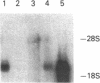
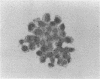
Abstract
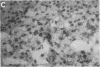
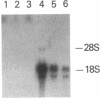
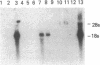
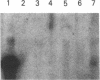
We used a recombinant cDNA probe for human chromogranin A to measure the expression of mRNA encoded by this gene in a variety of normal human tissues and tumor specimens using Northern blot and in situ hybridization analysis. With few exceptions, the expression of chromogranin A mRNA appears to be restricted to normal tissues and tumors of neuroendocrine lineage. However, we have detected mRNA expression of this gene in 1 of 14 cell lines and 2 of 13 tumor specimens of colon adenocarcinoma. The finding of chromogranin A expression in some colon carcinomas suggests that a previously unrecognized subgroup of these tumors has neuroendocrine features. The detection of this subgroup demonstrates the potential for improving tumor classification through the use of techniques and reagents developed by recombinant DNA technology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- DeStephano D. B., Lloyd R. V., Pike A. M., Wilson B. S. Pituitary adenomas. An immunohistochemical study of hormone production and chromogranin localization. Am J Pathol. 1984 Sep;116(3):464–472. [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr S. U., Kumarasamy R., Dean W. L., Cohn D. V. New suggestions for the physiological role of secretory protein-I. Bone Miner. 1987 Jul;2(4):251–255. [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Helman L. J., Thiele C. J., Linehan W. M., Nelkin B. D., Baylin S. B., Israel M. A. Molecular markers of neuroendocrine development and evidence of environmental regulation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2336–2339. doi: 10.1073/pnas.84.8.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Arnold A., Bakhshi A., Ravetch J. V., Siebenlist U., Hieter P. A., Sharrow S. O., LeBien T. W., Kersey J. H., Poplack D. G. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J Clin Invest. 1983 Feb;71(2):301–313. doi: 10.1172/JCI110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Mervak T., Schmidt K., Warner T. F., Wilson B. S. Immunohistochemical detection of chromogranin and neuron-specific enolase in pancreatic endocrine neoplasms. Am J Surg Pathol. 1984 Aug;8(8):607–614. doi: 10.1097/00000478-198408000-00004. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Wilson B. S. Specific endocrine tissue marker defined by a monoclonal antibody. Science. 1983 Nov 11;222(4624):628–630. doi: 10.1126/science.6635661. [DOI] [PubMed] [Google Scholar]
- Maples J. A. A method for the covalent attachment of cells to glass slides for use in immunohistochemical assays. Am J Clin Pathol. 1985 Mar;83(3):356–363. doi: 10.1093/ajcp/83.3.356. [DOI] [PubMed] [Google Scholar]
- Moertel C. G. Treatment of the carcinoid tumor and the malignant carcinoid syndrome. J Clin Oncol. 1983 Nov;1(11):727–740. doi: 10.1200/JCO.1983.1.11.727. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Korsmeyer S. J., Anderson K. C., Boyd A. W., Slaughenhoupt B., Park E., Jensen J., Coral F., Mayer R. J., Sallan S. E. B cell origin of non-T cell acute lymphoblastic leukemia. A model for discrete stages of neoplastic and normal pre-B cell differentiation. J Clin Invest. 1984 Aug;74(2):332–340. doi: 10.1172/JCI111428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Immunoreactive human chromogranin A in diverse polypeptide hormone producing human tumors and normal endocrine tissues. J Clin Endocrinol Metab. 1983 Nov;57(5):1084–1086. doi: 10.1210/jcem-57-5-1084. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Deftos L. J. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986 May 1;314(18):1145–1151. doi: 10.1056/NEJM198605013141803. [DOI] [PubMed] [Google Scholar]
- Park J. G., Oie H. K., Sugarbaker P. H., Henslee J. G., Chen T. R., Johnson B. E., Gazdar A. Characteristics of cell lines established from human colorectal carcinoma. Cancer Res. 1987 Dec 15;47(24 Pt 1):6710–6718. [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Schwab M., Ellison J., Busch M., Rosenau W., Varmus H. E., Bishop J. M. Enhanced expression of the human gene N-myc consequent to amplification of DNA may contribute to malignant progression of neuroblastoma. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4940–4944. doi: 10.1073/pnas.81.15.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. M., Orenstein J. M. Small-cell undifferentiated carcinoma of the rectosigmoid colon. Arch Pathol Lab Med. 1985 Jul;109(7):629–632. [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Sobol R. E., O'Connor D. T., Addison J., Suchocki K., Royston I., Deftos L. J. Elevated serum chromogranin A concentrations in small-cell lung carcinoma. Ann Intern Med. 1986 Nov;105(5):698–700. doi: 10.7326/0003-4819-105-5-698. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]