SUMMARY
The adverse health effects of environmental contaminants (ECs) are a rising public health concern, and a major threat to sustainable socioeconomic development. The developing fetuses and growing children are particularly vulnerable to the adverse effects of ECs. However, assessing the health impact of ECs presents a major challenge, given that multiple outcomes may arise from one exposure, multiple exposures may result in one outcome, and the complex interactions between ECs, and between ECs, nutrients and genetic factors, and the dynamic temporal changes in EC exposures during the life course. Large-scale prospective birth cohort studies collecting extensive data and specimen starting from the prenatal or pre-conception period, although costly, hold promise as a means to more clearly quantify the health effects of ECs, and to unravel the complex interactions between ECs, nutrients and genotypes. A number of such large-scale studies have been launched in some developed counties. We present an overview of “why”, “what” and “how” behind these efforts with an objective to uncover major unidentified limitations and needs. Three major limitations were identified: (1) limited data and bio-specimens regarding early life EC exposure assessments in some birth cohort studies; (2) heavy participant burdens in some birth cohort studies may bias participant recruitment, and risk substantial loss to follow-up, protocol deviations limiting the quality of data and specimens collection, with an overall potential bias towards the null effect; (3) lack of concerted efforts in building comparable birth cohorts across countries to take advantage of natural “experiments” (large EC exposure level differences between countries) for more in-depth assessments of dose–response relationships, threshold exposure levels, and positive and negative effect modifiers. Addressing these concerns in current or future large-scale birth cohort studies may help to produce better evidence on the health effects of ECs.
Introduction
The adverse health effects of exposures to environmental contaminants (ECs) are a rising public health concern, and a major threat to sustainable socioeconomic development. Pregnant woman and the developing fetus are particularly vulnerable to the adverse effects of ECs. This is partly due to the dramatic metabolic changes in the mother and fetus occurring during pregnancy. For example, life-time exposures to heavy metal contaminants (e.g. lead) accumulated in the bone could be mobilized into the circulation during pregnancy, subjecting both the mother and the developing fetus to potentially deleterious effects [1]. Impacts of ECs on child health (exposures during fetal or postnatal life) are the key considerations in developing environmental safety standards. However, assessing the health impacts of environmental contaminants presents a major challenge, given that multiple outcomes may arise from one exposure, multiple exposures may result in one outcome, the complex interactions between EC exposures, and between ECs, nutrients and genotypes, and the dynamic temporal changes in EC exposures over the life course.
In view of the growing health concerns regarding ECs, a number of large-scale prospective birth cohort studies have been launched in some developed countries, with a common goal of more clearly quantifying the exposure levels and health effects. Such studies hold the promise of providing critically important reference data for developing or modifying environmental chemical safety standards, and for designing effective early intervention measures to reduce population burdens of chronic illnesses such as asthma, diabetes, and cancers. The large sample sizes and longitudinal prospective data and specimen collection plans provide unprecedented study power to detect subtle health effects, relatively rare outcomes, interactions and effect modifiers, and for exploring causal mechanisms. This article aims to provide a critical overview of “why?”, “what” and “how” in these current efforts with an objective to uncover major unidentified limitations and needs. It is hoped that current or future such large-scale birth cohort studies addressing these concerns may help to produce better and more comprehensive evidence on the health effects of ECs.
What are environmental contaminants?
Environmental pollution, pollutants or contaminants had been a fact of life for centuries, but became a real problem only since the industrial revolution. Pollution is the introduction of contaminants into the environment that cause harm or discomfort to humans or other living organisms, and can come in the form of chemical substances or energy such as noise, heat or light (http://en.wikipedia.org/wiki/pollution). Pollutants can include not only man-made substances (ex. pesticides), but also naturally occurring substances when in excess of natural levels. However, pollution commonly refers to environmental contamination by man-made wastes or contaminants (http://www.merriam-webster.com/dictionary/pollution), which are of increasing public health concern owing to an increasing number of pollutants arising from modern biochemical industrial inventions. According to the US Environmental Protection Agency, there exist over 80,000 synthetic chemicals, most of which developed since the 1950s [2]. Pollutants exist in air, water, food, soil, various consumer products and other substances such as tobacco smoke, at public, work and home places. Some contaminants can enrich through the food chain, and accumulate in specific tissues, raising the exposure levels in human body well beyond their concentrations in natural habitats such as soil or water. These substances are called persistent, bio-accumulative toxins (PBT) [3]. For example, heavy metals (ex. lead and mercury) are enriched in marine fishes, and human life-time exposures are accumulated in bone tissues. Of particular health concern are those chemicals produced in large volumes and used extensively in consumer products, which would subject the mass population to the exposure. Some organic pollutants, such as polychlorinated biphenyls (PCBs), are very resistant to degradation in the natural environment, and therefore persist very long after their release into the environment. They are called persistent organic pollutants (POPs) [3].
What we have known about the health effects of environmental contaminants?
Numerous studies have been conducted concerning the carcinogenicity and teratogenicity of various chemicals, mostly in animal models. Only a limited number of man-made chemical compounds have been studied with respect to their health effects in humans. Although some chemicals are very toxic, their exposures are usually restricted to certain occupational sub-populations or residents close to specific plants or pollutant dumping sites. However, some pollutants are widespread, and exist almost ubiquitously, are thus of particular public health concern, and are the major EC health research targets. The most common such ubiquitous ECs include particulate matter (a mixture of various solid particles and liquid droplets), tobacco smoke (a mixture of chemicals), common gaseous pollutants [carbon monoxide (CO), nitrogen dioxide (NO2), sulphur dioxide (SO2), and ground-level ozone (O3)], heavy metals (such as lead, cadmium, mercury), polycyclic aromatic hydrocarbons (PAHs), PCBs, and pesticides such as dichlorodiphenyltrichloroethane (DDT), organic solvents such as ethyl alcohol and gasoline, plasticizers such as phthalates and bisphenol A, flame retardants such as polybrominated diphenyl ether congeners (PBDEs), and water disinfection by-products such as trihalomethanes (THMs).
Tobacco smoke is a well-known life style pollutant, and can cause many adverse health outcomes such as fetal growth restriction, asthma, chronic obstructive lung disease, atherosclerosis, coronary heart disease, lung and other cancers [4,5]. Tobacco smoke consists of a complex mixture of substances including PAHs, lead and cadmium that are also generated as air pollutants from other sources [4]. The adverse health effects of tobacco smoke are indicative of the toxic effects of its composite chemicals; many have been proved in subsequent studies [6]. The adverse health effects (such as increased mortality, cancers, respiratory problems) of poor air quality due to particular matter and gaseous contaminants have been well documented in many studies [4,7]. The main threats to human health from heavy metals are associated with exposure to lead, cadmium, mercury and arsenic [8]. Cadmium has been associated with cancers and kidney damage [9]. Mercury can cause neurological damage, and the developing fetus is most vulnerable [10]. Lead may have neurotoxic effects on infants even at the exposure levels previously considered safe [8,11]. Arsenic has been associated with increased risks of skin cancer and some other cancers, as well as other skin lesions such as hyperkeratosis [8]. Heavy metals pose a more serious threat to the developing fetus than to adults, because life-time exposures accumulated in the bone could be released into blood circulation during pregnancy, and the special vulnerability of the developing fetus [12].
The health effects of legacy POPs such as PAHs, DDT and PCBs have been well studied. PAHs have well recognized carcinogenic effects [6]. Although DDT (a persistent pesticide) is generally not toxic to humans, exposure to DDT at amounts that would be needed in malaria control might cause preterm birth, fetal growth restriction and birth defects, but the evidence remains inconclusive partly due to imprecision in exposure measures [13,14]. Numerous studies have reported negative associations between prenatal PCB exposure and measures of cognitive function in infancy and childhood [15–18]. Most of the research data on legacy contaminants come from studies in developed countries; there are relatively limited comparable data from developing countries where the exposure levels tend to be higher.
Industrial development in more recent decades has resulted in ubiquitous exposure to emerging new ubiquitous ECs such as flame retardants (ex. PBDEs) and plasticizers (ex. phalates, bisphenol A), but their health effects remain largely unknown. There is emerging evidence, mostly from animal models, that exposures to these new ubiquitous ECs (most associated with some endocrine function disrupting effects) may have adverse health effects such as decreased birth weight, decreased anogenital distance, and disruptions in thyroid functions [19–22].
Why large-scale prospective birth cohort studies?
The major health problems confronting modern populations are chronic conditions, such as type 2 diabetes, chronic hypertension, heart disease, cancers, asthma, and neuro-developmental disorders [23,24]. The etiology of these chronic conditions is complex, most likely involving multiple environmental and genetic factors and their interactive effects. There is substantial evidence that the prenatal and early postnatal environment may be critically important in the development of these various chronic disorders in adulthood [25,26]. Therefore, understanding the roles of various early life “environmental” exposures and “how they work” could pave the way for early prevention. However, knowledge on which specific environmental factors contribute to which specific chronic conditions through what specific mechanisms remain limited, and progress has been slow.
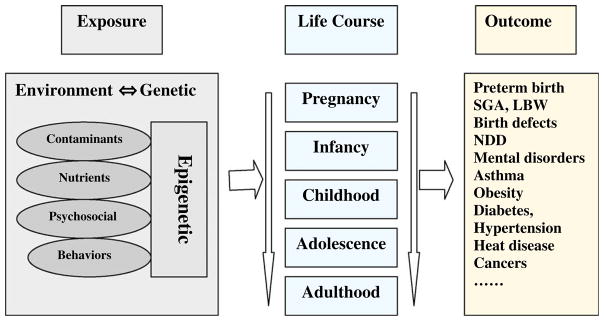
Although the randomized controlled trial (RCT) is the best design to determine causal relationships, it is not applicable in determining the health effects of environmental contaminants due to considerations of ethics. Prospective pregnancy/birth cohort study is the most powerful observational design in casual inference for understanding the roles of various environmental and genetic factors and their interactions in the development of various chronic diseases over the life course (Fig. 1). Such prospective birth cohort studies are much less vulnerable to exposure measurement biases and the reverse causality trap (an “exposure”, such as stress, may actually result from rather than result in the disease) than cross-sectional or case-control studies. Furthermore, the opportunities for collecting bio-specimens over the course of pregnancy would allow more precise measures of prenatal EC exposure levels, and biomarkers indicative of the causal pathways. Such prospective birth cohort studies can uncover important etiological factors early in the life course that could not have been possible using other study designs. For example, lead exposure during fetal life has been consistently associated with impaired neuro-cognitive development in childhood in birth cohort studies [27,28] (RCTs would be unethical). It is only in recent years that bio-monitoring of EC exposures in pregnancy has been incorporated in studying the long-term health effects in humans.
Fig. 1.
Prospective birth cohort design in studying the effects of environmental and genetic factors and their interactions over the life course in the etiology of chronic disease. LBW = low birth weight; SGA = small-for-gestational age; NDD = neuro-developmental disorders.
The goals
Previous birth cohort studies are limited by relatively small sample sizes and thus little statistical power to detect moderate effects, gene-environment interactions, and relatively rare outcomes, and are often limited to one or one group of chemicals at a time, and often with limited duration of follow-up. Further, most previous birth cohorts have no or very limited data on EC exposures during the prenatal or perinatal period, the suspected most critical and vulnerable exposure windows to the adverse health effects of environmental chemicals. Large-scale prospective birth cohort studies, with representative coverage and large sample sizes, would help to fill these gaps, providing a unique opportunity to clarify the roles of multiple EC exposures during early life in multiple health outcomes in later life. The opportunities to collect biological and environmental specimens during the pre-conception, perinatal and early postnatal life periods would allow more precise measures of the EC exposures (timing, frequency, magnitude and duration) for understanding their adverse health effects (short or long-term) during the critical developmental windows.
The challenges
In additional to the “regular” challenges faced by all prospective birth cohort studies, there are new major challenges in large-scale birth cohort studies:
Huge start-up and operating costs, and persistent challenges in securing research funds for future follow-ups: In a review of five small (n < 700) birth cohort studies on ECs funded by the National Institute of Environmental Sciences and the US Environmental Protection Agency, it was found that all study centers underestimated the needs for time, infrastructure and costs [29]. Cost is a much more serious concern in large-scale birth cohort EC studies which involve additional methodological, logistic and operational complexities. Underestimating the true costs would more likely occur in large-scale birth cohort studies, partly due to our lack of experience. In addition, researchers may have some desire to present a smaller budget for a better chance in obtaining the research funding, which would exacerbate the budget limitations. Because research funding commitments are made often for a short time frame, it is a persistent challenge to secure funding for continued follow-up of a large birth cohort with significant cost implications.
Data and specimen collection quality control: Large-scale birth cohort studies often involve many centers, which may vary considerably in research support capacity (staff, infrastructure, experience). Standardisation of operational procedures and quality control is a major challenge to alleviate the concern of “garbage-in, garbage-out”.
Participant burdens, recruitment and retention rates: Large-scale birth cohort studies are “mandated” to address many questions that could not be answered in other study designs. This calls for more extensive data and specimen collection, and heavier participant burden. However, heavy participant burdens may bias the sampling of study participants in that only those extremely research receptive subjects would be willing to participate; they may differ significantly from the general population, and consequently may compromise the sampling representativeness and generalizability of study findings. It has been documented that patients willing to participate in a study with only moderate participant burden may differ from the general population, biasing the results toward null association [30]. Heavy participant burden could result in low recruitment and low retention rates which might put the original research goals in jeopardy. There is a need to strike a delicate balance between participant burden and the public and scientific desire to obtain important new data and findings.
What’s going on in ongoing large-scale birth cohort studies?
There are a number of ongoing large pregnancy/birth cohort studies of variable designs mostly in developed countries, with a common general goal to more clearly define the EC exposure levels, the effects of environmental factors, and gene-environment interactions in the development of chronic health problems over the life course. Table 1 gives a summary of the major ongoing large birth cohort studies with at least some data collection during pregnancy. The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) in England recruited 14,541 pregnant women, but with extensive follow-up data on only 10–60% infants in postnatal years of life [31] (Table 1a). Despite being the birth cohort study with the largest number of scientific publications, the study has very little data on EC exposures. Also, most data are collected through mailed-in questionnaires – a relatively imprecise data collection method. The Generation R study in the Netherlands recruited 9778 pregnant women at non-specific gestational age windows, therefore the study entry time point, data and specimen collection varied for individual participants [32,33]. The study depended on routine child care centers for infant growth data, and in-person follow-up only a small subset of infants (12%) during the postnatal period. The Danish National Birth Cohort [34] and Norwegian Mother and Child Cohort Study [35,36] each has a mega sample size of 100,000; Both tried to capture minimal data with minimal burden to participants, mainly using relatively cheap computer-assisted and self-administered questionnaires. All of the above studies have very limited data and specimens for assessing early life EC exposures other than tobacco smoke, and had no priori considerations of bio-monitoring EC exposures during the pre- and perinatal periods. Accurate assays of many ECs, such as heavy metals, need special specimen collection and processing protocols. Therefore, these birth cohorts are not adapted, or somewhat handicapped for assessing the health impact of EC exposures during critical developmental time windows in early life course.
Table 1a.
Major ongoing large-scale birth cohort studies with a major component on environmental factors.
| Study | Country | Sample size | Description and comments |
|---|---|---|---|
| Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) [31] | England | 14,541 | Recruitment 1991–1992 (variable, from <10 wks to 40 wks). Maternal data collection through mailed questionnaires sent out before 14 wks, at 18, 32 wks and after delivery, and medical chart reviews. Infant follow-ups by mailed questionnaires every year from birth to 7 y, trained interviewers available for assistance if the mother/guardian requested. 14,062 Live births. <50% (n = 6500) had cord blood samples or DNAs (from blood or saliva) for child-mother pairs (n = 6755, including 700 mother-father-baby trios). In a subset (10%) of infants (n = 1432), in-person assessments on growth, development and health at 4, 8, 12, 18, 25, 31, 37, 43, 49 and 61 mo of age, and blood specimen at 8, 12, 18, 31, 43, 61 mo of age (Child in Focus sub-study). Subsequent follow-ups of a larger different subset (n = 2101–8297) at age 8, 9, 10, 11, 12, 13, 14 and 16 y. Inconsistent recruitment gestational age windows. Low specimen collection rates, low costs, moderately high attrition rates especially post-natally, limited data and bio-specimen for early life EC exposures. http://www.bris.ac.uk/alspac/sci-com/resource/recruit/ |
| The Generation R study [32,33] | Netherlands | 9778 (original target, n = 10,000) | Recruitment 2002–2006 (70% <18 wks, 19% 18–25 wks, 12% 25 wks-births). At least two ultrasound fetal growth measures. Maternal data collections by physical exams, questionnaires at 12–17 wks, 20–25 wks, 30 wks in research centers. Child data collection on growth at 2, 3, 4, 6, 11, 14, 18, 24, 26, 48 mo based on routine records at child health centers, and child questionnaires at 2, 6, 12, 18, 24, 36, and 48 mo. Bio-specimen include maternal blood and urine samples at <18 wks, 18–25 wks, paternal blood, cord blood. In a subset of 1232 children, more intensive follow-up data at 6, 14, 24, 36, 48 mo, blood and urine samples at 6 and 24 mo, neurological assessments at 14 mo (Focus cohort). Inconsistent timing for study visits in pregnancy. Feasible, low cost, low participant burdens in the core cohort, limited data and bio-specimen for early life EC exposures. Moderate attribution rates in infant follow-ups. http://www.generationr.nl/index.php?option=com_content&task=view&id=3 |
| Danish National Birth Cohort [34] | Denmark | 101,042 (original target: 100,000) | Recruitment 1996–2002 at the GP clinics (6–12 wks). Exposure data mainly collected by computer-assisted tele-interview (<20 min). Tele-interview women twice during pregnancy (12 wks, 30 wks), and infants at 6 mo and 18 mo of age. Whole maternal blood samples at 1st (6–12 wks) and 2nd (24 wks) trimester of gestation, cord blood at birth. Most outcome data are based on linkable routine health registers. Enrolled 101,042 pregnant women. Follow-up of the child at 7-y by self-administered questionnaires to mothers ongoing. 75% Follow-ups at 18 mo of age. Minimal data collection in pregnancy. Low participant burden, low costs, feasible, moderate attrition rates, limited data and bio-specimen for early life EC exposures. http://www.cls.ioe.ac.uk/text.asp?section=00010001000500090002 |
| Norwegian Mother and Child Cohort Study [35,36] | Norway | 100,000 | Recruitment 1999–2008 mainly by mails at hospital maternal units (13–17 wks). Maternal data are collected mainly by self filled-in Questionnaires twice in pregnancy (13–17 wks, 30 wks). Infant questionnaires at 6 mo, 18 mo and 3-y of age. Maternal blood and urine samples at 17–18 wks and delivery, paternal blood, and cord blood (or neonatal blood at 3–4 postnatal days of life, if cord blood unavailable) samples. Planned to follow-up the child at 7-y. Changed specimen collection tubes from heparin to EDTA in 2003. Data collection incompletely funded. Future follow-ups after age 7-y undetermined. Low participant burden, low costs, feasible, limited data and bio-specimen for early life EC exposures. http://www.fhi.no/eway/default.aspx?pid=238&trg=MainArea_5811&MainArea_5811=5903:0:15,4329:1:0:0:::0:0 |
More recently, the US launched the National Children’s Study (NCS) after 8-y of preparation, with a goal of recruiting 100,000 pregnancies from the pre-conception period and followed the pregnancy at each trimester of pregnancy, and the infant up to 21 y of age [37] (Table 1b). The NCS study is focused on environment factors especially the exposures during the prenatal and early postnatal life periods, including many questionnaires to capture diverse dimensions of various exposures, and many biological and environmental specimens collection anticipated. It has the unprecedented best-in-science design, but comes with unprecedented heavy participant burden (and costs). It is possible that only very selected patients might be able to accept such high participant burdens, which may result in some recruitment selection bias. The NCS study group encourages investigators to propose adjunct studies in different centers. The NCS study may need to exercise prudence in pigging back adjunct studies with additional participant burdens. Also, the implementation of complex protocols across over 100 sites may risk substantial protocol deviations from quality data and specimen collection. Additional resources (ex. on-site experienced research staff) may be required to insure high quality data and specimen collection. The study is now in pilot recruitment phase in several vanguard centers. Despite its 21-y study plan, future funding remains uncertain.
Table 1b.
Major ongoing large-scale birth cohort studies with a major component on environmental factors.
| Study | Country | Sample size | Characteristics and comments |
|---|---|---|---|
| National Children’s Study [37] | US | 100,000 | Recruitment 2009–2015? (pre-conception) in communities/families. Pilot recruitment phase. Follow ups of pregnancy at the 1st trimester (home, as early as possible), 2nd trimester (phone), 3rd trimester (28–30 wks, clinics), delivery, and the infant at 3, 6, 9, 12, 18, 24, 30, 36 mo. Data collection by in-person (home, clinics) and tele-interviews. Two fetal ultrasound exams (1st, 3rd trimester). Biologic specimens include blood, urine, hair, and nail clippings from mothers (pre-conception, 1st and 3rd trimester) and children (birth, 1-y, later?); blood, urine, and hair from fathers; cord blood, umbilical cord and placental tissues, and meconium collected at or around the time of delivery; vaginal swabs (pre-conception, 1st and 3rd trimester), saliva and breast milk from mothers. Environmental samples include air, dust, soil, food, and water at home places. Hope to follow-up infants at 5, 7, 9, 12, 16, 21 y. Center-specific adjunct studies expected. Best protocol in science on ECs. Best-in-science design, heavy participant burden (34 h/visit, many specimens). Most costly, future funding uncertain. http://www.nationalchildrensstudy.gov/Pages/default.aspx |
| Maternal and Infant Research on Environmental Contaminants (MIREC) pregnancy cohort [38] | Canada | 2000 | Recruitment 2008–2011 (1st trimester, 6–13 wks) in 10 obstetric hospitals in nine cities. Specifically focused on ECs. Follow ups of pregnancy at the 2nd trimester (16–21), 3rd trimester (32–34 wks), delivery, and the infant at 2–8 wks, 6 mo of age. Data collection by in-person interviews (mostly at hospitals, infant home visits at 2–6 wks). Biologic specimens include blood, urine and hairs from mothers (1st. 2nd, 3rd trimester of pregnancy, and delivery); cord blood, placental tissues, and meconium collected at the time of delivery; breast milk (2–6 wks) from mothers. Last follow-up at 6-mo to assess infant growth, neuro-behavioural and sensory development. Modest participant burdens (1–2 h/visit, many specimens). Low recruitment but high retention rates. Future infant follow-ups (>6 mo) and funding uncertain. http://mirec-canada.ca/ |
| The Integrated Research Network in Perinatology of Quebec (IRNPQ) pregnancy cohort [39] | Canada, Quebec and Eastern Ontario | 5000 | Recruitment 2009–2012 (1st trimester, 10–13 wks) in 8 obstetric hospitals in four cities. ECs are one of the primary exposures. Follow ups of pregnancy at the 2nd trimester (20–24), 3rd trimester (32–35 wks), delivery, and the infant at 3, 12 and 24 mo of age. 3rd trimester ultrasound estimated fetal weight, Doppler exam of placenta sufficiency for pregnancies at risk of FGR (fetal growth restriction). Data collection by in-person interviews in hospitals. Biologic specimens include blood, urine and hairs from mothers (1st, 2nd, 3rd trimester and delivery); Vaginal fluids at 1st and 2nd trimester; cord section, cord blood, placental tissues and meconium samples at delivery; paternal blood; infant blood at 2-y. Modest to high participant burden (1–3 h/visit, many specimens). Future infant follow-ups (>2 y) and funding uncertain. http://www.irnpqeo.ca/ |
In Canada, there are two ongoing medium scale pregnancy cohort studies focused on ECs. The Maternal and Infant Research on Environmental Contaminants (MIREC) pregnancy cohort study is recruiting 2000 pregnant women at the 1st trimester of pregnancy in nine cities (Table 1b) [38]. Like the NCS, it was specifically designed for capturing early life EC exposures, but targeted only a few primary outcomes due to its much smaller sample size. Extensive data and specimen are being collected at each trimester of pregnancy, delivery, the infant at 2–6 weeks and 6 months of age (focused on neuro-cognitive development). The participant burdens seem moderate, and the study experienced initially low recruitment but high retention rates. The sample size will provide sufficient powers for examining relatively common outcomes only, and future infant follow-up plans remain uncertain. The Integrated Research Network in Perinatology of Quebec (IRNPQ) pregnancy cohort study plans to recruit 5000 pregnant women at the 1st trimester of pregnancy in four cities in Quebec and Eastern Ontario [39]. ECs are one of the primary exposures, and will be captured by both questionnaire-based and bio-monitoring approaches. Extensive data and specimen are being collected at each trimester of pregnancy, delivery, the infant at 3, 12 and 24 mo of age (focused on neuro-cognitive development, and early indicators of “programming” metabolic and cardiovascular risks). The study participant burden seems moderate to high, and the study may experience low recruitment and moderately high attrition rates especially in postnatal visits.
The limitations
The above discussions have identified two major limitations in ongoing large-scale birth cohort studies on ECs: (1) some birth cohorts have limited data and specimens for assessing EC exposures in early life (perinatal and early postnatal) – the most critical exposure windows; (2) heavy participant burden in some birth cohort studies may bias participant recruitment, and risk substantial loss of follow-ups, protocol deviations from quality data collection, with an overall potential bias towards null effect. In addition, there is a lack of large-scale birth cohort studies on ECs in developing countries where ECs may pose an even greater health burden, and a lack of cross-country birth cohort EC studies. However, different research protocols may collect different data and specimens and obtain different assay results, and these variations would handicap the comparisons across birth cohorts. There exist large EC exposure level differences across countries. For example, in the US, 2.2% of the children had blood lead levels exceeding 10 μg/dl (the current US safety threshold) in 1999–2002 [40]; whereas, in China, 33% (15 times) of the children had blood lead levels exceeding 10 μg/dl in 1994–2003 [41]. There is a need to build comparable birth cohorts across countries to take advantage of such natural “experiment” for more in-depth assessments of dose–response relationships, threshold exposure levels, and positive and negative effect modifiers.
Conclusion
Large-scale birth cohort studies with long-term follow-up provide exciting and unprecedented opportunities to uncover the causes and prevention measures of chronic diseases. However, great opportunities come with great challenges. There are needs for: (1) minimizing participant burden while obtaining sufficient data and specimen in core EC exposures; (2) being very prudent in piggybacking ancillary studies that require additional participant burdens; (3) international collaborative efforts in assembling comparable birth cohorts using similar study designs and implementation protocols. Addressing these concerns may help us to better “manage” such opportunities to produce better and more comprehensive evidence on the health effects of ECs, and to know the potential early intervention measures to reduce the population burdens of many chronic diseases.
Acknowledgments
This work was supported by research grants from the Canadian Institutes of Health Research (CIHR Grant # 79896 – Dr. Luo, CIHR Grant # 81285 – Dr. Fraser). Dr. Luo is supported by a New Investigator Award from the Canadian Institutes of Health Research (CIHR) and a Clinical Epidemiology Junior Scholar Award from the Fonds de la Recherche en Santé du Québec (FRSQ), and Dr. Fraser by a CIHR Canada Research Chair award.
Footnotes
Conflicts of interest statement
None declared.
Authors’ contributions
All authors contributed to formulating the theoretic framework, and offered critical comments to improve successive drafts of the manuscript. Z.C. Luo was responsible for drafting and finalizing the manuscript.
References
- 1.Ettinger AS, Hu H, Hernandez-Avila M. Dietary calcium supplementation to lower blood lead levels in pregnancy and lactation. J Nutr Biochem. 2007;18:172–8. doi: 10.1016/j.jnutbio.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemicals-in-Commerce Information System. Chemical Update System (database online) Washington, DC: US Environmental Protection Agency; 1998. [Google Scholar]
- 3.Gobas FA, de WW, Burkhard LP, Verbruggen EM, Plotzke K. Revisiting bioaccumulation criteria for POPs and PBT assessments. Integr Environ Assess Manage. 2009:1. doi: 10.1897/IEAM_2008-089.1. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. 2004 Surgeon General’s report – the health consequences of smoking. Centers for disease control and prevention, National center for chronic disease prevention and health promotion, office on smoking and health; 2004. [Google Scholar]
- 5.Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol. 2008;59(Suppl 6):19–34. [PubMed] [Google Scholar]
- 6.Farmer PB, Singh R, Kaur B, Sram RJ, Binkova B, Kalina I, et al. Molecular epidemiology studies of carcinogenic environmental pollutants. Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage. Mutat Res. 2003;544:397–402. doi: 10.1016/j.mrrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Thurston GD. A critical review of PM10-mortality time-series studies. J Expo Anal Environ Epidemiol. 1996;6:3–21. [PubMed] [Google Scholar]
- 8.Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 9.Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure – a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- 10.Murata K, Grandjean P, Dakeishi M. Neurophysiological evidence of methylmercury neurotoxicity. Am J Ind Med. 2007;50:765–71. doi: 10.1002/ajim.20471. [DOI] [PubMed] [Google Scholar]
- 11.Prasher D. Heavy metals and noise exposure: health effects. Noise Health. 2009;11:141–4. doi: 10.4103/1463-1741.53358. [DOI] [PubMed] [Google Scholar]
- 12.Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB. Mobilization of lead from human bone tissue during pregnancy and lactation – a summary of long-term research. Sci Total Environ. 2003;303:79–104. doi: 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 13.Rogan WJ, Chen A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT) Lancet. 2005;366:763–73. doi: 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- 14.Weselak M, Arbuckle TE, Foster W. Pesticide exposures and developmental outcomes: the epidemiological evidence. J Toxicol Environ Health B Crit Rev. 2007;10:41–80. doi: 10.1080/10937400601034571. [DOI] [PubMed] [Google Scholar]
- 15.Boersma ER, Lanting CI. Environmental exposure to polychlorinated biphenyls (PCBs) and dioxins – consequences for longterm neurological and cognitive development of the child lactation. Adv Exp Med Biol. 2000;478:271–87. [PubMed] [Google Scholar]
- 16.Faroon O, Jones D, de RC. Effects of polychlorinated biphenyls on the nervous system. Toxicol Ind Health. 2001;16:305–33. doi: 10.1177/074823370001600708. [DOI] [PubMed] [Google Scholar]
- 17.Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol. 1996;18:217–27. doi: 10.1016/s0892-0362(96)90001-x. [DOI] [PubMed] [Google Scholar]
- 18.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–76. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoeller RT. Environmental chemicals impacting the thyroid: targets and consequences. Thyroid. 2007;17:811–7. doi: 10.1089/thy.2007.0107. [DOI] [PubMed] [Google Scholar]
- 20.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–84. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev. 2009;12:157–74. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- 22.Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–83. [PubMed] [Google Scholar]
- 23.McKenna MT, Michaud CM, Murray CJ, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28:415–23. doi: 10.1016/j.amepre.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 26.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Téllez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-García A, Schnaas-Arrieta L, et al. Longitudinal associations between blood lead concentrations lower than 10 μg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118:e323–30. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- 28.Jedrychowski W, Perera FP, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, et al. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: Krakow prospective cohort study. Neuroepidemiology. 2009;32:270–8. doi: 10.1159/000203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eskenazi B, Gladstone EA, Berkowitz GS, Drew CH, Faustman EM, Holland NT, et al. Methodologic and logistic issues in conducting longitudinal birth cohort studies: lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113:1419–29. doi: 10.1289/ehp.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer MS, Wilkins R, Goulet L, Seguin L, Lydon J, Kahn SR, et al. Investigating socio-economic disparities in preterm birth: evidence for selective study participation and selection bias. Paediatr Perinat Epidemiol. 2009;23:301–9. doi: 10.1111/j.1365-3016.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 31.Golding J, Pembrey M, Jones R. ALSPAC – the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 32.Jaddoe VW, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Verhulst FC, et al. The generation R study: design and cohort profile. Eur J Epidemiol. 2006;21:475–84. doi: 10.1007/s10654-006-9022-0. [DOI] [PubMed] [Google Scholar]
- 33.Jaddoe VW, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EA, et al. The generation R study: design and cohort update until the age of 4 years. Eur J Epidemiol. 2008;23:801–11. doi: 10.1007/s10654-008-9309-4. [DOI] [PubMed] [Google Scholar]
- 34.Olsen J, Melbye M, Olsen SF, Sørensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort – its background, structure and aim. Scand J Public Health. 2001;29:300–7. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 35.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian mother and child cohort study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 36.Rønningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, et al. The biobank of the Norwegian mother and child cohort study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–25. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landrigan PJ, Trasande L, Thorpe LE, Gwynn C, Lioy PJ, D’Alton ME, et al. The National children’s study: a 21-year prospective study of 100,000 American children. Pediatrics. 2006;118:2173–86. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]
- 38.Fraser WD, Arbuckle TE, Muckle G, Séguin J, Ayotte P, Julien P, Luo ZC, Ettinger AS, Mitchell G, Platt R, et al. Maternal and Infant Research on environmental Contaminants (MIREC) 2007–2012. Funded by Canadian Institutes of Health Research (CIHR) and Health Canada. [Google Scholar]
- 39.Fraser WD, Luo ZC, Muckle G, Jacquetta T, Séguin J, Moutquin JM, Michaud J, Julien P, Monnier P, Pasquier JC, Nuyt AM, Chaillet N, et al. Integrated Research Network in Perinatology of Quebec (IRNPQ) 2008–2013. Funded by Canadian Institutes of Health Research (CIHR) [Google Scholar]
- 40.Meyer PA, Pivetz T, Dignam TA, Homa DM, Schoonover J, Brody D. Surveillance for elevated blood lead levels among children – United States, 1997–2001. MMWR Surveill Summ. 2003;52:1–21. [PubMed] [Google Scholar]
- 41.Wang S, Zhang J. Blood lead levels in children, China. Environ Res. 2006;101:412–8. doi: 10.1016/j.envres.2005.11.007. [DOI] [PubMed] [Google Scholar]