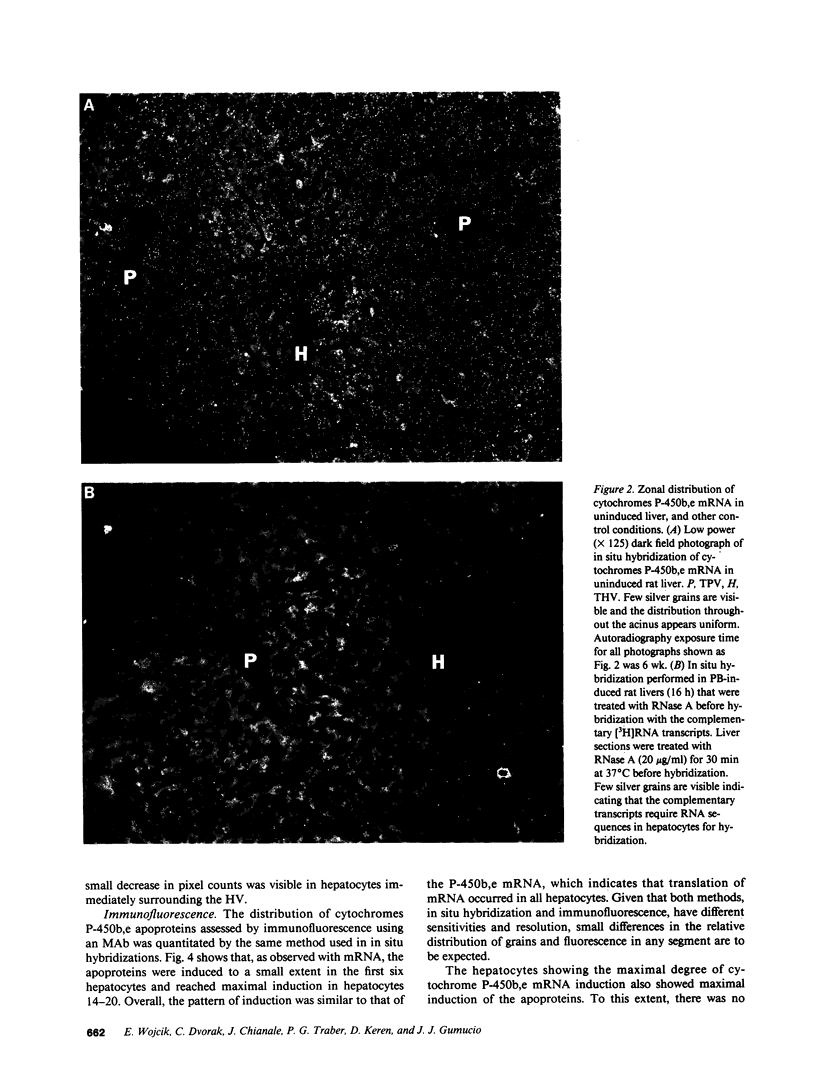
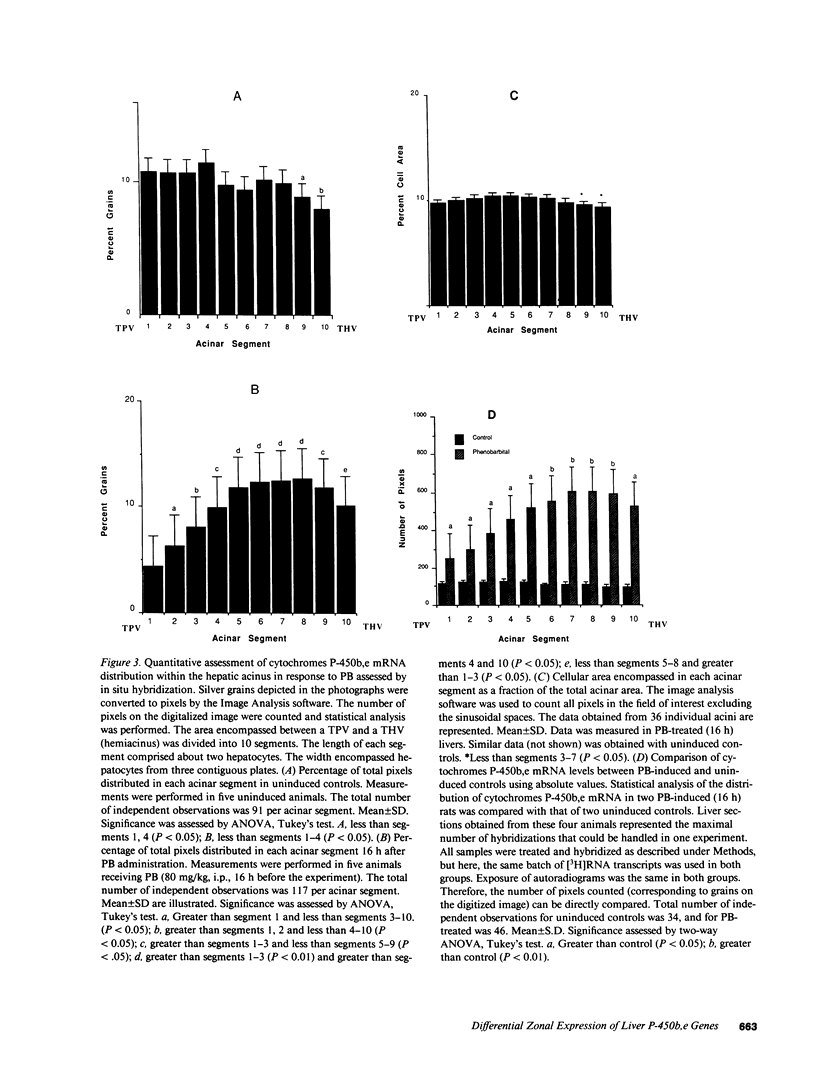
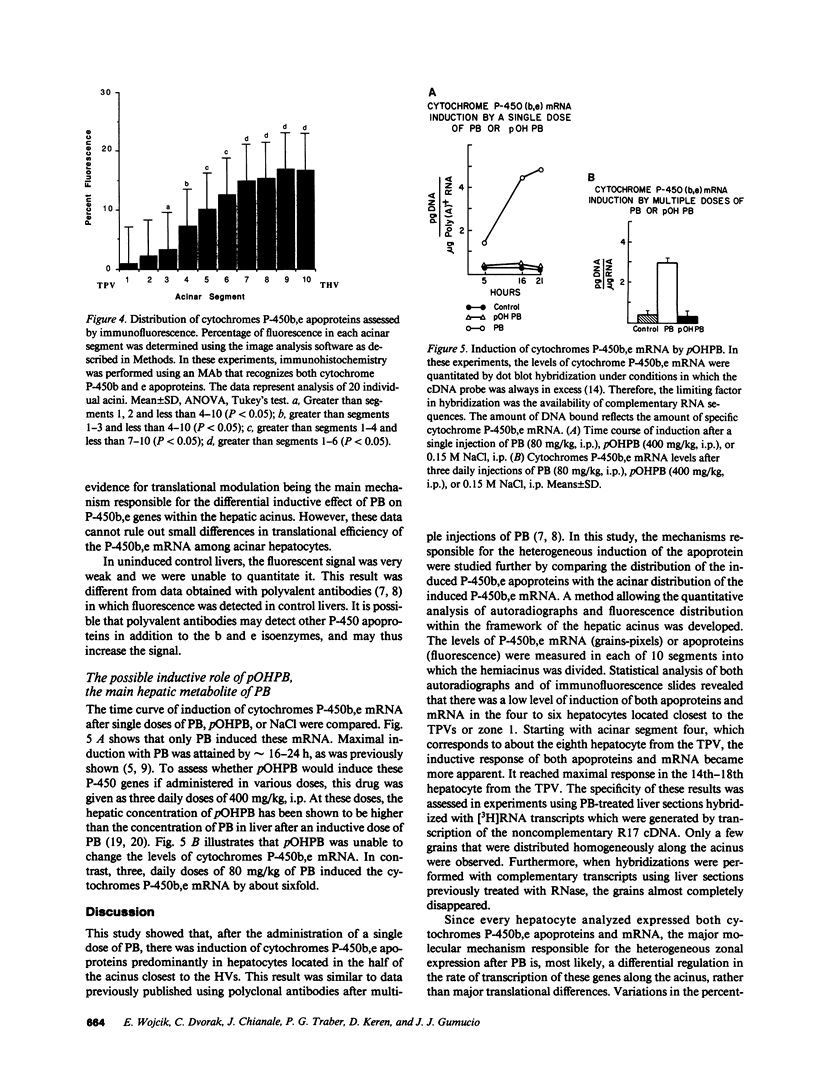
Abstract
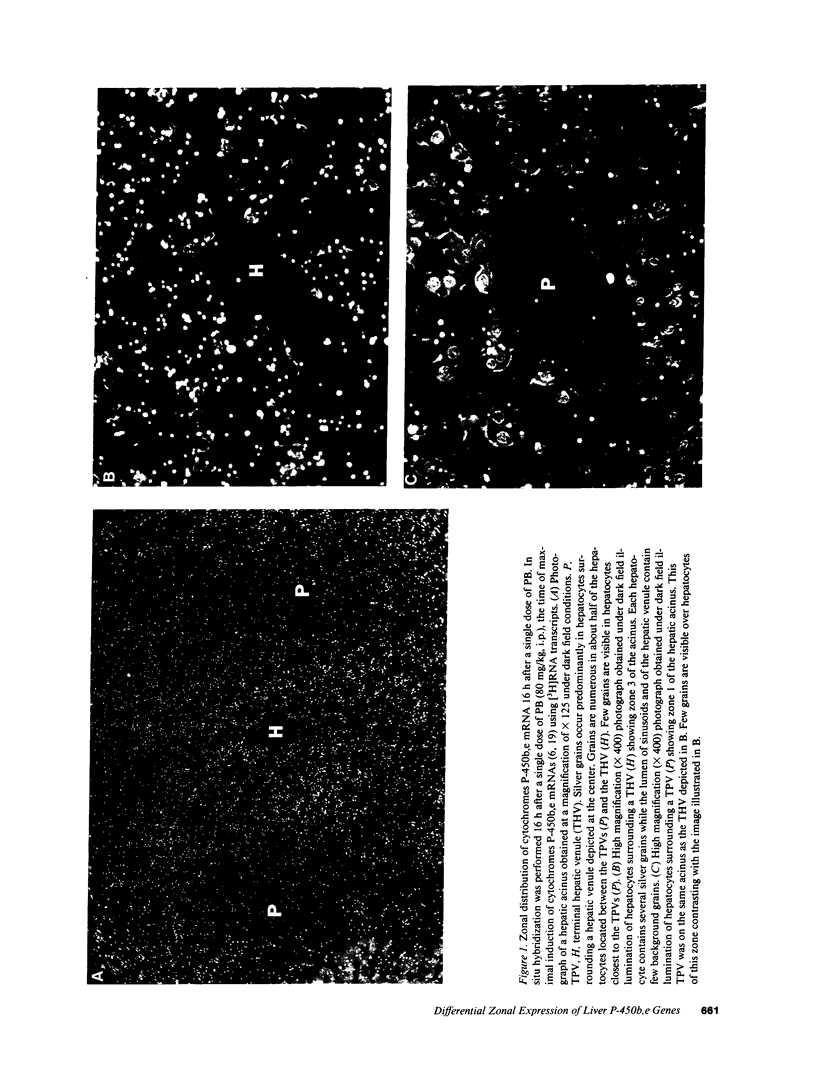
The various physiological processes that constitute liver function are compartmentalized within the hepatic acinus. The molecular mechanisms modulating the development and maintenance of this hepatocyte heterogeneity have not been defined. The objective of this study was to determine whether transcriptional or posttranscriptional zonal modulation of cytochromes P-450b,e gene expression was responsible for the heterogeneous induction of the P-450 proteins, which is observed after phenobarbital (PB) administration. The exact localization in liver tissue of hepatocytes responding to PB with induction of either P-450b,e mRNA or proteins was established by in situ hybridization and by immunofluorescence, respectively. As demonstrated by quantitative assessment of autoradiographs of approximately 20 hepatocytes located between a terminal portal venule and a hepatic venule, PB induced the P-450b,e mRNA up to sixfold in the 12-15 hepatocytes located closer to the hepatic venules (zones 2 and 3). In contrast, there was only a twofold induction in the 4-6 hepatocytes surrounding the terminal portal venules (zone 1). Quantitative immunofluorescence using an MAb showed that the acinar distribution of PB-induced P-450b,e proteins was similar to that of the mRNA. This combined approach indicated that, most likely, an increased rate of transcription of cytochromes P-450b,e genes in hepatocytes of zones 2 and 3 concomitantly, with a relative lack of activation, or repression, of these genes in hepatocytes of zone 1, were responsible for the heterogeneous phenotype observed after PB administration. Therefore, modulation of gene expression among hepatocytes of the liver acinus is one mechanism by which the functional heterogeneity of hepatocytes is attained. Experiments in which the induction of cytochromes P-450b,e genes was studied after administration of either PB or para-hydroxyphenobarbital, a main hepatic metabolite of PB, suggested that the species involved in the inductive process is the parent PB molecule rather than para-hydroxyphenobarbital.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Bar-Nun S., Maschio F., Zunich M., Lippman A., Bard E. Mechanism of induction of cytochrome P-450 by phenobarbital. J Biol Chem. 1981 Oct 25;256(20):10340–10345. [PubMed] [Google Scholar]
- Bonkovsky H. L., Hauri H. P., Marti U., Gasser R., Meyer U. A. Cytochrome P450 of small intestinal epithelial cells. Immunochemical characterization of the increase in cytochrome P450 caused by phenobarbital. Gastroenterology. 1985 Feb;88(2):458–467. doi: 10.1016/0016-5085(85)90507-4. [DOI] [PubMed] [Google Scholar]
- Chen E. H., Gumucio J. J., Ho N. H., Gumucio D. L. Hepatocytes of Zones 1 and 3 conjugate sulfobromophthalein with glutathione. Hepatology. 1984 May-Jun;4(3):467–476. doi: 10.1002/hep.1840040320. [DOI] [PubMed] [Google Scholar]
- Chianale J., Dvorak C., Farmer D. L., Michaels L., Gumucio J. J. Cytochrome P-450 gene expression in the functional units of the fetal liver. Hepatology. 1988 Mar-Apr;8(2):318–326. doi: 10.1002/hep.1840080222. [DOI] [PubMed] [Google Scholar]
- Chianale J., Dvorak C., May M., Gumucio J. J. Heterogeneous expression of phenobarbital-inducible cytochrome P-450 genes within the hepatic acinus in the rat. Hepatology. 1986 Sep-Oct;6(5):945–951. doi: 10.1002/hep.1840060522. [DOI] [PubMed] [Google Scholar]
- Chianale J., Mulholland L., Traber P. G., Gumucio J. J. Phenobarbital induction of cytochrome P-450 b,e genes is dependent on protein synthesis. Hepatology. 1988 Mar-Apr;8(2):327–331. doi: 10.1002/hep.1840080223. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cresteil T., Mahu J. L., Beaune P., Columelli S., Leroux J. P. Comparison of the effects of phenobarbital and its hydroxylated metabolites on drug-metabolizing enzymes during ontogenesis. Pediatr Pharmacol (New York) 1981;1(4):331–340. [PubMed] [Google Scholar]
- Cresteil T., Mahu J. L., Dansette P. M., Leroux J. P. In vivo administration of hydroxylated phenobarbital metabolites: effect on rat hepatic cytochromes P-450, epoxide hydrolase and UDP-glucuronosyltransferase. Biochem Pharmacol. 1980 Apr 15;29(8):1127–1133. doi: 10.1016/0006-2952(80)90407-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Groothuis G. M., Hardonk M. J., Keulemans K. P., Nieuwenhuis P., Meijer D. K. Autoradiographic and kinetic demonstration of acinar heterogeneity of taurocholate transport. Am J Physiol. 1982 Dec;243(6):G455–G462. doi: 10.1152/ajpgi.1982.243.6.G455. [DOI] [PubMed] [Google Scholar]
- Gumucio J. J., Miller D. L. Functional implications of liver cell heterogeneity. Gastroenterology. 1981 Feb;80(2):393–403. [PubMed] [Google Scholar]
- Gumucio J. J., Miller D. L., Krauss M. D., Zanolli C. C. Transport of fluorescent compounds into hepatocytes and the resultant zonal labeling of the hepatic acinus in the rat. Gastroenterology. 1981 Apr;80(4):639–646. [PubMed] [Google Scholar]
- Hardwick J. P., Gonzalez F. J., Kasper C. B. Transcriptional regulation of rat liver epoxide hydratase, NADPH-Cytochrome P-450 oxidoreductase, and cytochrome P-450b genes by phenobarbital. J Biol Chem. 1983 Jul 10;258(13):8081–8085. [PubMed] [Google Scholar]
- Kumar A., Raphael C., Adesnik M. Cloned cytochrome P-450 cDNA. Nucleotide sequence and homology to multiple phenobarbital-induced mRNA species. J Biol Chem. 1983 Sep 25;258(18):11280–11284. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi K., Mishima A., Okuda K. Immunofluorescence of phenobarbital inducible cytochrome P-450 in the hepatic lobule of normal and phenobarbital-treated rats. Hepatology. 1982 Nov-Dec;2(6):849–855. doi: 10.1002/hep.1840020619. [DOI] [PubMed] [Google Scholar]
- Pang K. S., Terrell J. A. Retrograde perfusion to probe the heterogeneous distribution of hepatic drug metabolizing enzymes in rats. J Pharmacol Exp Ther. 1981 Feb;216(2):339–346. [PubMed] [Google Scholar]
- Papavasiliou S. S., Zmeili S., Herbon L., Duncan-Weldon J., Marshall J. C., Landefeld T. D. Alpha and luteinizing hormone beta messenger ribonucleic acid (RNA) of male and female rats after castration: quantitation using an optimized RNA dot blot hybridization assay. Endocrinology. 1986 Aug;119(2):691–698. doi: 10.1210/endo-119-2-691. [DOI] [PubMed] [Google Scholar]
- Rappaport A. M. The microcirculatory hepatic unit. Microvasc Res. 1973 Sep;6(2):212–228. doi: 10.1016/0026-2862(73)90021-6. [DOI] [PubMed] [Google Scholar]
- Ravishankar H., Padmanaban G. Turnover of messenger RNA, apoprotein and haem of cytochrome P-450b + e induced by phenobarbitone in rat liver. Biochem J. 1985 Jul 1;229(1):73–79. doi: 10.1042/bj2290073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R. G., Kauffman F. C. Sublobular compartmentation of pharmacologic events (SCOPE): metabolic fluxes in periportal and pericentral regions of the liver lobule. Hepatology. 1985 Jan-Feb;5(1):144–151. doi: 10.1002/hep.1840050128. [DOI] [PubMed] [Google Scholar]