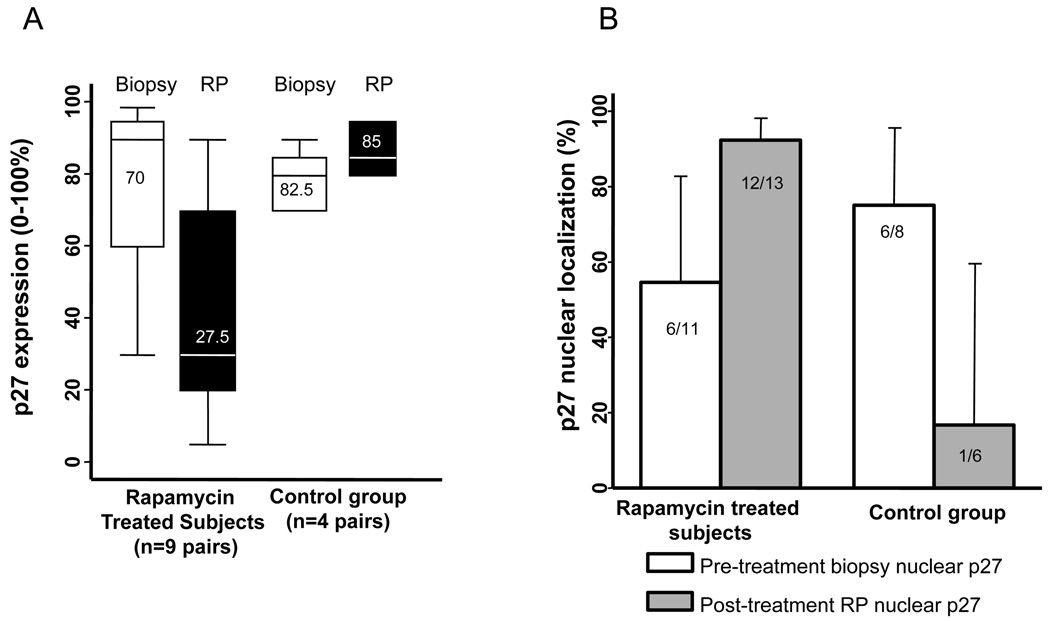
In rapamycin-treated subjects (n=9 with adequate paired tissue), p27 cytoplasmic staining frequency was reduced (mean pretreatment p27 expression 72% vs. 32% post-treatment), while no difference was noted in control subjects (n=4, 81% vs. 86%) (Figure 3a). An increase in p27 nuclear localization was noted in rapamycin-treated subjects (6/11 pre-treatment vs. 12/13 post-treatment) but not in control subjects (6/8 pre-treatment vs. 1/6 post-treatment), indicating no difference in baseline (biopsy) nuclear p27 localization but an increase in nuclear localization in post-treatment (RP) samples with rapamycin but not control subjects (Figure 3b).
Figure 3.
Box plots and bar graphs demonstrating: A) change in p27 expression in paired prostate tissues (biopsy and RP specimens) among rapamycin treated and control subjects. Figures shown within boxes represent median values. B) p27 nuclear localization in paired prostate tissues among rapamycin treated and control subjects.
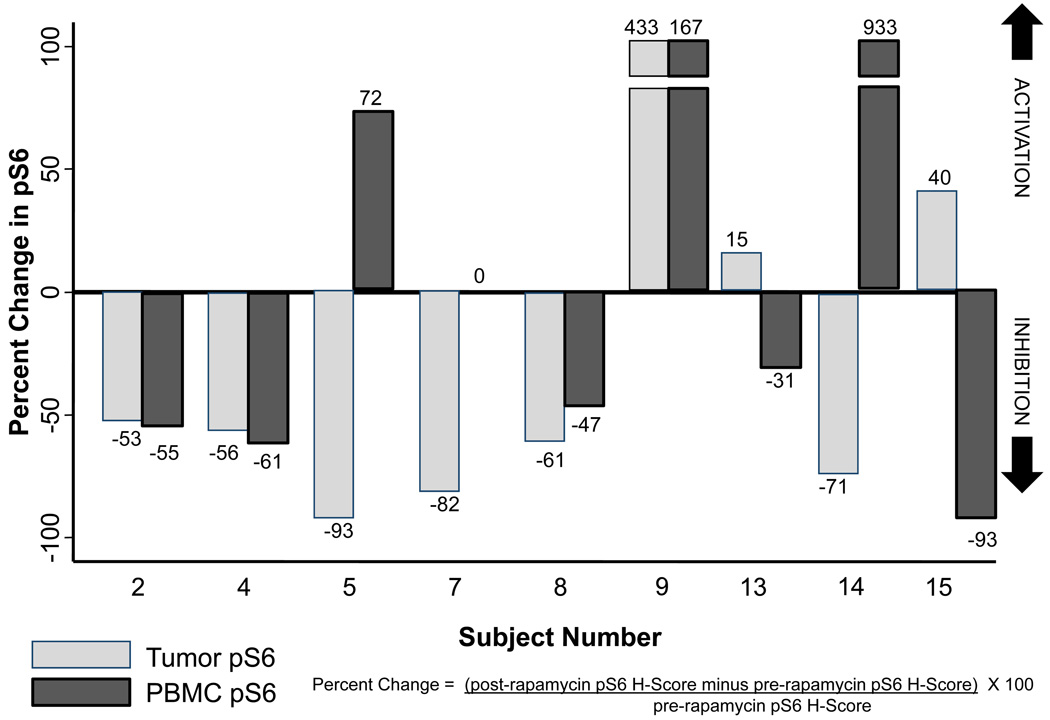
PBMC S6 kinase was inhibited on day 15 by a median of 32% in rapamycin-treated subjects (3 mg), and 9/19 subjects with evaluable paired PBMCs having ≥60% S6 inhibition post-treatment. PBMC S6 kinase inhibition did not correlate with tumor S6 kinase inhibition, and PD response in tumor vs. PBMCs did not correlate in nine subjects with evaluable paired tissues, with 6/9 subjects having discordant results. Only 4/9 paired PBMC-tumor results showed a similar directional trend (Figure 4). Induction of PBMC total S6 expression by rapamycin was observed in 44% of men. There was a modest correlation of baseline PBMC S6 kinase activity with day 15 S6 kinase inhibition in rapamycin-treated subjects (R2=−0.68).
Figure 4.
Paired bar plot demonstrating peripheral blood mononuclear cell (PBMC) and prostate tumor phospho-S6 inhibition among those rapamycin-treated subjects (n=9) who had available PBMC and tumor tissue for biomarker assessment. Phospho-S6 percent change (before and after rapamycin) is calculated using the equation depicted in the figure.
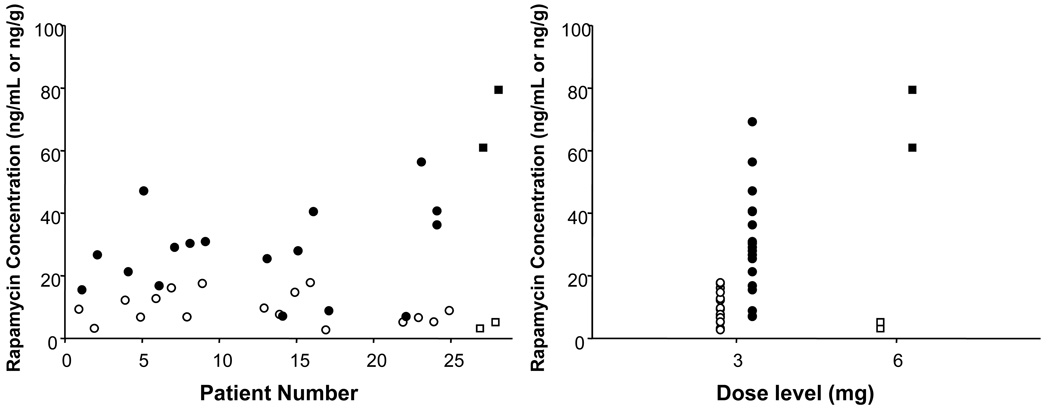
Mean day 15 blood rapamycin level was 9.9 ng/ml (range 2.7–17.8) in the 3 mg cohort, and mean day 15 prostate tissue rapamycin level was 28.7 ng/g (range 7.0–69.2, Figure 5). Mean relative systemic exposure (tumor/whole blood ratio) was 3.54 (range 0.9–8.5, median 2.09), indicating a 3- to 4-fold increase in rapamycin tissue levels over whole blood concentrations. We found no correlation between whole blood or tissue rapamycin levels and any PD effects in rapamycin-treated men.
Figure 5.
Individual rapamycin concentrations in radical prostatectomy tumor tissue (filled symbols, expressed as ng/g) and peripheral blood (open symbols, expressed as ng/g) grouped by subject number (left) and rapamycin dose cohorts (right): 3 mg (circles) or 6 mg per (squares).
Discussion
In this study, we found that rapamycin entered the prostate at physiologically relevant concentrations 3–4 fold higher than whole blood, despite the use of a 3 mg oral daily dose that is half the MTD in other settings(2). We found that rapamycin inhibited the activity of a downstream target of TORC1, S6 kinase, in over half of evaluable patients without dose limiting toxicity, thus demonstrating the intended target inhibition and meeting our primary endpoint. However, we found no physiologically relevant effects of rapamycin on tumor cellular proliferation, post-treatment tumor grade or stage, PSA, or apoptosis over a 2 week exposure period. Rapamycin administration was safe at 3 mg, and this dose can be considered the MTD in the pre-operative setting. In addition, we found no correlation between PBMC and tumor pharmacodynamic efficacy, which suggests a limited role for PBMCs as surrogate measures of efficacy and the need for tumor-based PD assessments of drug mechanism.
This study has several limitations. This was a multicenter study, and some tissues were non-evaluable, due to absence of tumor or limited biopsy material for analyses. These limitations significantly reduced the overall sample size for PD analysis for all endpoints and emphasizes the need for standard operating procedures for collection, prioritization, tracking, and shipping of tumor tissue to maintain integrity and statistical power in these studies. Thus, many of our secondary PD analyses are underpowered to detect moderate biomarker effect sizes and should be considered exploratory in nature. In addition, many assays had wide variability despite attempts to isolate identical tumor foci in the RP specimen compared to the original biopsy. Despite this, we were able to collect sufficient paired samples to meet our primary endpoint and investigate several secondary PD and PK endpoints of rapamycin effect and mechanism in vivo which have not previously been reported in the literature. We also demonstrated that robust and strong PD effects (S6 inhibition) after drug exposure in PC can be observed with relatively small sample sizes, given reduced statistical variability with paired tissues. We did not find a correlation between PTEN expression in biopsy and RP specimens, despite using a validated PTEN assay with genetic controls, indicating that different heterogeneous tumors were sampled and analyzed before and after rapamycin, that PTEN expression may have been altered by rapamycin, or a limited power to detect these changes. We additionally found that many of the PD biomarkers such as Akt, p27, caspase-3, Ki-67, and PTEN have wide variability in this setting during specimen collection and processing across multiple centers, highlighting the challenges inherent in these pharmacodynamic studies and the need for standardized collection, processing, and ascertainment of these markers, with consideration of final sample size based on evaluable tissues.
We found that rapamycin reduced cytoplasmic expression and increased nuclear localization of the cyclin-dependent kinase inhibitor p27 in RP specimens as compared to untreated men. The finding of increased p27 nuclear localization is intriguing but not clearly correlated with a change in cellular proliferation as might be expected. A role for p27 as a prognostic marker in PC is relatively strong, with multiple(39–42) but not all(43) studies demonstrating an adverse relationship between low nuclear p27 expression and recurrence after surgery. In addition, knockout of p27 combined with constitutive Akt activation in model systems leads to an aggressive, invasive PC phenotype(44). Variability in fixation techniques or localization of paired tumor foci may explain some of this heterogeneity; thus, a reduction in p27 nuclear localization is currently of unclear physiologic relevance(45). However, defective p27 localization has been linked to rapamycin resistance, and further genomic studies are planned to characterize these molecular alterations(46). Longer periods of rapamycin exposure or higher doses may also be required to observe an anti-proliferative cytostatic effect; however, higher doses were not well tolerated in this treatment naïve pre-operative setting.
These pharmacodynamic findings, along with clinical studies in the metastatic setting, call into question the clinical relevance of single agent TORC1 inhibition in unselected men with advanced PC, and point to strategies that dissect mechanisms of resistance(16;17). Given the pre-clinical efficacy of TORC1 inhibitors, it is essential to improve upon our models while investigating the PI3K pathway, and which components, including TORC1 or TORC2, are most relevant to PC progression.
The mechanistic evaluation of novel agents and biomarkers in PC requires careful consideration of many factors including disease state, tumor heterogeneity, reliability and validation of biomarker assays, and the physiologic and clinical significance of biomarkers in determining relevance of trial outcomes(47). Biomarkers should be associated with clinical outcomes and assessed using appropriate intra- and inter-patient controls, which account for assay variability. Consideration of duration of exposure is crucial, given that early changes may be missed if assessment is deferred beyond the point of maximal effect on a biomarker, and inadequate exposure windows may not allow for sufficient time to observe PD effects. Our choice of S6 activity as a stable and validated marker of TORC1 activity reflected the predominant thinking when this study was conceived; however, recent studies have questioned its clinical relevance, and suggest that other markers, such as measures of proliferation or apoptosis, 4EBP-1, TORC2, and AKT activity may be more relevant to assessing a favorable impact on the PI3K/AKT pathway(8;48–50). Our investigation of several of these markers strengthen the impact of our findings, and the absence of effects on these parameters supports the concept that target inhibition (as measured by reduced S6 phosphorylation) does not correlate necessarily with physiologic or clinical benefit. Further analyses across different disease states will further define an optimal biomarker and the cellular mechanisms of benefit from or resistance to mTOR inhibition to guide in the development of agents targeting this important survival pathway.
Statement of Translational Relevance
In this mechanistic study, we gave a short course of the mTOR (TORC1) inhibitor rapamycin to men with intermediate to high risk localized prostate cancer prior to radical prostatectomy. We found that while we were able to successfully inhibit the activity of the downstream mTOR target S6 in the majority of tumors, there was no change in measures of tumor apoptosis or proliferation with rapamycin, despite a reduction in p27 nuclear localization and no detectable increase in Akt activation. While an anti-angiogenic or novel anti-tumor mechanism of action for rapamycin cannot be excluded, these findings suggest that single agent TORC1 inhibition may be insufficient to impact on prostate tumor growth or survival.
Supplementary Material
Acknowledgements
We would like to thank members of our research team that were not acknowledged in the authorship section, who were integral to the conduct of this study: Judd Moul MD, Steven Freedland MD, Jessica Hicks, Regina Huminski, Judith Wheeler, Steven Hortopan, Lawrence Jones, Ryan Anderson, George Cusatis, Manuel Hidalgo MD, Carol Hartke, Ping He, and Ming Zhao PhD. Finally, we would like thank all the dedicated patients who participated in this study.
Funded through the Duke Comprehensive Cancer Center K12 program (5K12-CA-100639), the American Society of Clinical Oncology Young Investigator Award, the American Association for Cancer Research Clinical/Translational Fellowship, the Johns Hopkins General Clinical Research Center, the Department of Defense Prostate Cancer Clinical Trials Consortium, and the Johns Hopkins Brady Urologic Institute. In addition, support was provided by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer. Center (NIH P30CA069773 and UL1 RR025005). PGF receives research support from NCI123175.
Footnotes
Presented in part as an oral abstract presentation at ASCO 2009, Orlando, Florida, abstract 5001.
References
- 1.Figlin RA, Brown E, Armstrong AJ, Akerley W, Benson AB, III, Burstein HJ, et al. NCCN Task Force Report: mTOR inhibition in solid tumors. J Natl Compr Canc Netw. 2008 Sep 6; Suppl 5:S1–S20. [PubMed] [Google Scholar]
- 2.Jimeno A, Rudek MA, Kulesza P, Ma WW, Wheelhouse J, Howard A, et al. Pharmacodynamic-guided modified continuous reassessment method-based, dose-finding study of rapamycin in adult patients with solid tumors. J Clin Oncol. 2008 Sep 1;26(25):4172–4179. doi: 10.1200/JCO.2008.16.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007 May 31;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 4.Deocampo ND, Huang H, Tindall DJ. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003 Jun;28(2):145–153. [PubMed] [Google Scholar]
- 5.Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998 Jan 15;58(2):204–209. [PubMed] [Google Scholar]
- 6.Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, et al. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci U S A. 1998 Apr 28;95(9):5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999 Sep 1;59(17):4291–4296. [PubMed] [Google Scholar]
- 8.Thomas GV, Horvath S, Smith BL, Crosby K, Lebel LA, Schrage M, et al. Antibody-based profiling of the phosphoinositide 3-kinase pathway in clinical prostate cancer. Clin Cancer Res. 2004 Dec 15;10(24):8351–8356. doi: 10.1158/1078-0432.CCR-04-0130. [DOI] [PubMed] [Google Scholar]
- 9.Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004 Jan 23;279(4):2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 10.Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005 Apr 1;65(7):2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 12.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004 Jun;10(6):594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 13.Guba M, von BP, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002 Feb;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 14.Guba M, Koehl GE, Neppl E, Doenecke A, Steinbauer M, Schlitt HJ, et al. Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int. 2005 Jan;18(1):89–94. doi: 10.1111/j.1432-2277.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- 15.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006 Aug;10(2):159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George DJ, Armstrong AJ, Creel P, Morris K, Madden J, Turnbull J, et al. A phase II study of RAD001 in men with hormone-refractory metastatic prostate cancer (HRPC); 2008 Genitourinary Cancers Symposium; 2008. [Google Scholar]
- 17.Amato RJ, Jac J, Mohammad T, Saxena S. Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer. 2008 Sep;6(2):97–102. doi: 10.3816/CGC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- 18.Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999 Nov 17;91(22):1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 19.Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997 Dec 1;57(23):5221–5225. [PubMed] [Google Scholar]
- 20.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005 Aug 10;23(23):5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 21.Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005 Aug;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 22.Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005 Sep 1;104(5):1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 23.Pandya KJ, Dahlberg S, Hidalgo M, Cohen RB, Lee MW, Schiller JH, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500) J Thorac Oncol. 2007 Nov;2(11):1036–1041. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 24.Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009 Feb 3;15(2):148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004 Jul 27;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 26.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006 Apr 21;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006 Aug;10(2):159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006 Feb 1;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2006 Sep 25; doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 30.Balakumaran BS, Porrello A, Hsu DS, Glover W, Foye A, Leung JY, et al. MYC activity mitigates response to rapamycin in prostate cancer through eukaryotic initiation factor 4E-binding protein 1-mediated inhibition of autophagy. Cancer Res. 2009 Oct 1;69(19):7803–7810. doi: 10.1158/0008-5472.CAN-09-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008 Jul;7(7):1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 32.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008 Oct 1;68(19):8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 33.Lerut E, Roskams T, Goossens E, Bootle D, Dimitrijevic S, Stumm M, et al. Molecular pharmacodynamic (MPD) evaluation of dose and schedule of RAD001 (everolimus) in patients with operable prostate carcinoma (PC) J Clin Oncol (Meeting Abstracts) 2005 Jun 1;23(16_suppl):3071. [Google Scholar]
- 34.Thomas G, Speicher L, Reiter R, Ranganathan S, Hudes G, Strahs A, et al. Demonstration that Temsirolimus Preferentially Inhibits the mTOR Pathway in the Tumors of Prostate Cancer Patients with PTEN Deficiencies. AACR-NCI-EORTC Meeting 2005. 2005 [Google Scholar]
- 35.Chi KN, Eisenhauer E, Fazli L, Jones EC, Goldenberg SL, Powers J, et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2'-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst. 2005 Sep 7;97(17):1287–1296. doi: 10.1093/jnci/dji252. [DOI] [PubMed] [Google Scholar]
- 36.Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005 Jul 15;11(14):5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 37.Peralba JM, DeGraffenried L, Friedrichs W, Fulcher L, Grunwald V, Weiss G, et al. Pharmacodynamic Evaluation of CCI-779, an Inhibitor of mTOR, in Cancer Patients. Clin Cancer Res. 2003 Aug 1;9(8):2887–2892. [PubMed] [Google Scholar]
- 38.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 39.Freedland SJ, de GF, Sacoolidge JC, Elshimali YI, Csathy GS, Elashoff DA, et al. Predicting biochemical recurrence after radical prostatectomy for patients with organ-confined disease using p27 expression. Urology. 2003 Jun;61(6):1187–1192. doi: 10.1016/s0090-4295(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 40.Halvorsen OJ, Haukaas SA, Akslen LA. Combined loss of PTEN and p27 expression is associated with tumor cell proliferation by Ki-67 and increased risk of recurrent disease in localized prostate cancer. Clin Cancer Res. 2003 Apr;9(4):1474–1479. [PubMed] [Google Scholar]
- 41.Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, et al. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998 Feb 1;58(3):542–548. [PubMed] [Google Scholar]
- 42.Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, deKernion JB, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998 Mar;159(3):941–945. [PubMed] [Google Scholar]
- 43.Dreher T, Zentgraf H, Abel U, Kappeler A, Michel MS, Bleyl U, et al. Reduction of PTEN and p27kip1 expression correlates with tumor grade in prostate cancer. Analysis in radical prostatectomy specimens and needle biopsies. Virchows Arch. 2004 Jun;444(6):509–517. doi: 10.1007/s00428-004-1004-6. [DOI] [PubMed] [Google Scholar]
- 44.Majumder PK, Grisanzio C, O'Connell F, Barry M, Brito JM, Xu Q, et al. A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell. 2008 Aug 12;14(2):146–155. doi: 10.1016/j.ccr.2008.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Marzo AM, Fedor HH, Gage WR, Rubin MA. Inadequate formalin fixation decreases reliability of p27 immunohistochemical staining: probing optimal fixation time using high-density tissue microarrays. Hum Pathol. 2002 Jul;33(7):756–760. doi: 10.1053/hupa.2002.126187. [DOI] [PubMed] [Google Scholar]
- 46.Luo Y, Marx SO, Kiyokawa H, Koff A, Massague J, Marks AR. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996 Dec;16(12):6744–6751. doi: 10.1128/mcb.16.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Efstathiou E, Kim J, Logothetis CJ. Informative clinical investigation: a demanding taskmaster. J Clin Oncol. 2009 Oct 20;27(30):4937–4938. doi: 10.1200/JCO.2009.23.8063. [DOI] [PubMed] [Google Scholar]
- 48.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007 Jul;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009 Feb 3;15(2):148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rojo F, Najera L, Lirola J, Jimenez J, Guzman M, Sabadell MD, et al. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res. 2007 Jan 1;13(1):81–89. doi: 10.1158/1078-0432.CCR-06-1560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.