Abstract
Arterial stiffness predicts cardiovascular events beyond traditional risk factors. However, the relationship with aging of novel noninvasive measures of aortic function by MRI and their interrelationship with established markers of vascular stiffness remain unclear and currently limit their potential impact. Our aim was to compare age-related changes of central measures of aortic function with carotid distensibility, global carotid–femoral pulse wave velocity, and wave reflections. We determined aortic strain, distensibility, and aortic arch pulse wave velocity by MRI, carotid distensibility by ultrasound, and carotid–femoral pulse wave velocity by tonometry in 111 asymptomatic subjects (54 men, age range: 20 to 84 years). Central pressures were used to calculate aortic distensibility. Peripheral and central pulse pressure, augmentation index, and carotid–femoral pulse wave velocity increased with age, but aortic strain and aortic arch PWV were most closely and specifically related to aging. Ascending aortic (AA) strain and distensibility decreased, respectively, by 5.3±0.5% (R2 = 0.54, P<0.0001) and 13.6±1 kPa−1×10−3 (R2=0.62, P<0.0001), and aortic arch pulse wave velocity increased by 1.6±0.13 m/sec (R2=0.60, P<0.0001) for each decade of age after adjustment for gender, body size, and heart rate. We demonstrate in this study a dramatic decrease in AA distensibility before the fifth decade of life in individuals with diverse prevalence of risk factors free of overt cardiovascular disease. In particular, compared with other measures of aortic function, the best markers of subclinical large artery stiffening, were AA distensibility in younger and aortic arch pulse wave velocity in older individuals.
Keywords: MRI, aorta, aging, elasticity, pulse wave velocity
Age-related vascular changes in individuals without overt cardiovascular disease and normal blood pressure remain currently undetected. However, the ability to identify individuals having early deterioration of vascular and cardiac function, as well as progressive subclinical arterial disease, would allow to define a target population for preventive therapy in the hope of reducing vascular and cardiac remodeling and dysfunction, as well as preventing lethal or incapacitating events. Arterial stiffness is a main determinant of age-related systolic and pulse pressure increase, a major predictor of stroke and myocardial infarction, and has been associated with heart failure.1–3 The aorta accounts for most of global arterial stiffening and is central to the onset of atherosclerosis with its subsequent complications. The value of carotid–femoral pulse wave velocity (cfPWV) as a marker of arterial stiffness and a predictor of fatal and nonfatal cardiovascular events over traditional risk factors has been established both in patients and the general population.4 Similarly, the carotid augmentation index (AIx), a marker of waveform reflection and central pulse pressure (PP), have independent predictive value for mortality in end-stage renal disease patients5,6 and for cardiovascular events in hypertensive7 and coronary artery disease patients beyond peripheral blood pressure. Recent studies have stressed that both PP but also cfPWV increase occurred mainly after the 5th decade and that AIx is a better marker of arterial stiffening and potentially also of CV risk in individuals <50 years.8 MRI uniquely combines the assessment of ventricular and aortic geometry with direct high-resolution measurements of aortic strain, distensibility, and aortic arch PWV. Although MRI has been validated for reliable noninvasive assessment of aortic PWV9,10 and distensibility,11 these parameters have never been simultaneously compared to established measures of global vascular PWV and wave reflection by tonometry in a larger population with varied levels of cardiovascular risk. Our aim was to determine the most sensitive markers of arterial aging with the hypothesis that direct measures of aortic stiffness, distensibility with MRI and central PP and aortic arch PWV, would be relevant and early markers of the effect of aging that could complement more peripheral or indirect measures such as cfPWV, central PP alone, and AIx.
Methods
Study Subjects
We enrolled 122 subjects: 62 women, 60 men. Recruitment was based on local advertisement. All subjects were informed about the study protocol and provided written consent. The study was approved by The Johns Hopkins University Ethics Review Board. The procedures followed were in accordance with institutional guidelines and the Declaration of Helsinki. Inclusion criteria were: >18 years of age without contraindications to MRI, absence of acute, or chronic disease and without personal history or symptoms of cardiac disease, normal physical examination, and normal ECG. Height and weight were recorded, and body mass index (BMI) was used as a measure of overall adiposity.
Assessment of Central Aortic Function by MRI
Image Acquisition and Analysis
All images were acquired on a 3.0T scanner (Trio Tim, Siemens) using ECG gating and breath-holding with a 6-element thoracic coil for radiofrequency signal detection.
To visualize the aorta, we acquired 4 sagittal oblique views of the aortic arch using a black-blood spin echo sequence (slice thickness, 6 mm; matrix, 256×256). A gradient echo pulse sequence with through-plane velocity encoding simultaneously providing the velocities in the ascending and descending aorta was applied perpendicular to the aorta at the level of pulmonary artery bifurcation. Maximal velocity encoding was 150 cm/sec; slice thickness, 6 mm; matrix, 192×192; and temporal resolution, 20 ms. The same slice location was used to acquire an aortic cine using a fast retrospectively gated gradient echo sequence (slice thickness, 6 mm; matrix, 256×256; temporal resolution, 20 ms).
The contours of the ascending and proximal descending aorta were automatically traced for all phases of the cardiac cycle on both the modulus images of the phase contrast acquisition for flow analysis and on the cine images for aortic area analysis (Figure S1 in the online Data Supplement at http://hyper.ahajournals.org) using the ARTFUN software (INSERM U678). The only user intervention was to point the center of the aorta and any point close to the vessel wall on 1 image of the cine series, with the subsequent border detection and tracking for the whole cardiac cycle being fully automatic.
Aortic Distensibility and Aortic Arch PWV Calculation
The maximal (Amax) and minimal (Amin) aortic lumen areas and relative change in area (aortic strain), defined as ΔA=(Amax−Amin)/Amin, were used to calculate distensibility of the ascending and proximal descending aorta in each subject as follows:
where cPP is the central PP as described below. The average aortic diameters were calculated from the average of maximal and minimal aortic areas. To study the interrelationship between the local PWV in the AA and the regional aortic arch PWV, we calculated the former from AA distensibility using the Bramwell–Hill equation.12 Aortic arch PWV was calculated by using the transit time Δt of the flow curves and the distance D between the ascending and descending aortic locations of the phase-contrast acquisition.
As previously described,13 to minimize the variability of foot-to-foot measurement inherent to lower temporal resolution on flow curves compared to pressure curves, the transit time was calculated as the average time difference using the least squares estimate between all data points on the systolic upslopes of the ascending and descending aortic flow curves after peak flow normalization (Figure 1). The distance traveled by the pulse wave was measured as the centerline of the aorta using 8 to 10 markers on the central black blood view of the aortic arch. The first and last markers were placed at the center of respectively the ascending and descending aorta and in the plane used for the velocity acquisition (online Figure S2).
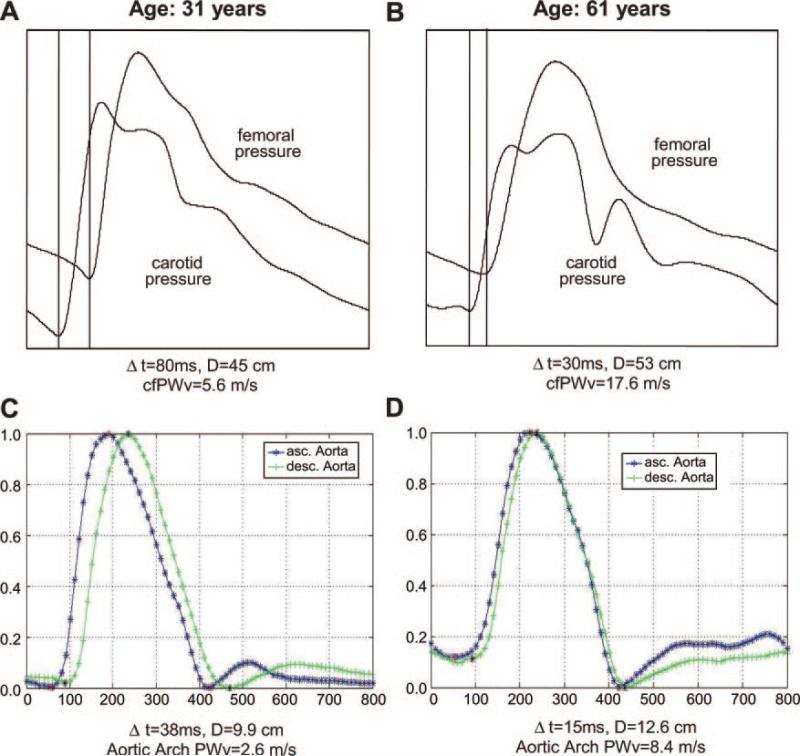
Figure 1.
Aortic arch PWV assessment with MRI and cfPWV measured by tonometry. cfPWV assessed with carotid–femoral tonometry in a young (A) and older participant (B). Corresponding measurements of aortic arch PWV (C and D) showing simultaneously acquired normalized flow curves by phase-contrast MRI in the ascending and descending aorta. The younger participant shows preserved aortic elasticity (normal pulse wave velocities), whereas the older participant presents increased pulse wave velocities in relation with a stiffer aorta. Δt indicates transit time; D, transit distance; x axis, time in milliseconds; y axis in arbitrary units.
Ultrasound Data Acquisition and Analysis
The right common carotid artery was scanned longitudinally with an 11-MHz vascular probe (Aplio, Toshiba, Japan) while the patient was in the supine position with head extended. The carotid systolic and diastolic diameters and the intima–media thickness (IMT) of the posterior wall (distance from the leading edge of lumen–intima interface to the leading edge of the media–adventitia interface) were measured on line from the digitized longitudinal images. Carotid distensibility was calculated as the ratio of relative carotid diameter change over carotid PP.
Tonometry Data Acquisition and Analysis
Tonometry was performed immediately after MRI at rest in supine position in a quiet, temperature-controlled room. We used a commercially available device (VP-2000, Colin Corp, Komaki, Japan) customized to output all physiological signals, including ECG, phonocardiogram, oscillometric signals from arms and ankles, and tonometric signals from right common carotid and right femoral arteries. Brachial systolic and diastolic pressures were the averages of 4 oscillometric measurements (2 on each side) and were used to calculate mean brachial pressure. All signals were digitized simultaneously at a sampling frequency of 250 Hz for off-line analysis. Carotid and femoral arterial pressure waveforms were stored for 30 seconds from applanation tonometry sensors. Right radial artery waveforms were recorded 10 seconds after carotid–femoral recording. The simultaneously digitized tonometric waveforms were analyzed with customized software to identify the foot of each waveform in every cardiac cycle. The pulse transit time of each segment was measured as the foot-to-foot interval of waveforms: between right carotid and right femoral arteries. Surface distances from the suprasternal notch to the right carotid tonometric sensor (A) and from the suprasternal notch to the right femoral sensor (B) were measured, by means of a tape ruler over the body surface. cfPWV was calculated from the distance between arterial recording sites divided by the pulse transit time: (B−A)/transit time.14 Central aortic pressure waveforms were reconstructed for each subject from the radial waveforms using a generalized transfer function as in the study by Chen et al.15 The AIx was analyzed from the right common carotid artery pressure wave contour. Carotid blood pressure was obtained from carotid waveforms calibrated with brachial mean and diastolic blood pressure. AIx was the ratio of the augmented pressure, which was determined as the height of the late systolic peak above the inflection point, to the PP in percentage.
Statistical Analysis
Baseline characteristics are provided as means±SD for continuous variables and percentages±SD for discrete variables. To present an exploratory analysis for the trends of the different arterial parameters over age, we grouped the subjects into six age bins of 10 years and calculated the conditional means±SD of the arterial parameters given each age bin. Statistical inferences for the general trends of the arterial parameters over age were evaluated by testing the equality of these conditional means over the age bins using the analysis variance F test at 5% significant level.
When age was taken as a continuous random variable, the relationship between age and the arterial parameters was studied using linear regression models and piecewise linear regression models. Goodness-of-fit tests for specific models versus the more general regression models were performed using the ANOVA F tests. These goodness-of-fit tests suggested that the 2-piece simple linear models with age cutoff at 50 years give adequate description of the relationships between age and the selected aortic variables. Multivariate regression models were used to evaluate the relationships between age and the arterial measures adjusted for other covariates. Potential covariates with clinical relevance, such as gender and BMI, were selected by examining their significance in univariate and multivariate models and the stepwise variable selection procedures. Univariate correlations between arterial measures was reported using Pearson's correlation coefficients. All reported probability values are 2-sided and a probability value of less than 0.05 is used to indicate statistical significance. Analysis was performed with STATA 10IC.
Results
Study Subjects
Of the 122 subjects enrolled, 9 subjects did not complete MRI because of claustrophobia and 2 failed to complete the protocol, leaving 111 subjects (57 women; 54 men; mean age: 47±17 years; range: 20 to 84 years) for analysis. Fifty-seven subjects free of cardiovascular risk factors and 54 subjects having at least 1 cardiovascular risk factor were studied of which 40 had hypertension (blood pressure, ≥140/90 mm Hg), 6 were past smokers, 9 were active smokers, 6 had diabetes mellitus, and 21 had hypercholesterolemia.
Study subject characteristics by age group are summarized in Table 1. The trend for gender, weight, heart rate, and BMI was not significantly different across age groups although height was moderately lower in the 6th decade because of a higher proportion of women in that group. As expected, carotid IMT, a surrogate measure of atherosclerosis, increased significantly with age, with a mean increase of 0.07 mm/10 years (P<0.0001) after adjustment for gender, BMI, and cardiovascular risk factors.
Table 1.
Patient Characteristics and Arterial Measures According to Age Category
| Patient Characteristics | 20–29 Yr (n=21) | 30–39 Yr (n=15) | 40–49 Yr (n=31) | 50–59 Yr (n=16) | 60–69 Yr (n=14) | ≥70 Yr (n=14) | P |
|---|---|---|---|---|---|---|---|
| Gender, female/male | 10/11 | 6/9 | 16/15 | 9/7 | 10/4 | 6/8 | 0.60 |
| Height, cm | 172±7 | 172±9 | 172±8 | 168±8 | 164±7 | 166±10 | 0.02 |
| Weight, kg | 73±16 | 75±15 | 82±19 | 76±13 | 73±16 | 76±17 | 0.36 |
| BMI | 25±5 | 25±5 | 28±7 | 27±4 | 27±5 | 27±4 | 0.29 |
| Heart rate, bpm | 64±9 | 68±9 | 67±12 | 61±8 | 64±10 | 60±13 | 0.17 |
| Carotid IMT, mm | 0.41±0.07 | 0.47±0.08 | 0.60±0.15 | 0.70±0.15 | 0.87±0.25 | 0.84±0.15 | <0.0001 |
| AIx, % | –10±15 | –4±15 | 16±18 | 31±9 | 26±9 | 32±13 | <0.0001 |
| Augmented pressure, mm Hg | –3.73±6.1 | –1.30±5.1 | 6.0±7.3 | 13.9±6.6 | 14.3±7.7 | 17.1±6.1 | <0.0001 |
| Brachial pressures, mm Hg | |||||||
| SBP | 109±9 | 113±12 | 122±16 | 134±20 | 143±21 | 135±13 | <0.0001 |
| DBP | 62±6 | 68±11 | 76±11 | 79±10 | 77±11 | 69±9 | <0.0001 |
| PP | 48±7 | 46±3 | 46±8 | 55±11 | 66±15 | 66±10 | <0.0001 |
| Central pressures, mm Hg | |||||||
| SBP | 94±10 | 100±14 | 113±16 | 126±20 | 134±24 | 129±12 | <0.0001 |
| DBP | 59±7 | 66±12 | 75±11 | 77±11 | 75±12 | 69±10 | <0.0001 |
| PP | 35±6 | 33±4 | 38±9 | 49±12 | 59±18 | 60±11 | <0.0001 |
| Carotid distensibility, kPa–1 · 10–3 | 12.6±3.9 | 11.6±3.6 | 8.7±2.2 | 6.8±2.4 | 5.6±2.9 | 5.3±1.6 | <0.0001 |
| Ascending aorta | |||||||
| Diameter, mm | 27.5±2.7 | 27.8±3.1 | 31.1±3.1 | 32.9±4.1 | 34.3±2.7 | 33.7±3.8 | <0.0001 |
| Strain, % | 33±10 | 27±10 | 15±8 | 11±4 | 9±4 | 8±4 | <0.0001 |
| Distensibility, kPa–1 · 10–3 | 74±23 | 61±23 | 31±18 | 18±7 | 12±7 | 10±6 | <0.0001 |
| Descending aorta | |||||||
| Diameter, mm | 20.5±1.6 | 21.1±2.7 | 23.5±2.4 | 24.1±2.6 | 24.7±1.8 | 24.7±2.5 | <0.0001 |
| Strain, % | 33±8 | 31±12 | 19±9 | 18±9 | 13±5 | 14±7 | <0.0001 |
| Distensibility, kPa–1 · 10–3 | 72±18 | 70±24 | 38±17 | 29±13 | 18±8 | 17±6 | <0.0001 |
| Aortic arch PWV, m/sec | 3.5±0.5 | 3.9±1.1 | 5.6±1.4 | 7.2±2.3 | 9.7±2.9 | 11.1±4.6 | <0.0001 |
| cfPWV, m/sec | 6.2±0.7 | 6.7±1.0 | 8.8±1.9 | 9.5±1.8 | 12.8±3.9 | 13.8±5.3 | <0.0001 |
Age, Peripheral, and Central Blood Pressure
For the detailed description of age-related changes in blood pressure, see online Figure S3.
Age and Measures of Arterial Function
Age-related structural changes of the aorta were attested by age-related increases in ascending and descending thoracic aorta diameters (Table 1). The AA increased significantly in diameter by an average of 1.5 mm per 10 years of age. Interestingly, aortic dilation was more marked in the ascending than in the descending thoracic aorta (23% increase in AA diameter between 2nd and 7th decade compared to 20% increase in descending aortic diameter). Importantly, the increase in aortic size was more marked after the 4th decade.
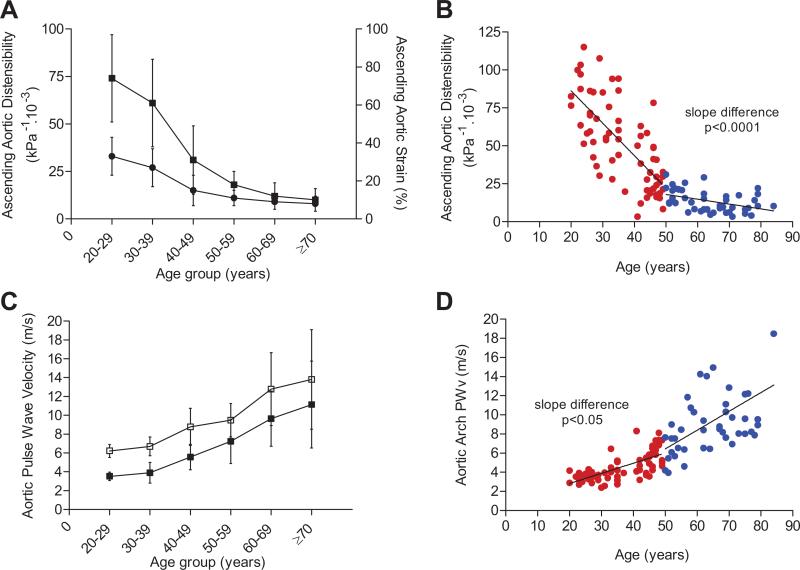
Age-related aortic functional impairment was evident, with marked age-related reductions of both ascending and descending aortic strain and distensibility (Table 1 and Figure 2A). Indeed, AA strain and distensibility decreased significantly with advancing age, with a respective average decrease of 5.3% and 13.6 kPa−1 · 10−3 per 10 years (Table 2). Moreover, although AA distensibility was the measure of aortic stiffness most closely related to aging, the relationship was markedly nonlinear. The reductions in aortic strain and distensibility were particularly marked in individuals <50 years of age, with a steep negative slope of −21.3, compared to −3.2 kPa−1 · 10−3/10 years in individuals ≥50 (Table 3 and Figure 2B). Carotid distensibility also decreased with aging but more gradually, with an average −1.6 kPa−1 · 10−3/10 years, and it was lower than aortic distensibility across all age groups.
Figure 2.
Effect of age on measures of proximal aortic function. A, AA strain (dot) and distensibility (squares) by decades of age. B, AA distensibility (age <50=red dots and ≥50 years=blue dots). C, Aortic arch PWV (dark squares) and cfPWV (open squares) by decades of age. D, Aortic arch PWV (red dots, <50 years of age; blue dots, ≥50 years of age).
Table 2.
Analysis of the Effect of Aging on Arterial Function Independent of Gender, Body Size, and Subclinical Atherosclerosis
| Model A |
Model B |
Model C |
||||||
|---|---|---|---|---|---|---|---|---|
| Arterial Function Measures | β | R2 | β | R2 | Significant Predictors | β | R2 | Significant Predictors |
| AA strain, % | –5.3±0.5* | 0.55* | –5.3±0.5* | 0.54* | Age* | –6±0.6* | 0.56* | Age*, HCl‡ |
| AA distensibility, kPa–1 · 10–3 | –13.6±1* | 0.62* | –13.6±1* | 0.62* | Age* | –15.7±1* | 0.66* | Age*, HCl† |
| Carotid distensibility, kPa–1 · 10–3 | –1.6±0.2* | 0.44* | –1.5±0.2* | 0.48* | Age*, BMI† | –1.5±0.2* | 0.48* | Age*, BMI† |
| Aortic arch PWV, m/sec | 1.60±0.13* | 0.60* | 1.60±0.13* | 0.60* | Age* | 1.48±0.12* | 0.58* | Age* |
| cfPWV, m/sec | 1.56±0.15* | 0.50* | 1.59±0.14* | 0.62* | Age*, HR†, BMI† | 1.61±0.14* | 0.61* | Age*, HR*, BMI† |
| AIx, % | 8.8±0.9* | 0.48* | 8.2±0.8* | 0.55* | Age*, Sex*, HR† | 8.6±0.8* | 0.57* | Age*, Sex*, HR† |
Regression coefficient β is given for 10 yr of aging. Model A: simple regression of each vascular variable with age. Model B: multivariable regression: the relationship with age of each variable is described with adjustment for gender (result for being male), BMI, and heart rate (HR). Model C=Model B with further adjustment for hypertension, diabetes, smoking, high cholesterol, and carotid IMT.
P<0.0001
P<0.01
P<0.05.
Table 3.
Comparison of the Effects of Aging by Ten Years on Arterial Function in Individuals <50 and ≥50 Years
| Age <50 Yr |
Age ≥50 Yr |
|||||
|---|---|---|---|---|---|---|
| Arterial Function Measures | β | P | β | P | R2 | Slope P |
| AA strain, % | –8.9±1 | <0.0001 | –1.2±1.1 | 0.29 | 0.63* | <0.0001 |
| AA distensibility, kPa–1 · 10–3 | –21.3±2 | <0.0001 | –3.2±2 | <0.0001 | 0.70* | <0.0001 |
| Carotid distensibility, kPa–1 · 10–3 | –1.80±0.4 | <0.0001 | –0.79±0.5 | 0.10 | 0.46* | 0.10 |
| Aortic arch PWV, m/sec | 1.06±0.28 | <0.0001 | 1.97±0.34 | <0.0001 | 0.62* | 0.04 |
| cfPWV, m/sec | 1.17±0.35 | 0.001 | 2.02±0.41 | <0.0001 | 0.51* | 0.12 |
| AIx, % | 13±1.8 | <0.0001 | –0.06±2.2 | 0.98 | 0.56* | <0.0001 |
Piecewise regression coefficient β is given for 10 yr of aging. Overall model significance R2
P<0.0001
significance of change in slope are given.
Aortic arch PWV and cfPWV increased with aging by an average of 1.60 and 1.56 m/sec per 10 years, respectively. There was a trend for a more marked increase of aortic arch PWV among individuals ≥50 years in piecewise regression, but gain over the linear model was modest because of higher variability in this age group (Tables 1 and 3; Figure 2D). The unadjusted relationship of cfPWV with aging is strong, although at a lower degree of significance compared to aortic arch PWV (Table 2).
The AIx increased significantly but nonlinearly with age (online Figure S4). The age increase in AIx averaged 9% per 10 years but was more marked in individuals <50 years and thereafter became nonsignificant with a plateau reflecting significant difference in slopes by piecewise regression (Table 3).
Determinants of Measures of Arterial Function
In simple regression analysis, the arterial variables that best correlated with age were AA strain and distensibility, aortic arch PWV, and cfPWV, as summarized in Table 2. However, the only arterial measures determined predominantly by the effects of aging independent from gender, heart rate and body size were AA strain and distensibility, as well as aortic arch PWV. Heart rate and gender were significant correlates of AIx independent of age. Heart rate and BMI significantly influenced cfPWV, whereas BMI was also directly related to carotid distensibility independent of age. Interestingly, aortic arch PWV was the only arterial variable solely predicted by age after adjustment for cardiovascular risk factors and carotid IMT. On the other hand, only hypercholesterolemia influenced AA strain and distensibility (inverse relationships) beyond the effect of age. However, in addition to aging, BMI and heart rate remained significant predictors of cfPWV. As expected, mean central pressure was found to be an independent determinant of all measures of aortic function when added to the regression model.
Correlation of Measures of Aortic Function
AA strain and distensibility were inversely correlated with AIx (respectively, r=−0.59 and r=−0.66; P<0.0001). Furthermore, the correlation between measures of aortic distensibility and AIx was highest for the AA compared to the descending aorta. In multivariable analysis, there was a trend for an association between AIx and AA strain (P=0.04) independent of age, gender, body size, and cardiovascular risk factors. Local AA PWV, derived from AA distensibility, correlated strongly with aortic arch PWV and cfPWV (r=0.73 and r=0.63, respectively; P<0.0001) and was an independent predictor of aortic arch PWV after adjustment for age, gender, body size, and risk factors (R2=0.65, P=0.002).
The positive correlation of AIx with PWV was significant for aortic arch PWV (r=0.53, P<0.0001) and for cfPWV (r=0.49, P<0.0001); however, AIx was not significantly associated with either measure after adjustment for age, gender, body size, and risk factors.
The unadjusted correlation between aortic arch PWV and cfPWV was very good (r=0.71, P<0.0001) and persisted after adjustment for potential cfPWV confounders, such as BMI and heart rate. Peripheral PWV (cfPWV) was consistently higher than aortic arch PWV (Figure 2C).
Discussion
Our study demonstrates an early subclinical alteration in AA distensibility and that AA distensibility is the most sensitive marker of arterial aging in individuals <50 years of age, whereas aortic arch PWV is more sensitive in individuals ≥50 years of age, beyond the influences of gender, body size, and cardiovascular risk factors.
There is growing evidence that altered vascular function is an important age-related determinant of morbidity and mortality.16,17 Interestingly, an emerging body of research seems to suggest that the aging process begins early in life, with evidence for alterations in vascular structural matrix proteins, mitochondrial bioenergetics, endothelial function, inflammation, coagulation processes, and cell senescence as early as the 3rd decade in healthy individuals.18,19 However, associating these interconnected pathophysiological pathways in an integrated arterial aging and disease process remains challenging. The reliance on cuff peripheral blood pressure measurement to assess vascular aging has led to underestimate the effects of age on the arterial system. More sensitive markers of arterial alteration are necessary if we are to detect earlier evidence of unusual or accelerated arterial aging and to better define the spectrum of “normal” vascular aging. We also documented the overestimation of central aortic pressure by peripheral measures of arterial pressure in young individuals. This is attributable to the physiological impedance mismatch between compliant elastic central arteries and resistive muscular peripheral arteries, which decreases with aging to the point of a reversal of the stiffness gradient along the arterial tree following arterial stiffening of the elastic arteries.12,20 Consequently, we report that central aortic PP is a better marker of aging than peripheral PP before and after adjustment for body size, gender, heart rate, and further adjustment for cardiovascular risk factors and carotid IMT. Consistently with McEniery et al,8 we found AIx to be nonlinearly related to age with a significant increase in individuals <50 years of age, followed by a plateau effect.
Interest in more direct measures of arterial function has been rising as the value of global aortic stiffness as an integrated marker of cardiovascular risk and mortality has been established in the general population.4 Carotid distensibility has been shown to be less predictive of arterial damage than cfPWV21 and to have no independent predictive value in high-risk patients.22 In this study, we compare the relationship to age of direct measures of MRI-defined aortic function with cfPWV and AIx measured by tonometry, to describe arterial remodeling over a broad age range and varied levels of cardiovascular risk.
A strength of MRI is the unique ability to provide both local and regional direct noninvasive measures of aortic function. In particular, lumen area changes (aortic strain) over the cardiac cycle can be acquired with high temporal and spatial resolution and pulse wave velocity can be measured in different locations. Using aortic strain combined with central (measured by tonometry) instead of peripheral PP allows, for aforementioned reasons, the calculation of more relevant measures of local aortic distensibility, especially in younger individuals. Using this methodology, we found AA distensibility to be the most sensitive marker of aortic aging in individuals <50 years of age. This can be explained in light of the relationship of the 2 components of AA distensibility with age. AA distensibility is calculated as the ratio of aortic strain, a measure of circumferential deformation, to the central PP as the main driver of luminal changes. We found aortic strain to have a nonlinear relationship with age and to steeply decrease before the age of 50 years, particularly in the AA, whereas the decrease remained significant but more gradual after 50 years. The decrease in circumferential strain was therefore the main determinant of reduced distensibility in individuals <50 years of age, because the increase in central PP was relatively modest before age 50. In individuals ≥50 years of age, central PP increased more markedly and strain continued to decline, although more gradually, explaining the lesser sensitivity of AA distensibility as a descriptor of arterial aging in this group. Other important findings reported in our study relate to the interrelationship among different measures of PWV. We found PWV in the AA, derived from local distensibility, to be strongly related with aortic arch PWV, independent of age, gender, body size, or risk factors. As also reported by McEniery et al,8 we found a nonlinear relationship between age and cfPWV but, in addition, that aortic arch PWV was a better correlate of pure arterial aging. In effect, aortic arch PWV was found to be the most significant measure of arterial aging in subjects ≥50 years of age. Aortic arch PWV was the only measure of arterial stiffness to be strongly determined by aging across the full age range independently of gender, BMI, heart rate, and cardiovascular risk factors, whereas cfPWV was confounded by BMI and heart rate. Measures of aortic arch PWV and cfPWV were strongly related but values of cfPWV were consistently higher. The latter finding can be explained by both physiological and technical reasons. Firstly, PWV increases from the aortic arch to the femoral arteries,23 and cfPWV is a global approximation of PWV in the entire aorta. Secondly, the calculation of cfPWV requires 2 anatomic assumptions: (1) that PWV from the aortic arch to the carotid artery corresponds to that in the AA (from the heart to the dome of the aortic arch) and (2) that the vascular distance traveled by the pulse wave is close to the length measured on the body surface, an assumption recently challenged.24 In that regard, we have found the length used for cfPWV calculation to be influenced by body shape and size as it correlated with BMI and body surface area (respectively: r=0.60, r=0.58, P<0.0001), leading to an overestimation of global aortic PWV. Consequently, the normal range for aortic arch PWV in older individuals in our study was approximately 9 to 10 m/sec, close to values found by Sugawara et al24 for cfPWV using vessel length measured with MRI, whereas cfPWV with surface measurements was around 13 m/sec. The potential clinical implications are to be considered because recent guidelines consider cfPWV >12 m/sec to be abnormally high.25 The determination of normal values with MRI for clinical use will require further studies.
Our finding of a dramatic decrease in AA strain and distensibility early in life is consistent with the mechanistic hypothesis by O'Rourke and Hashimoto of a decline in aortic elasticity in the 3rd decade,26 partly because of thinning and fragmentation of elastin fibers caused by inexorable pulsatile stress associated with a decline in elastin synthesis and activation of proteolysis. The stiffness of the matrix protein network is further enhanced by increased collagen deposition and cross-linking and fibrosis deposition. Calcium deposition in the media has also been demonstrated but at an older age.27 Consequently, we hypothesize that the loss of AA strain and distensibility is an early marker of age-related arterial alterations within a temporal continuum, leading to arterial dysfunction and stiffening further increased by atherosclerosis and cardiovascular risk factors in older individuals. Increased aortic arch PWV seems to be a sensitive and specific marker of proximal aortic stiffness and dysfunction during the aging process, particularly in middle-aged and older individuals.
The main limitation in our study was the inability to measure simultaneously aortic strain with MRI and central pressures with tonometry. MRI was immediately followed by tonometry measurements in all participants in a similar setting, and measurements were repeated to minimize individual variability in blood pressure as much as possible. Furthermore, the somewhat high proportion of overweight subjects in our random community sample increased the overestimation of arterial length by body surface measurements and therefore the overestimation of cfPWV.
Conclusions
AA strain and distensibility and aortic arch PWV determined by MRI were the most sensitive and specific markers of age-related arterial stiffness and demonstrated the dramatic and early alterations in central arterial function in individuals free of overt cardiovascular disease. The relationship with age of measures of aortic function is nonlinear, and AA distensibility determined by MRI and central pulse pressure was the most sensitive marker of large artery dysfunction in individuals less than 50 years.
Perspectives
The reliance on peripheral blood pressure as a marker of vascular alteration leads to underestimate vascular aging in asymptomatic individuals. In this study, we demonstrate the relevance of novel markers of central aortic function assessed noninvasively by MRI to explore vascular aging. In particular, we show regional aortic strain and distensibility to be significantly decreased early in life in healthy humans and to be stronger correlates of aging than more peripheral indices such as carotid distensibility, cfPWV, and the AIx. Aging is a complex and heterogeneous process intricately related to cardiovascular disease and its complications. Because most of the loss of central aortic elasticity happens before the 5th decade, when the prevalence of overt disease increases, it seems important to study potential differences in subclinical vascular function differences by using the most sensitive arterial markers in clinical research studying younger individuals. Future studies will be needed to establish the prognostic value of these novel central aortic function indices and define normal values for each age group to eventually guide prevention and treatment strategies.
Supplementary Material
Acknowledgments
We thank Elzbieta Chamera and Rosalie Cosgriff for their important contribution to data collection and participant management.
Sources of Funding A.R. received partial grant support from Fédération and Société Française de Cardiologie and Société Française de Radiologie.
Footnotes
Disclosures None.
References
- 1.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 2.Giannattasio C, Achilli F, Failla M, Capra A, Vincenzi A, Valagussa F, Mancia G. Radial, carotid and aortic distensibility in congestive heart failure: effects of high-dose angiotensin-converting enzyme inhibitor or low-dose association with angiotensin type 1 receptor blockade. J Am Coll Cardiol. 2002;39:1275–1282. doi: 10.1016/s0735-1097(02)01755-2. [DOI] [PubMed] [Google Scholar]
- 3.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 4.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 5.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 6.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc'h PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 7.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M, CAFE Investigators, Anglo-Scandinavian Cardiac Outcomes Trial Investigators, CAFE Steering Committee and Writing Committee Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 8.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, ACCT Investigators Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Bolster BD, Jr, Atalar E, Hardy CJ, McVeigh ER. Accuracy of arterial pulse-wave velocity measurement using MR. J Magn Reson Imaging. 1998;8:878–888. doi: 10.1002/jmri.1880080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers WJ, Hu YL, Coast D, Vido DA, Kramer CM, Pyeritz RE, Reichek N. Age-associated changes in regional aortic pulse wave velocity. J Am Coll Cardiol. 2001;38:1123–1129. doi: 10.1016/s0735-1097(01)01504-2. [DOI] [PubMed] [Google Scholar]
- 11.Metafratzi ZM, Efremidis SC, Skopelitou AS, De Roos A. The clinical significance of aortic compliance and its assessment with magnetic resonance imaging. J Cardiovasc Magn Reson. 2002;4:481–491. doi: 10.1081/jcmr-120016386. [DOI] [PubMed] [Google Scholar]
- 12.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 13.Ou P, Celermajer DS, Raisky O, Jolivet O, Buyens F, Herment A, Sidi D, Bonnet D, Mousseaux E. Angular (Gothic) aortic arch leads to enhanced systolic wave reflection, central aortic stiffness, and increased left ventricular mass late after aortic coarctation repair: evaluation with magnetic resonance flow mapping. J Thorac Cardiovasc Surg. 2008;135:62–68. doi: 10.1016/j.jtcvs.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Yu WC, Chuang SY, Lin YP, Chen CH. Brachial-ankle vs carotidfemoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens. 2008;22:24–31. doi: 10.1038/sj.jhh.1002259. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 16.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke MF. Clinical assessment of arterial stiffness. Am J Hypertens. 2007;20:839. doi: 10.1016/j.amjhyper.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Tracy RP. The five cardinal signs of inflammation: Calor, Dolor, Rubor, Tumor ... and Penuria (Apologies to Aulus Cornelius Celsus, De medicina, c. A.D. 25). J Gerontol A Biol Sci Med Sci. 2006;61:1051–1052. doi: 10.1093/gerona/61.10.1051. [DOI] [PubMed] [Google Scholar]
- 19.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 21.Paini A, Boutouyrie P, Calvet D, Tropeano AI, Laloux B, Laurent S. Carotid and aortic stiffness: determinants of discrepancies. Hypertension. 2006;47:371–376. doi: 10.1161/01.HYP.0000202052.25238.68. [DOI] [PubMed] [Google Scholar]
- 22.Dijk JM, Algra A, van der Graaf Y, Grobbee DE, Bots ML, SMART study group Carotid stiffness and the risk of new vascular events in patients with manifest cardiovascular disease. The SMART study. Eur Heart J. 2005;26:1213–1220. doi: 10.1093/eurheartj/ehi254. [DOI] [PubMed] [Google Scholar]
- 23.Nichols WW, O'Rourke MF, editors. McDonald's Blood Flow in Arteries. Theoretical, Experimental and Clinical Principles. 5th ed. Oxford University Press; Oxford: 2005. [Google Scholar]
- 24.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. J Am Coll Cardiol Cardiovasc Imaging. 2008;1:739–748. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 25.O'Rourke M, Farnsworth A, O'Rourke J. Aortic dimensions and stiffness in normal adults. J Am Coll Cardiol Cardiovasc Imaging. 2008;1:749–751. doi: 10.1016/j.jcmg.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 26.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 27.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.