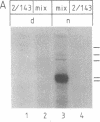
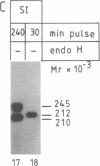
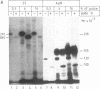
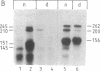
Abstract
Eight cases of congenital sucrase-isomaltase deficiency were studied at the subcellular and protein level with monoclonal antibodies against sucrase-isomaltase. At least three phenotypes were revealed: one in which sucrase-isomaltase protein accumulated intracellularly probably in the endoplasmic reticulum, as a membrane-associated high-mannose precursor, one in which the intracellular transport of the enzyme was apparently blocked in the Golgi apparatus, and one in which catalytically altered enzyme was transported to the cell surface. All patients expressed electrophoretically normal or near normal high-mannose sucrase-isomaltase. The results suggest that different, probably small, mutations in the sucrase-isomaltase gene lead to the synthesis of transport-incompetent or functionally altered enzyme which results in congenital sucrose intolerance.
Full text
PDF



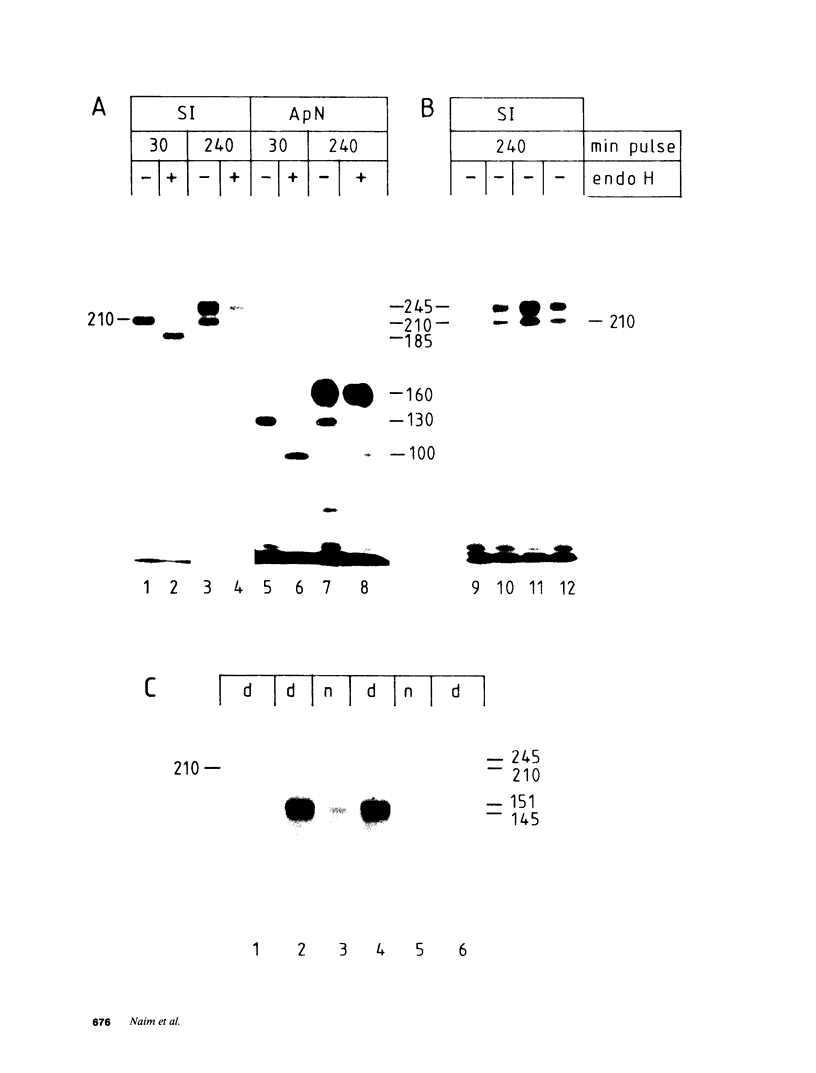



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonkovsky H. L., Hauri H. P., Marti U., Gasser R., Meyer U. A. Cytochrome P450 of small intestinal epithelial cells. Immunochemical characterization of the increase in cytochrome P450 caused by phenobarbital. Gastroenterology. 1985 Feb;88(2):458–467. doi: 10.1016/0016-5085(85)90507-4. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W. alpha 1-Antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986 Dec;78(6):1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell G. M., Tranum-Jensen J., Sjöström H., Norén O. Topology and quaternary structure of pro-sucrase/isomaltase and final-form sucrase/isomaltase. Biochem J. 1986 Jul 15;237(2):455–461. doi: 10.1042/bj2370455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M. Biosynthesis of intestinal microvillar proteins. The intracellular transport of aminopeptidase N and sucrase-isomaltase occurs at different rates pre-Golgi but at the same rate post-Golgi. FEBS Lett. 1985 Oct 7;190(1):69–72. doi: 10.1016/0014-5793(85)80429-4. [DOI] [PubMed] [Google Scholar]
- Dubs R., Steinmann B., Gitzelmann R. Demonstration of an inactive enzyme antigen in sucrase-isomaltase deficiency. Helv Paediatr Acta. 1973 Jul;28(3):187–198. [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G., Brunner J., Hauser H., Wacker H., Semenza G., Zuber H. The hydrophobic anchor of small-intestinal sucrase--isomaltase: N-terminal sequence of isomaltase subunit. FEBS Lett. 1978 Dec 1;96(1):183–188. doi: 10.1016/0014-5793(78)81090-4. [DOI] [PubMed] [Google Scholar]
- Freiburghaus A. U., Dubs R., Hadorn B., Gaze H., Hauri H. P., Gitzelmann R. The brush border membrane in hereditary sucrase-isomaltase deficiency: abnormal protein pattern and presence of immunoreactive enzyme. Eur J Clin Invest. 1977 Oct;7(5):455–459. doi: 10.1111/j.1365-2362.1977.tb01634.x. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
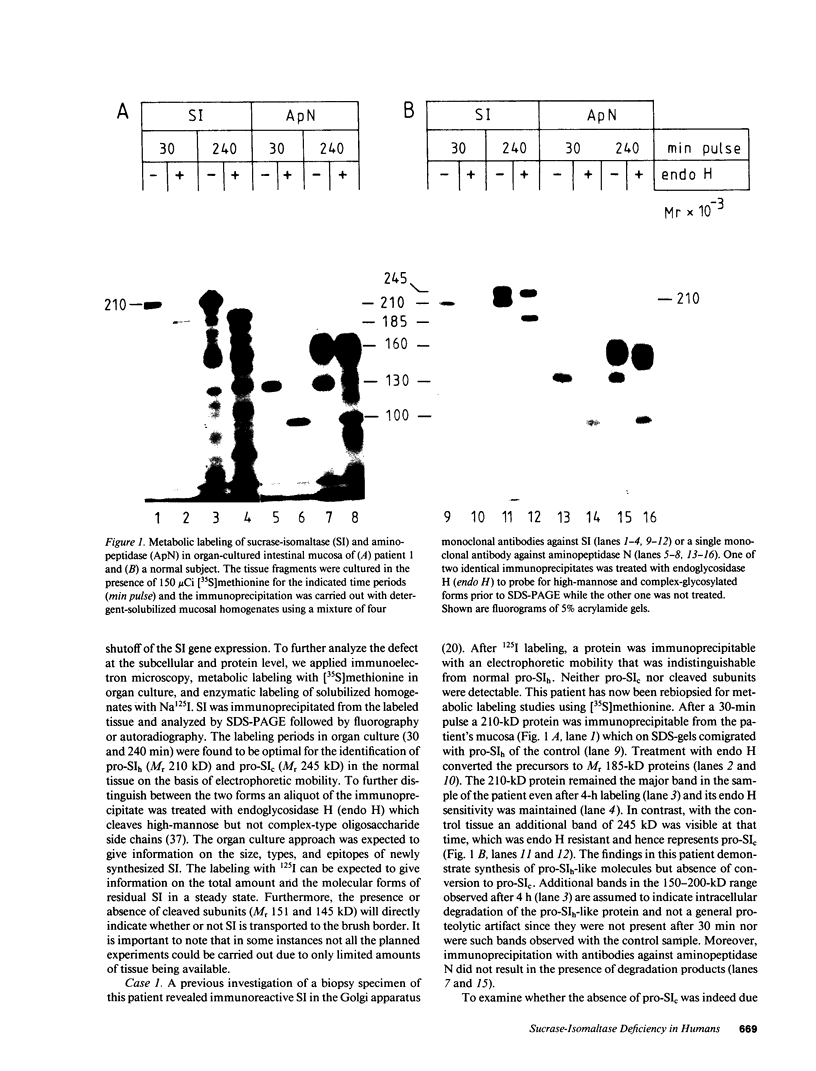
- Ghersa P., Huber P., Semenza G., Wacker H. Cell-free synthesis, membrane integration, and glycosylation of pro-sucrase-isomaltase. J Biol Chem. 1986 Jun 15;261(17):7969–7974. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gray G. M., Conklin K. A., Townley R. R. Sucrase-isomaltase deficiency. Absence of an inactive enzyme variant. N Engl J Med. 1976 Apr 1;294(14):750–753. doi: 10.1056/NEJM197604012941403. [DOI] [PubMed] [Google Scholar]
- Green F., Edwards Y., Hauri H. P., Povey S., Ho M. W., Pinto M., Swallow D. Isolation of a cDNA probe for a human jejunal brush-border hydrolase, sucrase-isomaltase, and assignment of the gene locus to chromosome 3. Gene. 1987;57(1):101–110. doi: 10.1016/0378-1119(87)90181-8. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Monoclonal antibodies to sucrase/isomaltase: probes for the study of postnatal development and biogenesis of the intestinal microvillus membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6629–6633. doi: 10.1073/pnas.77.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Roth J., Sterchi E. E., Lentze M. J. Transport to cell surface of intestinal sucrase-isomaltase is blocked in the Golgi apparatus in a patient with congenital sucrase-isomaltase deficiency. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4423–4427. doi: 10.1073/pnas.82.13.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P. Use of monoclonal antibodies to investigate the intracellular transport and biogenesis of intestinal brush-border proteins. Biochem Soc Trans. 1986 Feb;14(1):161–163. doi: 10.1042/bst0140161. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Wacker H., Rickli E. E., Bigler-Meier B., Quaroni A., Semenza G. Biosynthesis of sucrase-isomaltase. Purification and NH2-terminal amino acid sequence of the rat sucrase-isomaltase precursor (pro-sucrase-isomaltase) from fetal intestinal transplants. J Biol Chem. 1982 Apr 25;257(8):4522–4528. [PubMed] [Google Scholar]
- Hercz A., Katona E., Cutz E., Wilson J. R., Barton M. alpha1-Antitrypsin: the presence of excess mannose in the Z variant isolated from liver. Science. 1978 Sep 29;201(4362):1229–1232. doi: 10.1126/science.308696. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Quaroni A., Bugge B., Kirsch K. Partial characterization of the carbohydrate units of rat intestinal sucrase--isomaltase. Biochem J. 1981 Aug 1;197(2):511–514. doi: 10.1042/bj1970511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Kelly J. J., Alpers D. H. Blood group antigenicity of purified human intestinal disaccharidases. J Biol Chem. 1973 Dec 10;248(23):8216–8221. [PubMed] [Google Scholar]
- Kelly R. B. Protein transport. From organelle to organelle. Nature. 1987 Mar 5;326(6108):14–15. doi: 10.1038/326014a0. [DOI] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloyd M. L., Olsen W. A. A study of the molecular pathology of sucrase-isomaltase deficiency. A defect in the intracellular processing of the enzyme. N Engl J Med. 1987 Feb 19;316(8):438–442. doi: 10.1056/NEJM198702193160804. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Naim H. Y., Sterchi E. E., Lentze M. J. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem J. 1987 Jan 15;241(2):427–434. doi: 10.1042/bj2410427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F., Crumpton M. J. Biosynthesis and maturation of HLA-DR antigens in vivo. J Biol Chem. 1981 Sep 10;256(17):8987–8993. [PubMed] [Google Scholar]
- Palade G. E. Problems in intracellular membrane traffic. Ciba Found Symp. 1982;(92):1–14. doi: 10.1002/9780470720745.ch1. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Preiser H., Menard D., Crane R. K., Cerda J. J. Deletion of enzyme protein from the brush border membrane in sucrase-isomaltase deficiency. Biochim Biophys Acta. 1974 Sep 6;363(2):279–282. doi: 10.1016/0005-2736(74)90067-4. [DOI] [PubMed] [Google Scholar]
- Roth J. Post-embedding cytochemistry with gold-labelled reagents: a review. J Microsc. 1986 Aug;143(Pt 2):125–137. doi: 10.1111/j.1365-2818.1986.tb02771.x. [DOI] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Lehrman M. A., Südhof T. C., Yamamoto T., Davis C. G., Hobbs H. H., Brown M. S., Goldstein J. L. The LDL receptor in familial hypercholesterolemia: use of human mutations to dissect a membrane protein. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):811–819. doi: 10.1101/sqb.1986.051.01.094. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Bresson J. L., Triadou N., Bataille J., Rey J. Analyse en électrophorèse sur gel de polyacrylamide des protéines de la membrane microvillositaire et d'une fraction cytoplasmique dans 8 cas d'intolérance congénitale au saccharose. Gastroenterol Clin Biol. 1980 Apr;4(4):251–256. [PubMed] [Google Scholar]
- Schmitz J., Commegrain C., Maestracci D., Rey J. Absence of brush border sucrase-isomaltase complex in congenital sucrose intolerance. Biomedicine. 1974 Nov 20;21(11):440–443. [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Christiansen L., Wacker H., Semenza G. A fully active, two-active-site, single-chain sucrase.isomaltase from pig small intestine. Implications for the biosynthesis of a mammalian integral stalked membrane protein. J Biol Chem. 1980 Dec 10;255(23):11332–11338. [PubMed] [Google Scholar]
- Skovbjerg H., Krasilnikoff P. A. Immunoelectrophoretic studies on human small intestinal brush border proteins. The residual isomaltase in sucrose intolerant patients. Pediatr Res. 1981 Mar;15(3):214–218. doi: 10.1203/00006450-198103000-00004. [DOI] [PubMed] [Google Scholar]
- Skovbjerg H., Krasilnikoff P. A. Maltase-glucoamylase and residual isomaltase in sucrose intolerant patients. J Pediatr Gastroenterol Nutr. 1986 May-Jun;5(3):365–371. doi: 10.1097/00005176-198605000-00005. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Mutations that influence the secretory path in animal cells. Biochem J. 1983 Oct 15;216(1):1–9. doi: 10.1042/bj2160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadou N. Antigenic cross-reactions among human intestinal brush-border enzymes revealed by the immunoblotting method and rabbit anti-enzyme sera. J Immunol Methods. 1984 Oct 26;73(2):283–291. doi: 10.1016/0022-1759(84)90403-4. [DOI] [PubMed] [Google Scholar]
- Williams S. R., McIvor R. S., Martin D. W., Jr Molecular basis of a human purine nucleoside phosphorylase deficiency. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1059–1064. doi: 10.1101/sqb.1986.051.01.123. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]