Abstract
Background
Omega-3 fatty acid (O3FA) levels and dimensional personality measures have been associated with major depression and the course of depressive illness. We sought to study the utility of O3FA levels and dimensional personality measures as predictors of early improvement with escitalopram.
Methods
Twenty-four participants were enrolled in an open-label trial of escitalopram 10 mg/d for 4 weeks. Baseline erythrocyte O3 levels and dimensional personal assessments were obtained.
Results
Using a conservative, intention-to-treat analysis, baseline neuroticism (r = −0.57; P = .007), as measured by the Revised NEO Personality Inventory (NEO-PI-R) but not erythrocyte O3 levels, was correlated with improvements on escitalopram. A facet analysis of the neuroticism domain showed the relationship with antidepressant response to be focused on trait anxiety (r = −0.65; P = .002).
Conclusions
Anxiety may have important prognostic implications on subsequent response to selective serotonin reuptake inhibitors, such as escitalopram.
Keywords: omega-3 fatty acids, anxiety, major depression, neuroticism, antidepressant
Introduction
Although selective serotonin reuptake inhibitor (SSRI) antidepressants have been in wide use for more than 2 decades, there are no well-replicated and clinically practical predictors of response, other than overall chronicity and severity. In this article, we assess the potential of 2 measures—one based on erythrocyte omega-3 (O3) concentrations and the second on a self-report personality measure.
Growing evidence suggests a role of O3FA in major depression, although O3 values have not been assessed as predictors of antidepressant response. Ecologic studies demonstrated an inverse relationship between dietary O3 consumption and major depression prevalence.1 Case-control and cross-sectional studies have further associated low serum or erythrocyte O3 levels with depression.2–7 One large Finnish study failed to replicate these findings, although the average dietary O3 intake for the sample was quite high, at an estimated 2.2 g/d.8 Most,9–13 though not all,14–17 randomized control data have shown a benefit of O3FA in major depression. A meta-analysis of 8 studies using at least 1 g/d of essential fatty acids for mood disorders favored a benefit of O3.18 Another meta-analysis of 10 studies without dose criteria reached similar conclusions.19 However, a funnel plot of available data suggests the possibility of publication bias, with smaller, negative studies underrepresented in the literature.20
There has also been limited study of the well-established 5-factor model of personality on antidepressant response. Low levels of neuroticism have been found to favor antidepressant response in some21–24 but not all studies.25–28 Despite the general association between neuroticism—variously defined—and poor outcome in depression,29–31 the 3 studies using the well-validated 5-factor model in major depression did not support an association with antidepressant response.25,27,28 It has been suggested that neuroticism decreases with treatment response27,32,33 and may even mediate treatment response.34 Neuroticism also appears to reduce response to prophylactic lithium in mood disorders.35 Findings for factors beyond neuroticism have been even less consistent: Better outcomes were associated with high agreeableness in one study27 and with extraversion in another.28
With growing evidence for a role of O3FA in depression, we designed a protocol to determine whether erythrocyte measures of fatty acid membrane composition predict symptom improvement during treatment of major depressive disorder (MDD) with the SSRI escitalopram. We hypothesized that patients with high O3, specifically eicosapentaenoic acid (EPA), levels would be more likely to respond to antidepressants. We secondarily sought to determine if dimensional personality variables would predict antidepressant response, and hypothesized that neuroticism would be associated with an attenuated response.
Methods
The study consisted of a 2-phase clinical trial of O3FA for prospectively defined escitalopram nonresponders. This article presents findings from phase 1—a 4-week, open-label trial of escitalopram for major depression. All study procedures were reviewed and approved by the University of Iowa Institutional Review Board.
Participants, age 18 to 55 with a current diagnosis of MDD, were recruited by advertisements, brochures, and clinician referrals. Diagnosis was confirmed by a clinician diagnostic interview and the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I). Exclusion criteria included substance abuse in the past month or dependence in the past year, an eating disorder in the past year, an allergy to fish, a bleeding disorder, plans to initiate psychotherapy during the study, pregnancy, and taking medications known to produce affective symptoms. Patients were further excluded if they were taking warfarin or had taken O3FA supplements or antidepressants for 3 or more days in the past 4 months. Those taking a stable dose of the same antidepressant agent for at least 4 weeks were allowed entry in the study, but escitalopram was substituted. Any participant with a history of nonresponse to escitalopram or with more than 2 failed adequate antidepressant trials during the current episode was also excluded.
Participants were treated with open-label escitalopram, 10 mg/d at a fixed dose for 4 weeks. At intake, participants had a blood draw for erythrocyte O3 levels, and a dimensional assessment of personality was made with the Revised NEO Personality Inventory (NEO-PI-R),36 with scoring upon individual completion of the study. NEO-PI-R ratings were obtained by self-report to provide a dimensional assessment of 5 major domains of personality—neuroticism, extraversion, openness, agreeableness, and conscientiousness—with additional measures of the facets or traits that define each domain.37 One investigator (J.G.F.) performed mood ratings using the Montgomery-Åsberg Depression Rating Scale (MADRS),38 administered weekly and by telephone in weeks 1 and 3,39,40 as the primary outcome measure, with response defined as the total change in MADRS score.
Laboratory analysis
Fatty acid composition was determined in washed erythrocytes isolated from venous blood drawn into chilled polypropylene tubes containing EDTA (ethylenediaminetetraacetic acid) as the anticoagulant. Lipids were extracted with an isopropanol-chloroform mixture.41,42 Following addition of butylated hydroxytoluene as an antioxidant, the lipids were dried under nitrogen, margaric acid was added as an internal standard, and the fatty acids were hydrolyzed by saponification. After the nonsaponifiable lipid was removed, the isolated fatty acids were dried, methylated,43 taken up in carbon disulfide, and analyzed by gas-liquid chromatography.44,45 The erythrocyte fatty acids were identified from the retention times of fatty acid methyl esters standard mixtures (Supelco Inc., Bellefonte, PA) and the weight percentage of each fatty acid calculated. The average from samples obtained at baseline and week 4 was used in subsequent analyses.
Data analysis
Data were analyzed using a modified intention-to-treat (ITT) analysis, for which all participants with available data and at least 1 follow-up assessment were included and the last observation carried forward. For our primary aim, the relationship between improvements in MADRS score and fatty acids was assessed using Pearson correlations. Fatty acids of interest included total O3, EPA, docosahexaenoic acid (DHA), total omega-6 (O6), and arachidonic acid (AA). For the secondary aims, Pearson correlations assessed the relationship between change in MADRS and each of the 5 dimensional personality domains of the NEO-PI-R at baseline, using raw scores. If a significant association was observed for a domain, correlations with change in MADRS for facets of that domain were explored. Control for multiple comparisons with primary and secondary aims used Holm's sequential Bonferroni method to maintain experimental type I error rates at a desired 2-tailed α = .05. The assumptions of the ITT analysis were tested in a follow-up completer analysis.
Results
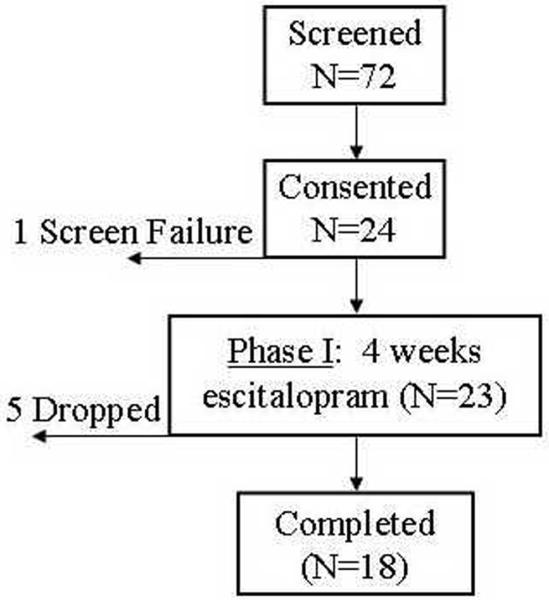
Of 72 participants screened, 24 ultimately qualified and provided consent for participation in the study. There was one screen failure for pregnancy. One participant did not complete any follow-up assessment, and another 4 participants dropped out before phase 1 was complete (Figure 1). One participant did not complete the NEO-PI-R at baseline, and another did not have an erythrocyte fatty acid analysis available.
Figure 1. Flow of participants.
A total of 72 potential participants were screened for eligibility, of whom 24 consented to participate in the study. There was one screen failure for pregnancy. Five participants dropped out before completion of the 4-week trial, 4 of whom had at least one follow-up assessment.
Demographic and clinical characteristics of the sample are detailed in the Table. The majority of participants were female. MADRS scores at baseline ranged from 18 to 43, with a mean score of 29, consistent with moderate depression. Of the 22 participants with at least one follow-up assessment, the mean (SD) improvement in MADRS was 11 (9). The mean (SD) MADRS at last study visit was 18 (9) and the response rate, defined as a 50% reduction in symptoms, was 27%. Based on self-report on intake history, participants did not respond to a mean of 1.0 prior antidepressant medications in their lifetime. Only 2 participants had 3 prior unsuccessful trials lifetime, and each had previously responded to one antidepressant.
Table.
Demographic and clinical data of participants beginning phase 1 of the study (n = 23)*
| Patient characteristic | Mean (SD) |
|---|---|
| Age | 31 (11) |
|
| |
| Years of education | 14 (2) |
|
| |
| MADRS | 29a (9) |
|
| |
| No. (%) | |
|
| |
| Gender | |
| Female | 17 (74%) |
|
| |
| Race | |
| African American | 3 (13%) |
| Asian/Pacific Islander | 2 (9%) |
| Caucasian | 18 (78%) |
|
| |
| Ethnicity | |
| Hispanic | 2 (9%) |
|
| |
| Marital status | |
| Married | 11 (48%) |
| Separated/divorced | 5 (22%) |
| Single | 6 (26%) |
| Widowed | 1 (4%) |
|
| |
| Psychiatric history | |
| Prior psychiatric hospitalization | 6 (26%) |
| Prior suicide attempt | 5 (22%) |
| Prior alcohol abuse/dependence | 4 (17%) |
| Prior drug abuse/dependence | 3 (13%) |
MADRS: Montgomery-Åsberg Depression Rating Scale.
Of 24 consenting participants, there was one screen failure (for pregnancy).
Consistent with moderate depression.
Participants had a mean (SD) percent distribution of total O3FA of 6.9% (3.4%), with 0.7% (0.8%) EPA and 3.2% (1.7%) DHA, comparable to reports from large studies of adults in the United States,46 although lower than those reported in Europe and Japan.47–49 Total O6FA was 34.8% (4.6%), with 16.3% (2.3%) AA. Neither total O3 nor EPA levels predicted response to escitalopram. In this modified ITT sample with available data (n = 21), MADRS improvement was not correlated with total O3 (r = −0.12; P = .6), EPA (r = −0.27; P = .2), DHA (r = −0.22; P = .3), O6 (r = −0.22; P = .3), or AA (r = 0.06; P = .8).
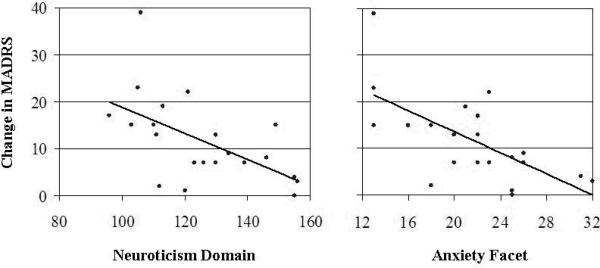
Mean (SD) raw scores for the 5 domains of the NEO-PI-R were 124 (19) for neuroticism, 92 (18) for extraversion, 119 (28) for openness, 121 (16) for agreeableness, and 93 (17) for conscientiousness. Of those with available data in the modified ITT sample (n = 21), neuroticism was inversely correlated with improvement in MADRS over 4 weeks of treatment with escitalopram (r = −0.57; P = .007). NEO-PI-R domains of extraversion, agreeableness, openness, and conscientiousness did not appear to be related to change in MADRS. Analysis of neuroticism facets revealed a statistically significant association only for the anxiety facet (r = −0.65; P = .002), not the angry hostility, depression, self-consciousness, impulsiveness, or vulnerability facets. This relationship between the broad domain of neuroticism and the focused facet of anxiety with subsequent changes in MADRS are illustrated in Figure 2. Similar, statistically significant results were seen in a follow-up completer analysis (n = 17).
Figure 2. Relationship between neuroticism and antidepressant response.
MADRS: Montgomery-Åsberg Depression Rating Scale; NEO-PI-R: Revised NEO Personality Inventory.
As evident in the scatterplot (left), the domain of neuroticism was highly correlated with change in the MADRS while on escitalopram 10 mg/d (r = −0.57; P = .007). This association was further confined to the neuroticism facet of anxiety (r = −0.65; P = .002), as illustrated in the adjacent scatterplot (right). Raw scores on the NEO-PI-R were used for domains and facets.
Discussion
Our results demonstrate a significantly negative relationship between neuroticism—more specifically, anxiety—with subsequent response to a 4-week course of escitalopram. Although the strong correlation may be spurious, this finding persisted on a follow-up completer analysis. There is also some, albeit inconsistent, support in the literature for this finding. Contrary to our initial hypothesis, O3FA levels did not predict early antidepressant response; in fact, a trend was observed in the opposite direction in this small sample. One could alternatively hypothesize that, given the associations between DHA depletion and low serotonin levels,50 those with lower O3FA levels may be more likely to respond to treatment with escitalopram.
Studies of personality and antidepressant response have produced varied results and have had substantial methodological variation, including that related to the measurement of neuroticism. Only 2 studies have assessed neuroticism using the NEO-PI, a more comprehensive and contemporary assessment.21,28 Of these studies, Bagby et al allowed treatment with any antidepressants over 5 weeks; they found extraversion but not neuroticism to predict response, although neuroticism was correlated with depression indices at the onset and conclusion of the study, and analyses of facets as predictors were not reported.28 Tanum et al used mianserin for the treatment of functional gastrointestinal disorders, not depression, and found that those whose pain did not remit had higher baseline neuroticism raw scores. Neither study used the current revision of the NEO, the NEO-PI-R. Two studies using the abbreviated NEO Five-Factor Inventory (NEO-FFI) found no differences in neuroticism indices between responders and nonresponders.25,27 The NEO-FFI provides only information on personality domains and does not allow a more refined facet analysis, as performed in our follow-up analysis. Other studies have used clinician assessment or the Eysenck Personality Inventory, in which neuroticism is less reliably or more broadly defined.23,24,26,51
Our refined analysis demonstrated the strongest association for the anxiety facet within the domain of neuroticism. The NEO-PI-R is a self-report measure, and questions for anxiety include—though are not limited to—“I am not a worrier” (reverse scored), “I am easily frightened,” and “I often feel tense and jittery.” Anxiety as measured by the self-report NEO-PI-R is a personality trait, which may be amplified in the setting of major depression.32 The apparent focus of our finding on a more narrowly defined facet within neuroticism may explain the discrepancy on prior findings. Trait anxiety or an anxious temperament has long been suggested as a predisposing factor for depression,52,53 although it has not been studied for antidepressant response as conceptualized by the NEO-PI-R. Recent reports from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial suggest that those with a categorically defined anxious depression differ in terms of baseline characteristics and are less likely to remit on antidepressants.54,55 Based on these findings, Fava et al concluded that anxious depression may be a valid diagnostic subtype of MDD.55 Extending these categorical conclusions, our findings suggest that a dimensional measure of anxiety may further have predictive validity.
Recent studies have suggested an association between serotonin receptor binding and neuroticism,56,57 and variants of the serotonin transporter gene have been associated both with neuroticism and anxiety-related traits.58,59 The lower functioning variants of the promoter for the serotonin transporter gene, reduced to the short allele in most studies, have further been linked to reduced antidepressant response.60 Our findings may imitate such variations in serotoninergic function or, more likely, reflect even more complex genotype or gene-environment interactions. Mechanistic considerations aside, these findings, if replicated, may be clinically useful.
There are several important limitations of this study. This sample size limits statistical power, although a statistically significant finding was identified. The magnitude of the observed effect is admittedly greater than would be expected and may be exaggerated in this small sample. Our sample included patients who had not been responding to a stable dose of an antidepressant, which may select for nonresponders in the current episode. Exclusion criteria were reported in brochures and to referring sources, so our enrollment rate following screening may overestimate the true generalizability of this finding to populations with major depression. Although our trial used recommended starting doses for escitalopram, rapid titration of serotonergic antidepressants may be associated with worsening anxiety, particularly in anxiety-prone individuals. This phenomenon has been referred to as the “jitteriness syndrome”61 and could perhaps have attenuated clinical improvement. A relatively low dose of escitalopram was used in this study, and some clinicians may have titrated more quickly to higher doses, particularly for those patients not previously responding to a stable dose of an antidepressant. However, this approach does not improve response rates,62 and given the elevated risk of worsening anxiety with rapid titration of antidepressants in anxiety-prone individuals, a more aggressive titration would have been unlikely to weaken the association observed. Patients on prior antidepressants were directly transitioned to escitalopram, which could result in a discontinuation syndrome, although no such symptoms were observed. Strengths of our study include our use of a rigorous erythrocyte O3FA analysis, use of a fixed-dose of a single antidepressant over a consistent time period, and use of the comprehensive and contemporary NEO-PI-R. Our use of continuous variables in the analysis further improved statistical power and mitigated the risk of our findings simply reflecting an artifact of dichotomization.
Conclusions
Although our analyses failed to support a relationship between higher O3FA levels and improvements with escitalopram, trait anxiety was revealed as a potential predictor of early improvement, with important prognostic implications regarding subsequent response to SSRIs, such as escitalopram. Psychological predictors of response have long been suspected by clinicians and could have considerable utility, although research has failed to deliver reliable psychological predictors. A larger, confirmatory study focusing on refined neuroticism facets is needed to confirm this finding, which must be considered preliminary.
Acknowledgements
The authors thank Shawn D. Harmon and Terry L. Kaduce for their technical assistance.
Disclosures Dr. Fiedorowicz is supported by L30 MH075180-02, 5KL2 RR024980, the Nellie Ball Trust Research Fund, and a NARSAD Young Investigator Award. He has also received research support for participating in a colleague's investigator-initiated study with Eli Lilly.
Footnotes
The authors have no potential conflicts of interest, financial or otherwise.
References
- 1.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 2.Tanskanen A, Hibbeln JR, Tuomilehto J, et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 3.Peet M, Murphy B, Shay J, et al. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, Christophe A, Delanghe J, et al. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 5.Silvers KM, Scott KM. Fish consumption and self-reported physical and mental health status. Public Health Nutr. 2002;5:427–431. doi: 10.1079/phn2001308. [DOI] [PubMed] [Google Scholar]
- 6.Adams PB, Lawson S, Sanigorski A, et al. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(suppl):S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 7.Edwards R, Peet M, Shay J, et al. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 8.Hakkarainen R, Partonen T, Haukka J, et al. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry. 2004;161:567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- 9.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 10.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 11.Su KP, Huang SY, Chiu CC, et al. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13:267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 12.Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 13.Nemets H, Nemets B, Apter A, et al. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 14.Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1393–1396. doi: 10.1016/j.pnpbp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Marangell LB, Martinez JM, Zboyan HA, et al. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 16.Silvers KM, Woolley CC, Hamilton FC, et al. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72:211–218. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99:421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 18.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 19.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 20.Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am J Clin Nutr. 2006;84:1308–1316. doi: 10.1093/ajcn/84.6.1308. [DOI] [PubMed] [Google Scholar]
- 21.Tanum L, Malt UF. Personality traits predict treatment outcome with an antidepressant in patients with functional gastrointestinal disorder. Scand J Gastroenterol. 2000;35:935–941. doi: 10.1080/003655200750022986. [DOI] [PubMed] [Google Scholar]
- 22.Berlanga C, Heinze G, Torres M, et al. Personality and clinical predictors of recurrence of depression. Psychiatr Serv. 1999;50:376–380. doi: 10.1176/ps.50.3.376. [DOI] [PubMed] [Google Scholar]
- 23.Davidson J, Lipper S, Pelton S, et al. The response of depressed inpatients to isocarboxazid. J Clin Psychopharmacol. 1988;8:100–107. [PubMed] [Google Scholar]
- 24.Boyce P, Parker G. Neuroticism as a predictor of outcome in depression. J Nerv Ment Dis. 1985;173:685–688. doi: 10.1097/00005053-198511000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Petersen T, Papakostas GI, Bottonari K, et al. NEO-FFI factor scores as predictors of clinical response to fluoxetine in depressed outpatients. Psychiatry Res. 2002;109:9–16. doi: 10.1016/s0165-1781(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 26.Joyce PR, Paykel ES. Predictors of drug response in depression. Arch Gen Psychiatry. 1989;46:89–99. doi: 10.1001/archpsyc.1989.01810010091014. [DOI] [PubMed] [Google Scholar]
- 27.Du L, Bakish D, Ravindran AV, et al. Does fluoxetine influence major depression by modifying five-factor personality traits? J Affect Disord. 2002;71:235–241. doi: 10.1016/s0165-0327(01)00370-6. [DOI] [PubMed] [Google Scholar]
- 28.Bagby RM, Joffe RT, Parker JDA, et al. Major depression and the five-factor model of personality. J Pers Disord. 1995;9:224–234. [Google Scholar]
- 29.Weissman MM, Prusoff BA, Klerman GL. Personality and the prediction of long-term outcome of depression. Am J Psychiatry. 1978;135:797–800. doi: 10.1176/ajp.135.7.797. [DOI] [PubMed] [Google Scholar]
- 30.Hirschfeld RM, Klerman GL, Andreasen NC, et al. Psycho-social predictors of chronicity in depressed patients. Br J Psychiatry. 1986;148:648–654. doi: 10.1192/bjp.148.6.648. [DOI] [PubMed] [Google Scholar]
- 31.Quilty LC, De Fruyt F, Rolland JP, et al. Dimensional personality traits and treatment outcome in patients with major depressive disorder. J Affect Disord. 2008;108:241–250. doi: 10.1016/j.jad.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Costa PT, Jr, Bagby RM, Herbst JH, et al. Personality self-reports are concurrently reliable and valid during acute depressive episodes. J Affect Disord. 2005;89:45–55. doi: 10.1016/j.jad.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Bagby RM, Levitan RD, Kennedy SH, et al. Selective alteration of personality in response to noradrenergic and serotonergic antidepressant medication in depressed sample: evidence of non-specificity. Psychiatry Res. 1999;86:211–216. doi: 10.1016/s0165-1781(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 34.Quilty LC, Meusel LA, Bagby RM. Neuroticism as a mediator of treatment response to SSRIs in major depressive disorder. J Affect Disord. 2008;111:67–73. doi: 10.1016/j.jad.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35:1685–1694. doi: 10.1017/S0033291705004484. [DOI] [PubMed] [Google Scholar]
- 36.Costa PT, Jr, McCrae RR. Domains and facets: hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess. 1995;64:21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- 37.Costa PT, Jr, McCrae RR. NEO PI-R professional manual. Psychological Assessment Resources, Inc.; Lutz, FL: 1992. [Google Scholar]
- 38.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 39.Kobak KA, Williams JB, Jeglic E, et al. Face-to-face versus remote administration of the Montgomery-Asberg Depression Rating Scale using videoconference and telephone. Depress Anxiety. 2008;25:913–919. doi: 10.1002/da.20392. [DOI] [PubMed] [Google Scholar]
- 40.Hermens ML, Adèr HJ, van Hout HP, et al. Administering the MADRS by telephone or face-to-face: a validity study. Ann Gen Psychiatry. 2006;5:3. doi: 10.1186/1744-859X-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res. 1965;6:428–431. [PubMed] [Google Scholar]
- 42.Spector AA, Ashbrook JD, Santos EC, et al. Quantitative analysis of uptake of free fatty acid by mammalian cells: lauric acid and human erythrocytes. J Lipid Res. 1972;13:445–451. [PubMed] [Google Scholar]
- 43.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride--methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 44.Martin DD, Robbins ME, Spector AA, et al. The fatty acid composition of human gliomas differs from that found in nonmalignant brain tissue. Lipids. 1996;31:1283–1288. doi: 10.1007/BF02587914. [DOI] [PubMed] [Google Scholar]
- 45.Williard DE, Harmon SD, Kaduce TL, et al. Docosahexaenoic acid synthesis from n-3 polyunsaturated fatty acids in differentiated rat brain astrocytes. J Lipid Res. 2001;42:1368–1376. [PubMed] [Google Scholar]
- 46.Block RC, Harris WS, Reid KJ, et al. Omega-6 and trans fatty acids in blood cell membranes: a risk factor for acute coronary syndromes? Am Heart J. 2008;156:1117–1123. doi: 10.1016/j.ahj.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wennberg M, Bergdahl IA, Stegmayr B, et al. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr. 2007;98:1038–1045. doi: 10.1017/S0007114507756519. [DOI] [PubMed] [Google Scholar]
- 48.Broadfield EC, McKeever TM, Whitehurst A, et al. A case-control study of dietary and erythrocyte membrane fatty acids in asthma. Clin Exp Allergy. 2004;34:1232–1236. doi: 10.1111/j.1365-2222.2004.02032.x. [DOI] [PubMed] [Google Scholar]
- 49.Iso H, Sato S, Umemura U, et al. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]
- 50.Levant B, Ozias MK, Davis PF, et al. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berlanga C, Heinze G, Torres M, et al. Personality and clinical predictors of recurrence of depression. Psychiatr Serv. 1999;50:376–380. doi: 10.1176/ps.50.3.376. [DOI] [PubMed] [Google Scholar]
- 52.Akiskal HS. Toward a definition of generalized anxiety disorder as an anxious temperament type. Acta Psychiatr Scand Suppl. 1998;393:66–73. doi: 10.1111/j.1600-0447.1998.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 53.Bruijn JA, Moleman P, van den Broek WW, et al. Trait anxiety and the effect of a single high dose of diazepam in unipolar depression. J Psychiatr Res. 2001;35:331–337. doi: 10.1016/s0022-3956(01)00035-8. [DOI] [PubMed] [Google Scholar]
- 54.Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 55.Fava M, Rush AJ, Alpert JE, et al. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry. 2006;51:823–835. doi: 10.1177/070674370605101304. [DOI] [PubMed] [Google Scholar]
- 56.Frokjaer VG, Mortensen EL, Nielsen FA, et al. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry. 2008;63:569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Takano A, Arakawa R, Hayashi M, et al. Relationship between neuroticism personality trait and serotonin transporter binding. Biol Psychiatry. 2007;62:588–592. doi: 10.1016/j.biopsych.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Fiedorowicz JG, Moser DJ, Hynes SM, Beglinger LJ, Schultz SK, Ellingrod VL. LA allelic heterozygosity of the 5HTTLPR polymorphism is associated with higher cognitive function and lower interpersonal sensitivity. Psychiatr Genet. 2007;17:3–4. doi: 10.1097/YPG.0b013e328010f498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonda X, Rihmer Z, Zsombok T, et al. The 5HTTLPR polymorphism of the serotonin transporter gene is associated with affective temperaments as measured by TEMPS-A. J Affect Disord. 2006;91:125–131. doi: 10.1016/j.jad.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 60.Smeraldi E, Zanardi R, Benedetti F, et al. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- 61.Pohl R, Yeragani VK, Balon R, et al. The jitteriness syndrome in panic disorder patients treated with antidepressants. J Clin Psychiatry. 1988;49:100–104. [PubMed] [Google Scholar]
- 62.Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63:331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]