Abstract
The integrity of central and peripheral nervous system myelin is affected in numerous lipid metabolism disorders. This vulnerability was so far mostly attributed to the extraordinarily high level of lipid synthesis that is required for the formation of myelin, and to the relative autonomy in lipid synthesis of myelinating glial cells because of blood barriers shielding the nervous system from circulating lipids. Recent insights from analysis of inherited lipid disorders, especially those with prevailing lipid depletion and from mouse models with glia-specific disruption of lipid metabolism, shed new light on this issue. The particular lipid composition of myelin, the transport of lipid-associated myelin proteins, and the necessity for timely assembly of the myelin sheath all contribute to the observed vulnerability of myelin to perturbed lipid metabolism. Furthermore, the uptake of external lipids may also play a role in the formation of myelin membranes. In addition to an improved understanding of basic myelin biology, these data provide a foundation for future therapeutic interventions aiming at preserving glial cell integrity in metabolic disorders.
MYELIN: A GIANT MEMBRANE ORGANELLE WITH SPECIFIC LIPID CHARACTERISTICS
The rapid saltatory conduction of action potentials along axons is crucially dependent on myelination. The myelin membrane is an extended and highly specialized plasma membrane synthesized by myelinating glial cells: oligodendrocytes in the central nervous system (CNS), and Schwann cells in the peripheral nervous system (PNS) (Fig. 1). The wrapping of myelin around an axonal segment increases axonal resistance and enables clustering of axonal ion channels at nodes of Ranvier (1). As such, myelinating glial cells shape the structural and electrical properties of axons resulting in a 10- to 100-fold increase in nerve conduction velocity (2, 3) and in a great reduction of axonal energy consumption (4).
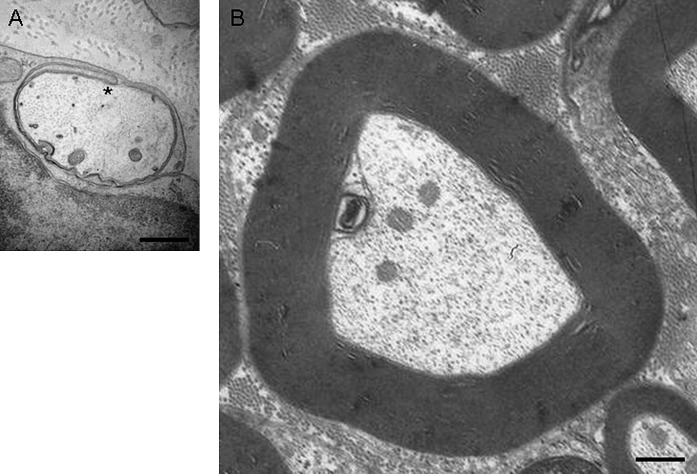
Fig. 1.
Ultrastructural comparison of the membranes of a promyelinating versus a mature myelinating glial cell. Electron micrographs of normal mouse sciatic nerves showing (A) promyelin figure at postnatal day 0 (P0) and (B) adult myelinated fiber (P100). The inner lip (also called inner mesaxon) of the myelinating glial cell, which turns around the axon to form the membranous spiral of myelin, is depicted with an asterisk (*). Scale bar, 0.5 μm. Sciatic nerve isolation and electron microscopy were done as described in (110).
One of the prominent biochemical characteristics that distinguishes myelin from other membranes is its high lipid-to-protein ratio; lipids account for at least 70% of the dry weight of myelin membranes (Table 1), which is also the physical basis for its biochemical purification by sucrose gradient centrifugation (5).
TABLE 1.
Approximate lipid composition of the myelin membrane
| Myelin Membranea | Liver CellPlasma Membraneb | |
|---|---|---|
| Lipid content (dry weight) | 71% | 34% |
| Lipid class | ||
| Cholesterol | 26% | 17% |
| Phospholipids | ||
| PE | 16% | 7% |
| PS | 6% | 4% |
| PC | 12% | 24% |
| PI | 1% | 4 |
| SM | 3% | 20% |
| Glycolipids | 31% | 7% |
| Other lipids | 5% | 17% |
From Norton and Poduslo 1973 (6): myelin of adult rat brain. Comparable lipid amounts are present in myelin of rodent peripheral nerve, with the exception of much higher SM levels (10–35%) (7).
From Dod and Gray, 1968 (164): plasma membrane of adult rat liver. The category ‘other lipids’ contains free fatty acids, triglycerides and cholesterol esters, which could be indicative for contamination by other membranes. Comparable lipid amounts were found by Ray et al., 1968 (165). Bold numbers indicate percentages of lipids enriched in myelin, as discussed in the text.
The myelin membrane contains myelin-specific proteins (e.g., myelin basic protein, myelin-associated glycoprotein, and proteolipid protein), but no truly myelin-specific lipids. Nevertheless, whereas all major lipid classes are present in myelin as in other membranes, myelin has its characteristic lipid composition. The myelin membrane contains a high level of cholesterol of at least 26% by weight (or 52 mol%) (Table 1) (6, 7). The importance of cholesterol for myelin production and maintenance has recently been reviewed (8). The myelin membrane is also substantially enriched in galactolipids (31% vs. 7% for liver cell plasma membranes). Two glycosphingolipids, the monogalactosylsphingolipids cerebroside and sulfatide account for 14%–26% and 2%–7% of myelin lipids, respectively (9–11). When compared with hepatocyte and erythrocyte membranes, myelin membranes also contain a higher proportion of saturated long-chain fatty acids (Fig. 2). Finally, the lipid content of myelin is also enriched in plasmalogens (etherlipids). These glycerophospholipids, defined by a vinyl ether double bond at the sn-1 position, account for 20% of the myelin phospholipid mass (compared with 18% in the average human phospholipid mass). Here, 70% of the total phosphatidylethanolamine in white matter is plasmalogen, in contrast to only 5% in liver (12, 13). These lipid characteristics of myelin, together with the presence of myelin-specific proteins, are likely required for myelin wrapping and/or to confer the specific biophysical properties of myelin as an electrical “insulator”, as will be discussed below.
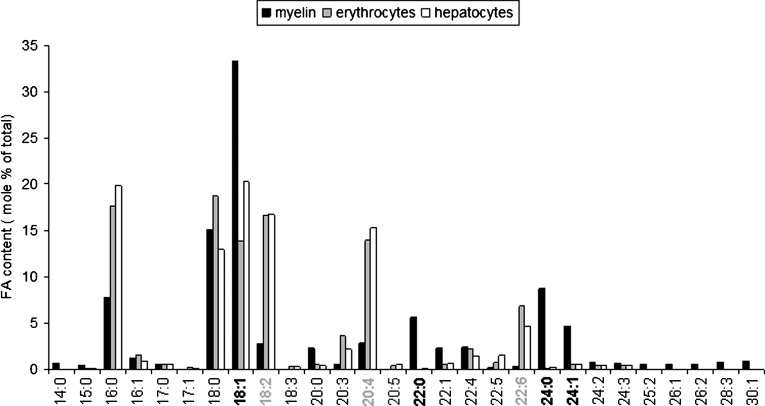
Fig. 2.
Fatty acid composition of myelin compared with other membranes. Depicted is the amount of fatty acid in mol percentage of total amount of fatty acids. Bold numbered and gray numbered lipids depict, respectively, strongly higher or lower levels of these fatty acids in myelin compared with hepatocytes or erythrocyte membranes. Myelin membrane of mouse sciatic nerve were isolated as described in (110, 163). Mouse erythrocyte membranes and hepatocyte phospholipids were isolated and analyzed according to (110, 163). It should be noted that under the isolation conditions used, amide bonds in sphingolipids are relatively stable. Hence, the very long-chain fatty acids found in myelin are not reflecting the high level of galactosphingolipids in myelin, but are more likely to result from a particular fatty acyl composition of the glycerophospholipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidylserine). In addition, with the detection method used, the bar representing 18:1 fatty acids does not include 18:1 alcohol of plasmalogens, which nevertheless was previously detected to be very low in PNS myelin in mouse (110).
During development of the human nervous system, myelination starts in the motor roots of the PNS (fifth fetal month) and is followed by myelination of the spinal cord and brain (CNS). The majority of myelin is assembled during the first two years of postnatal life (14), but myelination continues for 2–3 decades in the human cerebral white matter (15, 16). In rodents, myelination occurs predominantly during the first month of life (17). The magnitude of the tasks that glial cells have to accomplish in a short period of time is easily appreciated when visualizing the membrane expansion taking place during myelination (Fig. 1). In rodents, it has been estimated that the myelin-membrane surface area of one glial cell expands at a rate of 5–50 × 103 μm2/day, compared with the surface area of the cell soma (i.e., the plasma membrane) of ∼ 0.3 × 103 μm2 (18). This corresponds to an estimated 6,500-fold increase in membrane surface between an immature and a fully myelinated oligodendrocyte (19). In rodents, the expansion of the myelin membrane correlates with substantial accumulation of cholesterol and lipids in both the developing CNS and PNS (17, 20–22). More recently, transcriptional profiling of developing peripheral nerves shed light on the molecular cascades involved in PNS myelin assembly (23, 24). It was observed that transcripts encoding structural myelin proteins and enzymes involved in myelin lipid biosynthesis were expressed in a highly synchronized and timely fashion, suggesting a strict control of a balanced and local production of these two key components of myelin assembly. Relatively little is known about myelin lipid turnover in vivo, but studies in mice indicate a necessity to renew myelin lipids in adult life (25).
MYELIN DEFECTS IN INHERITED DISEASES AFFECTING LIPID METABOLISM
The lipid-rich composition of myelin is likely to contribute to the frequent occurrence of myelin defects in lipid metabolism disorders, including hypomyelination (decreased myelin production), dysmyelination (abnormally formed myelin), and demyelination (degenerative loss of myelin). Inherited forms of these diseases are particularly informative and provide direct insight into the underlying molecular mechanisms. These involve defects in metabolism of all myelin enriched lipids: cholesterol, glycosphingolipid, and long-chain fatty acids (Table 2). In several of these disorders the defects are caused by lipotoxicity, a result of the accumulation of various metabolic precursors involved in lipid biosynthesis to levels that are toxic to the myelinating glial cells or at least interfere with normal myelin membrane structure [reviewed in (26–28)]. Here, we will particularly discuss the disorders in which myelin defects are more likely caused by a primary defect in myelinating glia that causes reduced levels of myelin-enriched lipids, and are therefore more informative as to the contribution of these lipids to the synthesis and function of myelin under normal and pathological conditions (see section ‘Why are myelinating glial cells particularly vulnerable to lipid metabolism disorders?’).
TABLE 2.
Inherited lipid disorders with myelin abnormalities
| Presumed Incidence or Number of Patients | General Clinical Features | Myelin Defect |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease | OMIM# | Inheritance | Mutated Gene | Function | Onset | CNS | PNS | Remarks | ||
| Smith-Lemli-Opitz syndrome | 270400 | Autosomal recessive | 1-2:40,000 | sterol delta-7-reductase | cholesterol metabolism | microcephaly mental retardation hypotoniamicrognathia polydactyly ambiguous genitalia cleft palate | mostly within the first month of life (early lethality is common) | ++ | + | - absence or hypoplasia of corpus callosum detected- reduced NCV in PNS (rare) |
| Cerebrotendinous xanthomatosis | 213700 | Autosomal recessive | 1:50,000 (166) | sterol 27-hydroxylase | cholesterol metabolism | tendon xanthomas mental retardation cerebellar ataxia spasticity cataracts | variable (6 to 60 years) | ++ | ++ | - diffuse or focal cerebral and cerebellar white matter disease detected- reduced NCV in PNS |
| Tangier disease | 205400 | Autosomal recessive | ∼100 patients (167) | ATP-binding cassette transporter A1 | cholesterol transport | yellow-orange tonsils splenomegaly hepatomegaly peripheral neuropathy | variable (2 to 67 years) | +++ | - PNS diagnostics: neuromuscular symptoms, mostly normal NCV | |
| Dysmyelinating leukodystrophy and spastic paraparesis with or without dystonia | 612443 | Autosomal recessive | 5 families (58) | fatty acid 2-hydroxylase | sphingolipid metabolism | spasticity gait difficulties dystonia cognitive decline | 4 to 11 years | + | - hyperintensities in parietal and occipital white matter were detected | |
| Metachromatic leukodystrophy | 250100 | Autosomal recessive | 0.6-2,5:100,000 (168, 169) | arylsulfatase A | sphingolipid metabolism | ataxia muscle weakness optic atrophy mental deterioration white matter abnormalities peripheral neuropathy | variable (late infantile to adult onset) | +++ | +++ | - hyperintensities detected in white matter- reduced NCV in PNS |
| Krabbe disease | 245200 | Autosomal recessive | 1:100,000 | galactosylceramidase | sphingolipid metabolism | developmental regression hyperirritability seizuresoptic atrophy | variable, but 90% within first 6 months of life (in this case the lethality before the age of 2 years is common) | +++ | +++ | - diffuse cerebral atrophy detected- reduced NCV in PNS |
| Niemann-Pick disease type A | 257200 | Autosomal recessive | 0.5-1:100,000 (general population)3:100,000 (Ashkenazi Jews) (170) | sphingomyelin phosphodiesterase-1 | sphingolipid metabolism | cherry-red maculae hepatomegaly xanthomasmuscle weakness psychomotor retardation large vacuolated foam cells | early onset (infancy)lethality before the age of 3 years is common | +++ | + | - reduced NCV in PNS |
| Sjogren-Larsson syndrome | 270200 | Autosomal recessive | 0.6:100,000in Sweden (171) | fatty aldehyde dehydrogenase | fatty acid metabolism | spasticity mental retardation macular degeneration short stature enamel hypoplasia | neurologic symptoms usually develop within 2 years after birth | ++ | - hyperintensities detected in white matter | |
| Peroxisome biogenesis disorder | 214100 | Autosomal recessive | 1:100,000in USA (172) | peroxin 1 to 12 | fatty acid metabolism plasmalogen synthesis | facial dysmorphism severe mental retardation hypotonia seizures hyporeflexia/areflexiahepatomegaly | lethality before the age of 1 year is common | +++ | + | - colpocephaly and mild impairment of myelination detected |
| Refsum disease | 266500 | Autosomal recessive | unknown | phytanoyl-CoA hydroxylase | fatty acid metabolism | retinitis pigmentosaperipheral neuropathycerebellar ataxia deafness | majority 20 to 30 years | + | ++ | - white matter changes detected- reduced NCV in PNS |
| Adrenoleukodystrophy | 300100 | X-linked | 1:42,000 (173) | ATP-binding cassette transporter D1 | fatty acid metabolism | adrenal insufficiency neurodegeneration blindness hearing lossspastic paraplegiaparaparesis | 7 to 20 years | +++ | + | - white matter changes detected |
| Canavan disease | 271900 | Autosomal recessive | generally rare1:13,000 inAshkenazi Jews (174) | aspartoacylase | lipogenesis | atonia of neck muscles hypotoniaseizures blindnesssevere mental defect | 2 to 4 months (death within first decade) | +++ | - white matter changes detected | |
| Pyruvate carboxylase deficiency | 266150 | Autosomal recessive | 1:250,000 (175) | pyruvate carboxylase gene | lipogenesis | hepatomegaly mental retardation psychomotor retardation lactic acidemia | Onset at birth or in early infancy | +++ | - white matter changes detected | |
Information provided is based on OMIM database (www.ncbi.nlm.nih.gov/omim/), additional references on presumed incidence, and references on myelin defects as provided in the text.
Cholesterol disorders
At least three different cholesterol-related disorders cause defects in myelin (Table 2). Of these, Cerebrotendinous xanthomatosis (CTX) and Tangier disease (TD) lead to accumulation of 7α-hydroxy-4-cholesten-3-one and cholestanol (in CTX) and cholesterolesters (in TD), which are likely to underlie the myelin pathology [(29–32), Fig. 3]. Myelin defects due to reduced cholesterol levels are possibly found in Smith-Lemli-Opitz syndrome (SLOS), which is caused by mutations in the gene encoding sterol delta-7-reductase (DHCR7), the enzyme catalyzing the last step of cholesterol biosynthesis. This results in the elevation of the cholesterol precursors 7-dehydro-cholesterol and 8-dehydro-cholesterol and in cholesterol deficiency in all tissues (33–35). Patients with SLOS have multiple malformations, cognitive impairment, and behavioral deficits. CNS defects in white matter are common, often involving corpus callosum absence or hypoplasia (36–42), and reported to involve absence of myelin (42) and demyelination (43), but detailed analysis of myelin structure has not yet been described. The relative contribution of either cholesterol depletion or 7-dehydro-cholesterol and 8-dehydro-cholesterol accumulation in white matter abnormalities is currently unclear. Interestingly, dietary cholesterol supplementation has become the standard therapy for SLOS and was reported to partially improve the neurological status and white matter lipid abnormalities in some patients (36, 44–46). Remarkably, PNS myelin defects are rarely reported for SLOS. The reason for this is currently unclear, but dietary lipid supplementation has also been reported to improve a case of SLOS-associated demyelinating polyneuropathy (47).
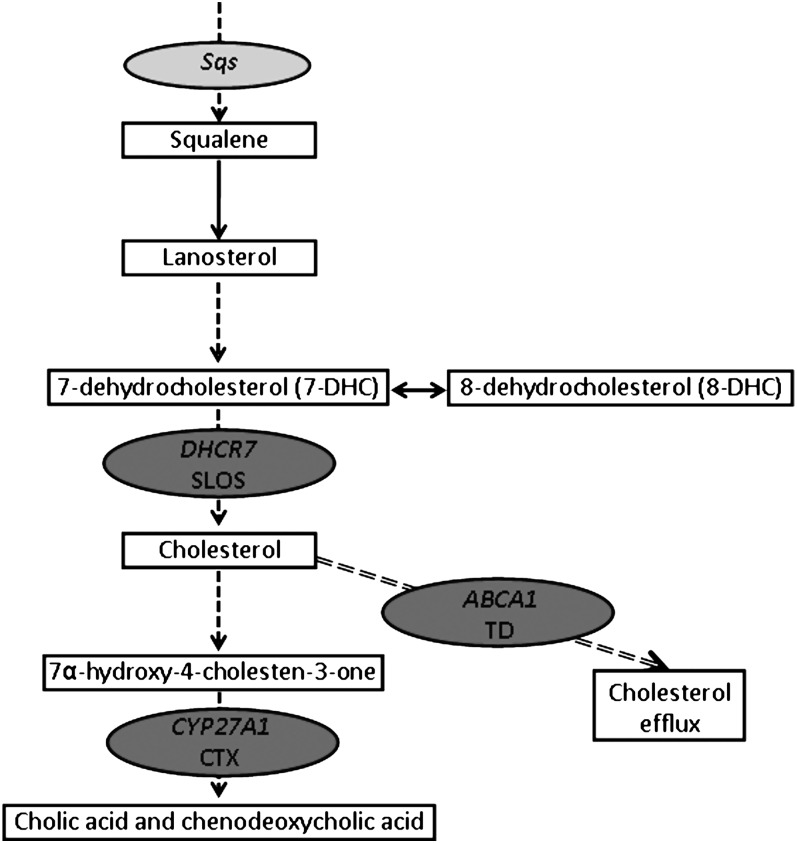
Fig. 3.
Human inherited and mouse experimental defects in the cholesterol biosynthesis pathway that cause myelin disorders. Solid line arrows depict a direct link between two steps, dashed line arrows imply intermediate steps that are not shown. Dark gray ovals show the positions of human disease genes; light gray oval shows the position of mutated genes in experimental mouse models. Sqs, squalene synthase; DHCR7, sterol delta-7-reductase; SLOS, Smith-Lemli-Opitz syndrome; CYP27A1, sterol 27-hydroxylase; CTX, Cerebrotendinous xanthomatosis; ABC1, ATP-binding cassette transporter A1; TD, Tangier disease.
Mouse models of cholesterol disorders.
Multiple mouse models of SLOS recapitulate morphological and biochemical aspects of the neuropathology of SLOS patients (48–50). Although ventricular dilatation and partial agenesis of corpus callosum was observed in one of these models (50), the status of myelination was so far not analyzed in DHCR7 mutant animals. The importance of local cholesterol biosynthesis in myelinating glial cells was, however, addressed by conditional inactivation of squalene synthase (SQS; gene symbol fdft1) specifically in oligodendrocytes and Schwann cells of fdftflox/flox/cnp1+/cre mice (51, 52) in which expression of cre recombinase (cre) was under control of the 2’:3′-cyclic nucleotide 3′-phosphodiesterase (cnp) promoter. In the CNS, SQS inactivation led to substantial hypomyelination and reduced motor performance in 20-day-old SQS conditional mutants. The discrepancy between the robust hypomyelination observed in SQS mouse mutants and the mild myelin defects observed in human SLOS is unclear, but may be related to the low amounts of cholesterol that are still produced in some forms of SLOS (33) and/or to the position of the defects leading to disruption of the cholesterol biosynthesis pathway, which is more upstream in SQS mouse mutants. Whereas the amount of myelin in SQS conditional mutants was decreased, its ultrastructure, lipid, and protein composition were not affected, suggesting a tight control mechanism matching the amount of myelin proteins produced by glial cells to the available cholesterol. Interestingly, both hypomyelination and motor deficits improved over time. These observations suggest that cell autonomous synthesis of cholesterol in oligodendrocytes is crucial for normal onset and progression of myelination. Cholesterol biosynthesis-deficient oligodendrocytes are, however, able to take up some cholesterol from their surrounding for limited myelin formation (51). The exact mechanism of this horizontal cholesterol transfer remains to be clarified. In the PNS, the inactivation of cholesterol biosynthesis in Schwann cells also led to substantial hypomyelination as detected by morphometric analysis of sciatic nerves from fdftflox/flox/cnp1+/cre animals. Similar to oligodendrocytes, also Schwann cells are able to take up extracellular cholesterol because many axons in peripheral nerves from fdftflox/flox/cnp1+/cre animals are ensheathed by (albeit thin) myelin. These data have been corroborated by in vitro experiments showing that extracellular cholesterol increased myelination in both wild-type and mutant Schwann cells (52). Interestingly, detailed ultrastructural and biochemical analysis of mutant myelin revealed substantial increase in the amount of noncompact myelin, probably as a consequence of defective myelin protein transport from the endoplasmic reticulum to the myelin sheath (see below). These data, together with a synchronized downregulation of the mRNA expression for multiple myelin proteins, suggest that in addition to its role as a major structural component of myelin, cholesterol plays a crucial role in coordinating myelin membrane assembly.
Glycosphingolipid disorders
Most of the myelin defects in glycosphingolipid disorders are due to accumulation of glycosphingolipids or their precursors (Table 2). Metachromatic leukodystrophy, Krabbe disease, and Niemann-Pick disease type A lead to the accumulation of respectively sulfatides, galactocerebrosides (and sphingosine), and sphingomyelin [(53–56) Fig. 4). The accumulation of these lipids in myelinating glial cells leads predominantly to cytotoxicity and demyelination. Myelin defects present in dysmyelinating leukodystrophy and spastic paraparesis with or without dystonia (57, 58), which is caused by a mutation in the gene encoding fatty acid 2-hydroxylase (FA2H), are, however, probably a consequence of reduced glycosphingolipid levels. The 2-hydroxylation of sphingolipid N-acyl chains catalyzed by the FA2H occurs during de novo ceramide synthesis. In accordance with the high level of FA2H in mammalian central and peripheral nervous systems, both galactosylceramides and sulfatides contain high proportions (up to 50%) of 2-hydroxy fatty acids (59). Surprisingly, the FA2H mutations result in demyelination of the CNS, whereas PNS myelin abnormalities are not found, suggesting there is a second fatty acid 2-hydroxylating activity in human Schwann cells (57).
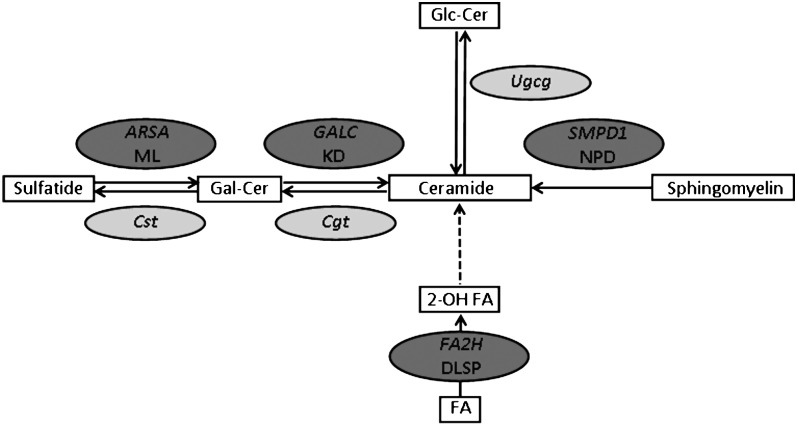
Fig. 4.
Human inherited and mouse experimental defects in the glycosphingolipids synthesis pathway that cause myelin disorders. Solid line arrows depict direct link between two steps, dashed line arrow implies intermediate steps that are not shown. Dark gray ovals show positions of human diseases; light gray ovals show the position of mutated genes in experimental mouse models. ARSA, arylsulfatase A; ML, Metachromatic leukodystrophy; Cst, cerebroside sulfotransferase; GALC, galactosylceramidase; KD, Krabbe disease; Cgt, UDP-galactose:ceramide galactosyltransferase; Ugcg, UDP-glucose ceramide glucosyltransferase; SMPD1, sphingomyelin phosphodiesterase-1; NPD, Niemann-Pick disease type A; FA2H, fatty acid 2-hydroxylase; DLSP, Dysmyelinating leukodystrophy and spastic paraparesis with or without dystonia; Gal-Cer, galactocerebroside; Glc-Cer: glucocerebroside; 2-OH FA, 2-hydroxy fatty acids; FA, fatty acids.
Mouse models of glycosphingolipid disorders.
FA2H deficient mouse mutants lack 2-hydroxylated sphingolipids [hydroxyl fatty acid (HFA)-GalC and HFA-sulfatide] as evaluated by thin-layer chromatography and MALDI-time-of-flight (TOF) MS (60). The absence of 2-hydroxylated sphingolipids in mice does not cause any detectable myelin changes up to adulthood. However, myelin decompaction was detected in aged (18 months) brain and was even more severe in peripheral nerve samples where signs of myelin degeneration (e.g., decompaction and myelin loss) were observed, clearly suggesting a role of HFAs in long-term myelin stability. The presence of this discrete phenotype, or its late onset, suggests the possibility that other models of glycosphingolipid disorders, including a mouse model of Gaucher disease (61) or a knockout mouse for sphingomyelin synthase 2 (62), may have ultrastructural myelin changes that so far have not been detected.
The role of the most abundant galactosphingolipids in myelin was analyzed in mouse models disrupting either sulfatide biosynthesis alone [cerebroside sulfotransferase (CST) knockout mice] or disrupting both galactocerebroside and sulfatide biosynthesis [UDP-galactose:ceramide galactosyltransferase (CGT) knockout mice, Fig. 4]. Characterization of CST-deficient mice revealed that sulfatide plays a crucial role in maintenance of myelin structure and organization of node and paranode (63, 64). Interestingly, whereas apparently normal myelin can be assembled in CST mutant animals, the myelin structure is disturbed with age, as reflected by uncompacted myelin sheaths in the CNS and nodal and paranodal abnormalities in both the CNS and PNS. The defective sulfatide biosynthesis also leads to axonal changes, as demonstrated by the loss of large axons in the CNS and the presence of axonal protrusions in the PNS. In comparison, CGT mutant animals, which are unable to synthesize both galactocerebroside and sulfatide, have a similar but more marked myelin phenotype because myelin abnormalities (including uncompacted areas and redundant myelin profiles) are already present at P10 and evolve to substantial demyelination at P43 (65). Both CGT−/− and CST−/− animals develop a similar nodal phenotype characterized by disorganized paranodal loops and enlarged nodal gaps (63). Because the CGT−/− animals accumulate HFA-Glc-Cer (which is normally absent) in their myelin, it was suggested that HFA-Glc-Cer could compensate partially for the absence of galactolipids in these animals. However, inactivation of UDP-glucose ceramide glucosyltransferase (Ugcg), which prevented accumulation of HFA-Glc-Cer, did not lead to any aggravation of myelin phenotype in double knockout Ugcgflox/flox; Cnp/Cre; CGT−/− mice (66). These data therefore suggest that glial cells are able to assemble myelin in the absence of glycolipids.
Fatty acid and plasmalogen disorders
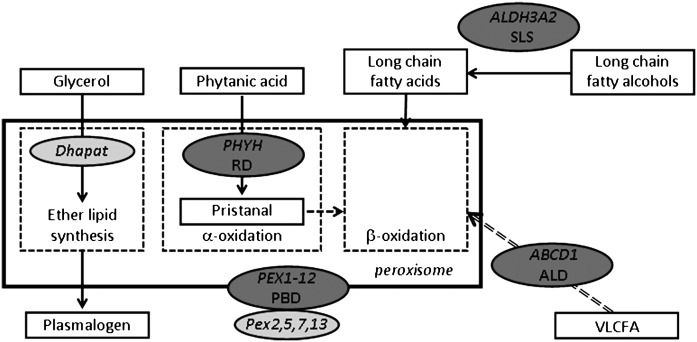
Several disorders in fatty acid metabolism are associated with defects in myelin that are mostly caused by the accumulation of lipids (Table 2, Fig. 5). In Sjogren-Larsson syndrome (SLS) mutations in fatty aldehyde dehydrogenase result in the accumulation of long-chain fatty alcohols (67), leading to CNS white matter abnormalities that are related to dysmyelination and hypomyelination (68, 69). Lipid accumulation is also found in peroxisome disorders, such as Refsum disease [RD (70)], adrenoleukodystrophy [ALD (53)], and peroxisome biogenesis disorders (71). In RD, mutation of phytanoyl-CoA hydroxylase leads to the accumulation of phytanic acid, a plant-derived fatty acid that is normally degraded by α-oxidation in peroxisomes. Accumulation of phytanic acid causes demyelination, especially in the PNS. ALD is caused by mutation of the ABCD1 protein which functions as a transporter for the uptake of very long-chain fatty acids (VLCFAs) in peroxisomes. Because VLCFAs are metabolized in peroxisomes via β-oxidation, myelinating glial cells in ALD-patients accumulate VLCFA, which causes dysmyelination but also inflammatory-induced demyelination. The latter may be caused by the failure to degrade arachidonic acid-derived eicosanoids (72, 73). Peroxisome biogenesis disorders of Zellweger syndrome occur when any one of 12 peroxins involved in the import of peroxisomal proteins is mutated [for a recent review, see (74)]. The consequent partial or complete peroxisome dysfunction results in defective α- and β-oxidation, subsequent accumulation of fatty acids, leading to myelin defects as also seen in ALD or RD. In addition, myelin defects due to reduced lipid levels are found in Zellweger syndrome as well, because the peroxisomal anabolic lipid pathway involved in ether lipid synthesis is disrupted (Fig. 5), resulting in plasmalogen deficiency and consequent mild hypomyelination and dysmyelination, especially in the CNS.
Fig. 5.
Human inherited and mouse experimental defects in fatty acids and plasmalogen metabolism that cause myelin disorders. Solid line arrows depict direct link between two steps, dashed line arrow implies intermediate steps that are not shown. Dashed line rectangles represent various lipid metabolic pathways in the peroxisome. Dark gray ovals show positions of human disease genes; light gray ovals show the position of mutated genes in experimental mouse models. Dhapat, dihydroxyacetone phosphate acyltransferase; PHYH, phytanoyl-CoA hydroxylase; RD, Refsum disease; PEX1-12, peroxins; PBD, peroxisome biogenesis disorders; ALDH3A2, fatty aldehyde dehydrogenase; SLS, Sjogren-Larsson syndrome; ABCD1, ATP-binding cassette transporter D1; ALD, adrenoleukodystrophy; VLCFA, very long-chain fatty acids.
Mouse models of fatty acid disorders.
As shown in Fig. 2, myelin is specifically enriched in saturated VLCFAs (C22:0-C24:0). Mice carrying a deletion of ceramide synthase 2, an enzyme that is mostly involved in synthesis of ceramides with VLCFAs, show myelin with ceramide species that no longer have VLCFAs (≥C22) but instead are enriched in short-chain fatty acids (75). These changes where accompanied by progressive loss of CNS and PNS myelin levels from early adulthood on and dysmyelination predominantly in the PNS. It remains to be determined whether myelinating glial cells are the primary cause, but these data are indicative of the involvement of VLCFA in maintenance of the myelin membrane (75).
Several mouse models have been generated for different peroxisomal disorders. Many of those recapitulate the main pathological or biochemical defects observed in patients, most often associated with lipotoxicity caused by accumulation of metabolic intermediates, like VLCFA in RD and in ALD (76–81). It should be noted that saturated VLCFAs, which are enriched in myelin (Fig. 2), rely fully on peroxisomes for β-oxidation, which could underlie the vulnerability of myelin for VLCFA accumulation in these disorders. Peroxisome biogenesis disorders have been modeled by inactivating different Pex genes (Pex2, Pex5, Pex13), which resulted in an early postnatal death of affected animals, preventing myelin analysis (78–81). Mice carrying an oligodendrocyte specific deletion of Pex 5 (CNPcre-Pex5 (82)) assembled normal myelin, but surprisingly within a few months, developed axonal defects followed by inflammatory demyelination. The observation that the PEX5-deficient oligodendrocytes are not able to maintain axonal integrity, even in the absence of obvious demyelination, indicates a role of oligodendrocyte peroxisome-associated lipid metabolism in axonal support. In addition, the remarkable inflammatory demyelination observed in CNPcre-Pex5 mice suggest a role for abnormal lipid metabolism in inflammation (82), as also observed in human ALD disease (72, 83, 84). Interestingly, polyunsaturated VLCFAs, such as arachidonic acid, are substrates for the synthesis of eicosanoids, which are lipid inflammatory mediators (85, 86). Peroxisomes play a major role in the degradation of eicosanoids (72, 87, 88). The fact that the VLCFAs in myelin are saturated and not unsaturated might reduce the risk of generating too high levels of lipid inflammatory mediators. Together, these data suggest that peroxisomes in myelinating glial cells are likely to be important for protection against lipid induced inflammation, by degrading VLCFA and eicosanoids. The observations on CNP-cre-PEX5 mice furthermore indicate that peroxisome-associated lipid metabolism is not required in oligodendrocytes for myelin membrane synthesis per se. However, mice in which Pex5 is lacking in all neural cells [Nestin-cre PEX5 mice (80)] do show dysmyelination, indicating that, in CNP-cre-PEX5 mice, oligodendrocytes may take up peroxisome-derived lipids from other neural cells and utilize these lipids for myelin membrane synthesis.
Mouse models of plasmalogen disorders.
The Pex2, Pex5, or Pex13 knockout mouse models of peroxisome biogenesis disorder have reduced nervous system plasmalogen levels (78–81), but how this contributes to myelin lipid defects is difficult to establish due to the contribution of the other defects in peroxisome lipid metabolism (Fig. 5). The Pex7 (77) and dihydroxyacetone phosphate acyl transferase (89) knockout mice were generated as mouse models of rhizomeluic chondrodysplasia type 1 and 2, respectively, both peroxisomal disorders that are characterized by a shortage of ether lipids and delays in myelination (80). In Pex7 knockout mice, a small reduction in CNS myelin protein levels was observed, and PNS nerves showed thinning of the myelin sheath and a reduced motor nerve conduction velocity (76). In the knockout mouse for Dhapat, a peroxisomal enzyme essential for plasmalogen synthesis, reduced CNS myelination was observed together with abnormal paranodal structure and reduced corpus callosum conduction velocity (90). Together, these studies suggest a structural role of plasmalogens in myelin membrane, although ultrastructural studies remain necessary to unravel the precise role of plasmalogens in myelination. Furthermore, plasmalogens are described to function as a sink of polyunsaturated fatty acids (PUFAs) (91) and as such proposed to have a protective role against the effects of PUFA accumulation as seen in peroxisome disorders (92). Indeed, Pex7:Abcd1 knockout mice show an increase in VLCFA accumulation and inflammatory demyelination (76).
General lipogenesis disorders
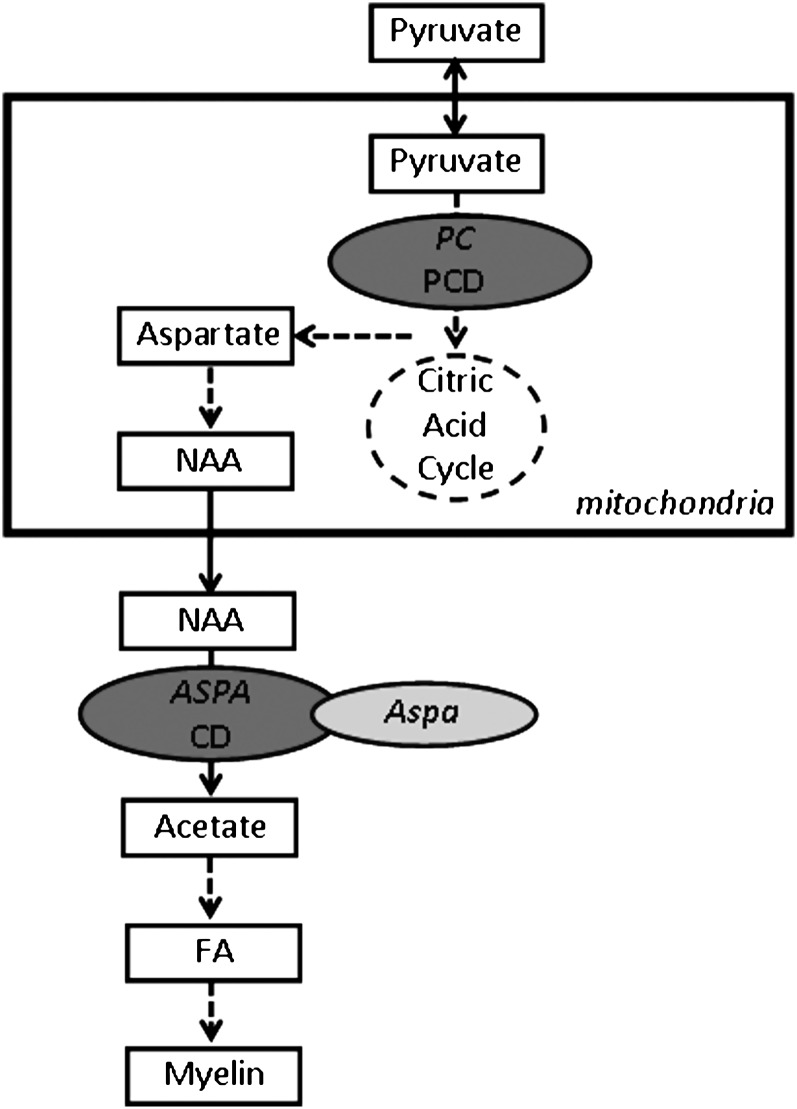
Several myelin disorders are caused by defects in early steps in the synthesis of lipids (Table 2, Fig. 6). Pyruvate carboxylase deficiency [PCD (93, 94)] is caused by a mutation in pyruvate carboxylase, an enzyme required for the synthesis of oxaloacetate of pyruvate in the tricarboxylic acid cycle, which is found in glial cells, predominantly astrocytes (95–97). Several biochemical pathways rely on the tricarboxylic acid cycle, such as biosynthesis of fatty acids, nonessential amino acids, and gluconeogenesis. Consequently, PCD patients show CNS hypomyelination, whereas PNS myelin defects have not been described. Canavan disease [CD (98)] is caused by a mutation in aspartoacylase (ASPA), an enzyme that in the rodent nervous system was shown to be predominantly expressed in oligodendrocytes (99) and is involved in the deacetylation of the nervous system specific metabolite N-acetylaspartic acid (NAA), thus generating free acetate in the brain. Patients affected by CD show accumulation of neuronal NAA and reduced levels of acetate in oligodendrocytes, the latter is generally thought to limit fatty acid and membrane lipid synthesis and to underlie the spongiform myelin degeneration, while leaving neurons intact (100, 101).
Fig. 6.
Human inherited and mouse experimental defects in general lipid metabolism that cause myelin disorders. Solid line arrows depict direct link between two steps, dashed line arrows implies intermediate steps that are not shown. Dashed line circle represents the citric acid cycle in mitochondria. Dark gray oval shows positions of human disease genes; light gray oval shows the position of mutated genes in an experimental mouse model. PC, pyruvate carboxylase; PCD, pyruvate carboxylase deficiency; ASPA, aspartoacetylase; CD, Canavan disease; NAA, N-acetylaspartic acid; FA, fatty acids.
Mouse models of general lipid disorders.
Two mouse models of CD, in which ASPA is deleted, show myelin vacuolization similar to human Canavan patients (102, 103), and reduced brain levels for acetate, myelin lipids, and myelin proteins (104, 105). Labeling studies have shown that NAA is a major source of acetate for lipid synthesis during brain development and that neuronal-derived NAA supplies acetyl groups for myelin lipid synthesis (106, 107). Its quantitative significance for myelin synthesis is not completely clear because in most cell types, the enzyme ATP-citrate lyase provides the acetyl groups for fatty acid synthesis. Madhavarao and colleagues (104) recently suggested that the lowering of acetate in ASPA knockout mice is sufficient to decrease myelin synthesis. However, ultrastructural analysis of myelin during development remains to be done in order to determine the precise consequence of ASPA deficiency on myelin formation. Recently, it was shown that defective survival and differentiation of immature oligodendrocytes may also be implicated in CD (108). Furthermore, the toxic effects of NAA in myelin vacuolization should not be neglected (109).
The role of glial lipid synthesis in myelination was recently studied by Verheijen et al. (110). It was shown that the acute phase of myelin lipid synthesis is regulated by sterol regulatory element-binding protein (SREBP) cleavage activating protein (SCAP), an activator of SREBPs. P0cre-SCAP mice, which carry a Schwann cell specific deletion of SCAP, showed congenital hypomyelination, and had a loss of SREBP-mediated gene expression involved in cholesterol and fatty acids synthesis. Interestingly, SCAP mutant Schwann cells were able to slowly synthesize myelin in an external lipid-dependent fashion, resulting in myelin membrane defects that were associated with abnormal lipid composition. Saturated VLCFAs (C22:0 and C24:0) were found to be reduced in myelin membranes, while poly-unsaturated fatty acids (C18:2, C22:6, C24:2, C25:2 and C26:2) were increased. The reduced saturation level of long-chain fatty acids resulted in an increase in disorder of acyl groups in the myelin lipid bilayer, which may lead to altered packaging of proteins in the membrane (111) and consequent ultrastructural myelin abnormalities. These observations also showed that glial cells are able to take up lipids from the extracellular environment and utilize these for myelin membrane synthesis, which is in line with the observation of Saher et al. (51, 52) on CNPcre-SQS mice in which cholesterol deficient myelinating glial cells are able to slowly synthesize myelin.
WHY ARE MYELINATING GLIAL CELLS PARTICULARLY VULNERABLE TO LIPID METABOLISM DISORDERS?
The unique lipid composition of myelin critically contributes to its function
Much of the vulnerability of myelin for lipid defects is caused by the fact that myelin membrane assembly requires an extraordinary amount of lipids, especially for the lipids that are enriched in myelin: galactolipids, cholesterol, plasmalogens, and fatty acids. As described above, for each of these lipid classes, human myelin disorders have been described in which the pathophysiology is related to lipotoxicity as a result of the accumulation of metabolic precursors for myelin lipids. The vulnerability of myelin for lipid disorders could, however, also relate to biochemical properties of each lipid class for functioning of the myelin membrane.
Cholesterol is one of the most important regulators of lipid organization in the membrane. The hydroxyl group of cholesterol interacts with the polar head group of other lipids, whereas the rigid body of cholesterol is situated along side the fatty acid tails of lipids in the membrane and can help to order these tails. The increased lateral ordering of lipids that is caused by cholesterol consequently affects the biophysical properties of the membrane by decreasing fluidity and reducing the permeability of polar molecules, like ions, for example (112–115). By contrast, too much ordering is detrimental because this could slow down the diffusion of membrane proteins and decrease the bending capacity of the membrane (112, 114, 116, 117). Furthermore, some membrane proteins bind tightly to cholesterol as discussed below. Together, this gives cholesterol the capacity to regulate membrane fluidity and to stabilize and seal the membrane, all functions that seem critical for the insulating function of the myelin membrane (8).
Glycosphingolipids consist of a ceramide backbone, formed by a long-chain fatty acyl residue linked by its amide bond to a long-chain sphingosine base that binds the functional group galactosyl or sulfatide. They pack tightly and have the remarkable property that their fluid/solid phase transition temperature is above body temperature, so that glycolipids have a solid ‘gel’ phase at body temperature (118–120). Accordingly, CGT knockout mice, which lack glycosphingolipids, have increased myelin membrane fluidity and therefore increased ion permeability, which may disrupt saltatory conduction (121). In addition to this, the anionic glycol group may provide an electronic shielding on the outside of the myelin membrane and thereby contribute to the electric isolation by the myelin membrane and thus nerve conduction velocity. Interestingly, glycosphingolipids are solubilized by cholesterol (122), and both lipids are predominantly present in the outer leaflet of the myelin membrane. Asymmetric distribution of lipids between two bilayer leaflets contributes to curving of membranes (122). This suggests a role of glycosphingolipids and cholesterol in regulating fluidity and curving of myelin membranes, which could be particularly relevant for the paranodal loops structure. In line with this is the nodal phenotype of galactolipids-lacking CGT and CST knockout mice (63).
Plasmalogens are a sub-class of ether phospholipids, which are glycerol-derived compounds that carry an ether-linked carbohydrate chain on the first carbon of glycerol as opposed to ester-linked fatty acid chain in classical phospholipid containing a vinyl group next to the ether bond. The particular role of plasmalogens in membranes has not been elucidated, but they are thought to play a role in membrane fusion processes and membrane dynamics. They increase membrane fluidity by lowering the transition of phospholipid mixtures from a lamellar to a liquid crystalline phase and stimulate the formation of nonbilayer lipid structures when present in high concentration (123). Accordingly, plasmalogens could be involved in myelin membrane formation or maintenance by potentiating membrane dynamics (124).
The brain is enriched in long PUFAs like DHA (c22:6) (125). However, this enrichment is not manifested in myelin, which instead contains high levels of saturated VLCFA, and has a particularly high C18:1/C18:2 ratio (see Fig. 2). Because of its high degree of saturation, phospholipids containing these fatty acids may decrease membrane fluidity and give a higher degree of fatty acid ordering in the membrane, which prevents intercalation of polar molecules into the lipids. Together with the extra-long length of the acyl chains, this will provide a thick permeability barrier for ions and as such contribute to electric insulation of the axon.
Considering their structural role in the membrane, we view that many of the lipids that are enriched in myelin play a role in its axon-insulating property: the electric shielding in the outer leaflet by glycolipids, the thick and ordered bilayer made by the long and saturated FA, and the further sealing of the outer leaflet by cholesterol. It should be noted that the specific myelin lipid composition is also likely to be related to proper packing of the myelin membrane. Changes in lipid composition affect lipid-protein interactions causing an altered packing of proteins in the membrane (111). For instance, proteins and lipids may work synergistically to provide proper structure to myelin membranes (126). In line with this, interference with endogenous Schwann cell lipid metabolism in P0cre-SCAP mutant mice increases the unsaturation level of fatty acids in myelin and is accompanied by ultrastructural defects in myelin membrane packing (110).
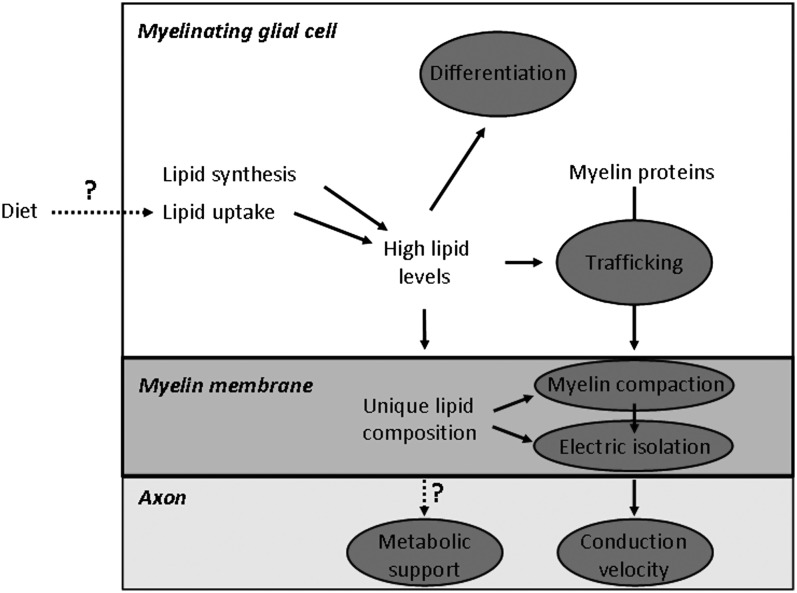
Together, these data suggest that lipids may control insulation of the axon and proper structure of the myelin membrane (Fig. 7). Both of these aspects are clearly affected in many lipid metabolic disorders, but it remains to be determined whether small functional defects on nerve function in certain cases are caused by more subtle changes in myelin lipid composition that affect axonal insulation. It is furthermore tempting to speculate that myelinating glial cells may control nerve conduction velocity by regulation of its myelin lipid content.
Fig. 7.
Schematic diagram of the various roles of lipids in myelinating glial cells. Gray ovals show the processes that are affected by lipid metabolism and involved in myelinated fiber function. High lipid levels, which are required for synthesis of a full myelin membrane, are ensured by both the endogenous synthesis of lipids as by the uptake of lipids from the extracellular environment. Lipids influence myelinating glial cell differentiation and trafficking of myelin proteins to the myelin membrane. The unique lipid composition of the myelin membrane is required for proper myelin membrane wrapping (compaction) and electrostatic isolation of the axon from extracellular environment, thereby promoting saltatory conduction and conduction velocity. Question marks show processes with a potential effect on myelin fiber function: the influence of lipid-specific diets on myelin lipid dependent processes, and the possible role of myelin lipids in metabolic support of the axon. See text for further explanation.
Timely myelination requires extraordinarily high levels of lipid synthesis together with uptake of extracellular lipids
Much of the vulnerability of myelin for lipid defects is caused by the fact that myelin membrane assembly requires an extraordinary amount of lipids. The observations on CNPcre-SQS ko mice (51, 52) and P0cre-SCAP ko mice (110) have led to two important conclusions: 1) myelinating glial cells are synthesizing most of the lipids themselves, but 2) myelinating glial cells are still able to synthesize myelin when endogenous lipid synthesis has been shut down, albeit at a very low level. This indicates that these cells have the capacity to take up lipids from the extracellular environment. Accordingly, transcription analysis in rodents showed that lipoprotein receptors are expressed by oligodendrocytes (127) and that expression of the low density lipoprotein receptor (LDLR) gene in the PNS is elevated with myelination (24) although the LDLR gene was found not to be required for PNS remyelination (128). Which lipoprotein receptors are involved in the uptake of lipids and exactly where on the glial cell they can be found to support membrane growth (Fig. 1) is currently unclear. The exact contribution of extracellular lipid supply for myelin membrane synthesis under normal conditions remains to be determined, but is likely to be small (Fig. 7). Defects in glial lipid synthesis will therefore consequently lead to defective myelin membrane synthesis.
Myelin lipids regulate the transport and localization of myelin proteins
In addition to the above mentioned synchronized expression of transcripts encoding structural myelin proteins and enzymes involved in myelin lipid biosynthesis, recent data suggest that glial lipid levels regulate myelin protein trafficking and thus also myelin assembly (52)(129). In oligodendrocytes, proteolipid protein (PLP) associates with lipid rafts before exiting the Golgi apparatus, suggesting that myelin lipid and proteins assemble in the Golgi complex. Indeed, the cholesterol- and galactosylceramide-rich lipid rafts were shown to be required for proper sorting of PLP to myelin (129). In line with this, certain mutations in PLP perturb interactions with cholesterol and lipid rafts, which may contribute to dysmyelination as found in spastic paraplegia (130). Similarly, disrupted Schwann cell cholesterol biosynthesis led to partial mislocalization of myelin protein P0, resulting in noncompaction of the myelin membrane and hypomyelination (52). Additional cell culture experiments demonstrated that this defect could be restored by cholesterol supplementation, and showed the critical role of the myelin protein P0 cholesterol recognition/interaction amino acid consensus motif for its correct trafficking from endoplasmic reticulum to the myelin compartment. Furthermore, elevation of extracellular cholesterol or lipoprotein levels was demonstrated to increase myelination by Schwann cells in vitro (52, 110). Together, these data suggest that lipids may control myelin protein trafficking and thereby assembly and compaction of the myelin membrane (Fig. 7). This lipid-mediated control mechanism of myelin protein sorting is likely to be deregulated in lipid metabolic disorders, but whether and how it is used by myelinating glial cells to control myelin membrane synthesis under healthy conditions remains to be determined.
Lipids regulate differentiation of myelinating glial cells
The regulatory role of lipids in differentiation of myelinating glial cells was demonstrated in oligodendrocytes and in Schwann cells. Both the cell culture experiments and the data from CGT and CST knockout animals revealed an increased number of terminally differentiated oligodendrocytes (OLs) in the absence of galactosphingolipids (131, 132). Based on these results, two putative regulatory mechanisms involving galactosphingolipids were suggested: either they could play a direct role in cell adhesion necessary for correct timing of OL differentiation or they can have an indirect role affecting signaling proteins present in plasma membrane thus disturbing OL differentiation (132). In the PNS, phosphatidic acid (PA) was shown to affect Schwann cell differentiation (133). Demyelination in Lpin1 mutant mice was suggested to be mediated by increased levels of PA. Subsequent cell culture experiments demonstrated that PA induces Schwann cell dedifferentiation via activation of the MEK-Erk pathway revealing its role in glial cell fate determination (133) (Fig. 7).
MYELIN LIPIDS AS DETERMINANTS FOR NERVE FIBER FUNCTION
The process of myelination represents an interesting paradigm to study the metabolism and transport of lipids. Myelination by rodent glial cells is, both in vivo and in vitro, finalized within a one- to two-week interval. Because during this period a myelinating glial cell needs to expand its membrane up to 6,500 times, one can consider these cells as “lipid producing factories”. For instance, transcriptional analysis of myelinating glial cells clearly revealed that the most coregulated group of genes expressed during the period of myelination are lipid biosynthesis-related transcripts (23, 24).
Lipids as markers for myelin membrane integrity and function
Much of the structure and function of myelin is dependent on its lipid content. Lipids may therefore be used as markers for myelin membrane integrity and associated nerve fiber function. The C18:1/C18:2 ratio is a well-described marker for the progression of myelination of the PNS (7) and the CNS (134). In addition, a high level of PUFA in PNS myelin suggests a strong contribution of exogenous lipids in building of the myelin membrane (110). High cholesterol levels are required for the formation of compact myelin (with high P0), with low cholesterol levels resulting in increased amounts of noncompact myelin [with myelin-associated glycoprotein (MAG)] (52). This suggests that the cholesterol content of myelin is indicative for its compaction status. Dedifferentiation of myelinating Schwann cells in the Lpin1 mutant appeared to be mediated by high PA levels (133). This suggests that high PA levels are indicative for an active anti-myelination program, although it remains to be determined whether PA is also instrumental in regulating (de)differentiation in other paradigms, e.g., during development or injury.
Myelin lipids provide direct support to axonal function; a hypothesis
A possible role of myelinating glial cells in trophic support of underlying axons was recently suggested (135). Previous data demonstrated that inactivation of peroxisomal biogenesis factor 5 (Pex5) led to generation of animals with disrupted peroxisomal function in oligodendrocytes and Schwann cells [see above (82)]. Interestingly, although the affected animals were able to assemble normal myelin, they developed progressive axonal loss followed by demyelination. Based on these data it was suggested that the observed axonal loss could be, at least partially, a consequence of disrupted glial β-oxidation normally executed in peroxisomes, leading to a diminished capacity to metabolically support the underlying axons (73). A similar hypothesis suggesting the role of lipids in “energy-on-demand” supply for active axons was proposed based on expression analysis of genes encoding proteins involved in lipid metabolism in the adult peripheral nerve (24, 136). Although further clarification of the involvement of lipids in axonal support is still needed, it was shown that the expression of sterol response binding protein 1c (Srebp1c), which is the key transcriptional regulator of storage lipid metabolism, is affected in Schwann cells of a rodent model of diabetic peripheral neuropathy (137). Together, therefore, these data suggest that local glial lipid metabolism plays a crucial role not only in myelination but also in glial support of underlying axons.
Implications for therapeutic interventions that target lipid metabolism
A working strategy against lipotoxicity would be to use drugs that inhibit lipid accumulation. For instance, pharmacological targeting of the cholesterol pathway, by statin-induced inhibition of HMG-CoA reductase, is used in CTX to inhibit the accumulation of cholestan (138). Importantly, statins are widely used for the treatment of hypercholesterolemia, and some statins, e.g., Lovastatin and Simvastatin, are able to cross the blood-brain barrier (139). Remarkably, statins can ameliorate remyelination in an animal model of multiple sclerosis (MS) (140–143), possibly via augmenting survival and differentiation of oligodendrocyte progenitors, and therefore have been tested in MS trials (144, 145). However, statins induce the formation of abnormal myelin-like membrane sheets in primary oligodendrocytes in vitro, due to impairment of cholesterol-dependent myelin protein transport (146), which indicates a risk of the use of statins for myelin membrane integrity. In addition, statins are reported to increase the risk of developing peripheral neuropathy (147), although there is some controversy concerning the exact amplitude of this risk, which furthermore appeared to be reversible and will need further study (148, 149). The major contribution of endogenous lipid metabolism by myelinating glial cells implies that inhibition of glial lipid metabolism, especially during the active period of myelination, may underlie the development of demyelinating neuropathy, and as such warrants for care in the use of lipid-lowering drugs especially during late pregnancies and early postnatal development.
Extracellular supply of lipids may be a working strategy for treatment of lipid deficit in myelinating glial cells. Observations of mice with lipid-deficient glial cells show that myelinating glial cells are able to take up lipids from the extracellular environment (51, 52, 150). This is, however, complicated by the blood-nerve barrier and the blood-brain barrier that shield, respectively, the PNS and CNS from lipids in the circulation (151, 152). Therefore, the nervous system is classically viewed as being largely autonomous in lipid metabolism. Despite this dogma, dietary approaches to rescue lipid deficits are used in several lipid disorders to treat myelin defects. In SLOS, cholesterol supplementation leads to good biochemical and physical changes in nonneuronal tissues (153). Changes in neuronal tissues are limited, also because of the inability to reverse developmental defects, although striking behavioral improvements involving both CNS and PNS functioning were found (44, 45, 154). In a rodent model for CD, glyceryltriacetate supplementation was recently reported to improve motor performance and myelin lipid content (155). In Zellweger patients, alkyl-glycerol supplementation to rescue plasmalogen deficiency has been performed with only little success (156), which may be related to the other peroxisomal defects that are not related to plasmalogen deficiency in these patients. Interestingly, in Pex7 knockout mice, a model for rhizomeluic chondrodysplasia type 1, alkyl-glycerol supplementation improves PNS plasmalogen levels and nerve function (P. Brites, personal communication).
The above-described changes may not involve lipid uptake by the nervous system, but instead be indirect consequences of lipid supplementation. For instance, lipid metabolism in Schwann cells is under the influence of the nutritional status of mice through a mechanism probably involving insulin signaling (137). Recent studies have also shed light on novel mechanisms of cholesterol exchange between the CNS and circulating lipoproteins under certain physiological or pathological conditions. Saito et al. (157) recently showed that deletion of SQS in neural stem cells led to neuronal cell death, however, not of progenitor cells at the subventricular zone that protected themselves against cholesterol deprivation by promoting angiogenesis and consequently raising their lipoprotein cholesterol supply from the circulation. Furthermore, it was reported that specific loss of brain ABCA1, which is required for efflux of cellular cholesterol, not only produced the expected reduction in brain cholesterol, but remarkably, also led to an apparently compensatory increase in the specific uptake of esterified cholesterol from plasma HDL particles into the CNS (158). The observed increase in HDL receptor SR-B1 in brain capillaries of these mice, together with the recent finding that lipoprotein receptors in brain capillary cells can be used for targeting of compounds across the blood-brain barrier (159), indicate that the shielding of the CNS from circulating lipids may not be as strict as thought for a long time. This is certainly the case for circulating unsaturated fatty acids. For instance, PNS myelin in P0cre-SCAP mutant mice contains more polyunsaturated fatty acids, and have higher C18:2 levels, which is consistent with an increased uptake of essential fatty acids from external sources (160). Consistent with this, circulating fatty acids are incorporated into adult myelin (161, 162). Taken together, in the situation of local hypolipidemia induced by pathological conditions like genetic lipid disorders, myelinating glial cells may be able to obtain lipids derived from the circulation or from other nervous system compartments, albeit at a very low level.
As summarized in this review, our knowledge about the role of lipids in myelin biology increased substantially from the analysis of inherited myelin disorders with defective lipid metabolism and their related mouse models. These new data point to a far more complex role of lipids in myelinating glial cells than solely being building blocks for the myelin membrane (Fig. 7). Lipid metabolism is important for glial cell development and function; lipids affect glial cell differentiation, are involved in myelin protein trafficking and myelin compaction, and may potentially contribute to mature myelinating glial cell support of enwrapped axons. Importantly, these new data also show that despite the requirement of timely local glial lipid synthesis for normal myelination, myelinating glial cells are able to take up lipids from the surrounding environment. Together, these insights may contribute to the development of therapeutic approaches aiming at the preservation of myelin under pathological conditions that affect lipid metabolism.
Acknowledgments
The authors thank Dr. J. F. Brouwers for advice and fatty acid analysis.
Footnotes
Abbreviations:
- ALD
- adrenoleukodystrophy
- ASPA
- aspartoacylase
- CD
- Canavan disease
- CGT
- ceramide galactosyltransferase
- CNS
- central nervous system
- CST
- cerebroside sulfotransferase
- CTX
- Cerebrotendinous xanthomatosis
- DHCR7
- delta-7-reductase
- FA2H
- fatty acid 2-hydroxylase
- HFA
- hydroxyl fatty acid
- NAA
- N-acetylaspartic acid
- OL
- oligodendrocyte
- PA
- phosphatidic acid
- PCD
- pyruvate carboxylase deficiency
- PLP
- proteolipid protein
- PNS
- peripheral nervous system
- RD
- Refsum disease
- SCAP
- SREBP cleavage activating protein
- SLOS
- Smith-Lemli-Opitz syndrome
- SREBP
- sterol regulatory element-binding protein
- SQS
- squalene synthase
- TD
- Tangier disease
- VLCFA
- very long-chain fatty acid
This work is supported by the European Union Grant EU-NEST 12702 (to M.H.G.V.), Swiss National Science Foundation Grant PP00P3-124833/1 (to R.C.), Deutsche Forschungsgemeinschaft (CMPB, to K-A.N.), and EU-FP7 (NGIDD, Leukotreat, to K-A.N.).
REFERENCES
- 1.Barres B. A. 2008. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 60: 430–440. [DOI] [PubMed] [Google Scholar]
- 2.Franz D. N., Iggo A. 1968. Conduction failure in myelinated and non-myelinated axons at low temperatures. J. Physiol. 199: 319–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waxman S. G. 1980. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 3: 141–150. [DOI] [PubMed] [Google Scholar]
- 4.Hartline D. K., Colman D. R. 2007. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 17: R29–R35. [DOI] [PubMed] [Google Scholar]
- 5.Norton W. T., Poduslo S. E. 1973. Myelination in rat brain: method of myelin isolation. J. Neurochem. 21: 749–757. [DOI] [PubMed] [Google Scholar]
- 6.Norton W. T., Poduslo S. E. 1973. Myelination in rat brain: changes in myelin composition during brain maturation. J. Neurochem. 21: 759–773. [DOI] [PubMed] [Google Scholar]
- 7.Garbay B., Heape A. M., Sargueil F., Cassagne C. 2000. Myelin synthesis in the peripheral nervous system. Prog. Neurobiol. 61: 267–304. [DOI] [PubMed] [Google Scholar]
- 8.Saher G., Simons M. 2010. Cholesterol and myelin biogenesis. Subcell. Biochem. 51: 489–508. [DOI] [PubMed] [Google Scholar]
- 9.Norton W. T., Cammer W. 1984. Isolation and characterization of myelin. Myelin P., Morell, editor Plenum, New York: 147–180. [Google Scholar]
- 10.Stoffel W., Bosio A. 1997. Myelin glycolipids and their functions. Curr. Opin. Neurobiol. 7: 654–661. [DOI] [PubMed] [Google Scholar]
- 11.Eckhardt M. 2008. The role and metabolism of sulfatide in the nervous system. Mol. Neurobiol. 37: 93–103. [DOI] [PubMed] [Google Scholar]
- 12.Nagan N., Zoeller R. A. 2001. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 40: 199–229. [DOI] [PubMed] [Google Scholar]
- 13.Balakrishnan S., Goodwin H., Cumings J. N. 1961. The distribution of phosphorus-containing lipid compounds in the human brain. J. Neurochem. 8: 276–284. [DOI] [PubMed] [Google Scholar]
- 14.Morell P., Quarles R.H. 1999. Myelin formation, structure and biochemistry. Basic Neurochemistry. George J. S., Agranoff B. W., Albers R. W., Fisher S. F., Uhler M. D., Lippincott-Raven, Philedelphia. [Google Scholar]
- 15.Sowell E. R., Peterson B. S., Thompson P. M., Welcome S. E., Henkenius A. L., Toga A. W. 2003. Mapping cortical change across the human life span. Nat. Neurosci. 6: 309–315. [DOI] [PubMed] [Google Scholar]
- 16.Yakovlev P. I., Lecours A. R. 1967. The myelogenetic cycles of regional maturation of the brain. Regional Development of the Brain in Early Life. Minkowski A., editor Blackwell Scientific, Oxford: 3–70. [Google Scholar]
- 17.Muse E. D., Jurevics H., Toews A. D., Matsushima G. K., Morell P. 2001. Parameters related to lipid metabolism as markers of myelination in mouse brain. J. Neurochem. 76: 77–86. [DOI] [PubMed] [Google Scholar]
- 18.Baron W., Hoekstra D. 2009. On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett. 584: 1760–1770. [DOI] [PubMed] [Google Scholar]
- 19.Webster H. D. 1971. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J. Cell Biol. 48: 348–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heape A., Juguelin H., Fabre M., Boiron F., Garbay B., Fournier M., Bonnet J., Cassagne C. 1986. Correlation between the morphology and the lipid and protein compositions in the peripheral nervous system of individual 8-day-old normal and trembler mice. Brain Res. 390: 173–180. [DOI] [PubMed] [Google Scholar]
- 21.Heape A., Juguelin H., Fabre M., Boiron F., Cassagne C. 1986. A quantitative developmental study of the peripheral nerve lipid composition during myelinogenesis in normal and trembler mice. Brain Res. 390: 181–189. [DOI] [PubMed] [Google Scholar]
- 22.Heape A., Boiron F., Cassagne C. 1987. A developmental study of fatty acyl group contents in the peripheral nervous system of normal and trembler mice. Neurochem. Pathol. 7: 157–167. [DOI] [PubMed] [Google Scholar]
- 23.Nagarajan R., Le N., Mahoney H., Araki T., Milbrandt J. 2002. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proc. Natl. Acad. Sci. USA. 99: 8998–9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verheijen M. H., Chrast R., Burrola P., Lemke G. 2003. Local regulation of fat metabolism in peripheral nerves. Genes Dev. 17: 2450–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ando S., Tanaka Y., Toyoda Y., Kon K. 2003. Turnover of myelin lipids in aging brain. Neurochem. Res. 28: 5–13. [DOI] [PubMed] [Google Scholar]
- 26.Dyck P. J., Thomas P. K. 2005. Lysosomal and peroxisomal disorders. Peripheral Neuropathy. Elsevier Saunders, Philadelphia, PA: 1845–1882. [Google Scholar]
- 27.Wanders R. J., Ferdinandusse S., Brites P., Kemp S. 2010. Peroxisomes, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta. 1801: 272–280. [DOI] [PubMed] [Google Scholar]
- 28.Horster F., Surtees R., Hoffmann G. F. 2005. Disorders of intermediary metabolism: toxic leukoencephalopathies. J. Inherit. Metab. Dis. 28: 345–356. [DOI] [PubMed] [Google Scholar]
- 29.Verrips A., Hoefsloot L. H., Steenbergen G. C., Theelen J. P., Wevers R. A., Gabreels F. J., van Engelen B. G., van den Heuvel L. P. 2000. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 123: 908–919. [DOI] [PubMed] [Google Scholar]
- 30.Gallus G. N., Dotti M. T., Federico A. 2006. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol. Sci. 27: 143–149. [DOI] [PubMed] [Google Scholar]
- 31.Pietrini V., Rizzuto N., Vergani C., Zen F., Ferro Milone F. 1985. Neuropathy in Tangier disease: A clinicopathologic study and a review of the literature. Acta Neurol. Scand. 72: 495–505. [DOI] [PubMed] [Google Scholar]
- 32.Pollock M., Nukada H., Frith R. W., Simcock J. P., Allpress S. 1983. Peripheral neuropathy in Tangier disease. Brain. 106: 911–928. [DOI] [PubMed] [Google Scholar]
- 33.Neklason D. W., Andrews K. M., Kelley R. I., Metherall J. E. 1999. Biochemical variants of Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 85: 517–523. [DOI] [PubMed] [Google Scholar]
- 34.Witsch-Baumgartner M., Fitzky B. U., Ogorelkova M., Kraft H. G., Moebius F. F., Glossmann H., Seedorf U., Gillessen-Kaesbach G., Hoffmann G. F., Clayton P., et al. 2000. Mutational spectrum in the Delta7-sterol reductase gene and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am. J. Hum. Genet. 66: 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciara E., Nowaczyk M. J., Witsch-Baumgartner M., Malunowicz E., Popowska E., Jezela-Stanek A., Piotrowicz M., Waye J. S., Utermann G., Krajewska-Walasek M. 2004. DHCR7 mutations and genotype-phenotype correlation in 37 Polish patients with Smith-Lemli-Opitz syndrome. Clin. Genet. 66: 517–524. [DOI] [PubMed] [Google Scholar]
- 36.Caruso P. A., Poussaint T. Y., Tzika A. A., Zurakowski D., Astrakas L. G., Elias E. R., Bay C., Irons M. B. 2004. MRI and 1H MRS findings in Smith-Lemli-Opitz syndrome. Neuroradiology. 46: 3–14. [DOI] [PubMed] [Google Scholar]
- 37.Kelley R. I., Hennekam R. C. 2000. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 37: 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curry C. J., Carey J. C., Holland J. S., Chopra D., Fineman R., Golabi M., Sherman S., Pagon R. A., Allanson J., Shulman S., et al. 1987. Smith-Lemli-Opitz syndrome-type II: multiple congenital anomalies with male pseudohermaphroditism and frequent early lethality. Am. J. Med. Genet. 26: 45–57. [DOI] [PubMed] [Google Scholar]
- 39.Ryan A. K., Bartlett K., Clayton P., Eaton S., Mills L., Donnai D., Winter R. M., Burn J. 1998. Smith-Lemli-Opitz syndrome: a variable clinical and biochemical phenotype. J. Med. Genet. 35: 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry R., Wilson H., Robinson J., Sandlin C., Tyson W., Campbell J., Porreco R., Manchester D. 1989. Apparent Smith-Lemli-Opitz syndrome and Miller-Dieker syndrome in a family with segregating translocation t(7;17)(q34;p13.1). Am. J. Med. Genet. 34: 358–365. [DOI] [PubMed] [Google Scholar]
- 41.Cherstvoy E. D., Lazjuk G. I., Ostrovskaya T. I., Shved I. A., Kravtzova G. I., Lurie I. W., Gerasimovich A. I. 1984. The Smith-Lemli-Opitz syndrome. A detailed pathological study as a clue to a etiological heterogeneity. Virchows Arch. 404: 413–425. [DOI] [PubMed] [Google Scholar]
- 42.Ness G. C., Lopez D., Borrego O., Gilbert-Barness E. 1997. Increased expression of low-density lipoprotein receptors in a Smith-Lemli-Opitz infant with elevated bilirubin levels. Am. J. Med. Genet. 68: 294–299. [DOI] [PubMed] [Google Scholar]
- 43.Fierro M., Martinez A. J., Harbison J. W., Hay S. H. 1977. Smith-Lemli-Opitz syndrome: neuropathological and ophthalmological observations. Dev. Med. Child Neurol. 19: 57–62. [DOI] [PubMed] [Google Scholar]
- 44.Elias E. R., Irons M. B., Hurley A. D., Tint G. S., Salen G. 1997. Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS). Am. J. Med. Genet. 68: 305–310. [DOI] [PubMed] [Google Scholar]
- 45.Irons M., Elias E. R., Abuelo D., Bull M. J., Greene C. L., Johnson V. P., Keppen L., Schanen C., Tint G. S., Salen G. 1997. Treatment of Smith-Lemli-Opitz syndrome: results of a multicenter trial. Am. J. Med. Genet. 68: 311–314. [PubMed] [Google Scholar]
- 46.Porter F. D. 2000. RSH/Smith-Lemli-Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol. Genet. Metab. 71: 163–174. [DOI] [PubMed] [Google Scholar]
- 47.Starck L., Bjorkhem I., Ritzen E. M., Nilsson B. Y., von Dobeln U. 1999. Beneficial effects of dietary supplementation in a disorder with defective synthesis of cholesterol. A case report of a girl with Smith-Lemli-Opitz syndrome, polyneuropathy and precocious puberty. Acta Paediatr. 88: 729–733. [DOI] [PubMed] [Google Scholar]
- 48.Fitzky B. U., Moebius F. F., Asaoka H., Waage-Baudet H., Xu L., Xu G., Maeda N., Kluckman K., Hiller S., Yu H., et al. 2001. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J. Clin. Invest. 108: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wassif C. A., Zhu P., Kratz L., Krakowiak P. A., Battaile K. P., Weight F. F., Grinberg A., Steiner R. D., Nwokoro N. A., Kelley R. I., et al. 2001. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum. Mol. Genet. 10: 555–564. [DOI] [PubMed] [Google Scholar]
- 50.Correa-Cerro L. S., Wassif C. A., Kratz L., Miller G. F., Munasinghe J. P., Grinberg A., Fliesler S. J., Porter F. D. 2006. Development and characterization of a hypomorphic Smith-Lemli-Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum. Mol. Genet. 15: 839–851. [DOI] [PubMed] [Google Scholar]
- 51.Saher G., Brugger B., Lappe-Siefke C., Mobius W., Tozawa R., Wehr M. C., Wieland F., Ishibashi S., Nave K. A. 2005. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 8: 468–475. [DOI] [PubMed] [Google Scholar]
- 52.Saher G., Quintes S., Mobius W., Wehr M. C., Kramer-Albers E. M., Brugger B., Nave K. A. 2009. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J. Neurosci. 29: 6094–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Figura K., Gieselmann V., Jaeken J. 2001. Metachromatic leukodystrophy. The Metabolic And Molecular Basis of Inherited Disease. Scriver C. R., Beaudet A. L., Valle D., Sly W. S., McGraw Hill, New York: 3695–3724. [Google Scholar]
- 54.Landrieu P., Said G. 1984. Peripheral neuropathy in type A Niemann-Pick disease. A morphological study. Acta Neuropathol. 63: 66–71. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki K. 2003. Globoid cell leukodystrophy (Krabbe's disease): update. J. Child Neurol. 18: 595–603. [DOI] [PubMed] [Google Scholar]
- 56.Schuchman E. H., Desnick R. J. 2001. Niemann-Pick disease types A and B: acid sphingomyelinase deficiencies. The Metabolic and Molecular Basis of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Childs B., Kinzler K. W., Vogelstein B., McGraw-Hill, New York: 3589–3610. [Google Scholar]
- 57.Edvardson S., Hama H., Shaag A., Gomori J. M., Berger I., Soffer D., Korman S. H., Taustein I., Saada A., Elpeleg O. 2008. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am. J. Hum. Genet. 83: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dick K. J., Eckhardt M., Paisan-Ruiz C., Alshehhi A. A., Proukakis C., Sibtain N. A., Maier H., Sharifi R., Patton M. A., Bashir W., et al. 2010. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35). Hum. Mutat. 31: E1251–E1260. [DOI] [PubMed] [Google Scholar]
- 59.Alderson N. L., Rembiesa B. M., Walla M. D., Bielawska A., Bielawski J., Hama H. 2004. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 279: 48562–48568. [DOI] [PubMed] [Google Scholar]
- 60.Zoller I., Meixner M., Hartmann D., Bussow H., Meyer R., Gieselmann V., Eckhardt M. 2008. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 28: 9741–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enquist I. B., Lo Bianco C., Ooka A., Nilsson E., Mansson J. E., Ehinger M., Richter J., Brady R. O., Kirik D., Karlsson S. 2007. Murine models of acute neuronopathic Gaucher disease. Proc. Natl. Acad. Sci. USA. 104: 17483–17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hailemariam T. K., Huan C., Liu J., Li Z., Roman C., Kalbfeisch M., Bui H. H., Peake D. A., Kuo M. S., Cao G., et al. 2008. Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler. Thromb. Vasc. Biol. 28: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 63.Marcus J., Honigbaum S., Shroff S., Honke K., Rosenbluth J., Dupree J. L. 2006. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 53: 372–381. [DOI] [PubMed] [Google Scholar]
- 64.Hoshi T., Suzuki A., Hayashi S., Tohyama K., Hayashi A., Yamaguchi Y., Takeuchi K., Baba H. 2007. Nodal protrusions, increased Schmidt-Lanterman incisures, and paranodal disorganization are characteristic features of sulfatide-deficient peripheral nerves. Glia. 55: 584–594. [DOI] [PubMed] [Google Scholar]
- 65.Coetzee T., Fujita N., Dupree J., Shi R., Blight A., Suzuki K., Popko B. 1996. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 86: 209–219. [DOI] [PubMed] [Google Scholar]
- 66.Saadat L., Dupree J. L., Kilkus J., Han X., Traka M., Proia R. L., Dawson G., Popko B. 2010. Absence of oligodendroglial glucosylceramide synthesis does not result in CNS myelin abnormalities or alter the dysmyelinating phenotype of CGT-deficient mice. Glia. 58: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizzo W. B., Carney G., Lin Z. 1999. The molecular basis of Sjogren-Larsson syndrome: mutation analysis of the fatty aldehyde dehydrogenase gene. Am. J. Hum. Genet. 65: 1547–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willemsen M. A., Van Der Graaf M., Van Der Knaap M. S., Heerschap A., Van Domburg P. H., Gabreels F. J., Rotteveel J. J. 2004. MR imaging and proton MR spectroscopic studies in Sjogren-Larsson syndrome: characterization of the leukoencephalopathy. AJNR Am. J. Neuroradiol. 25: 649–657. [PMC free article] [PubMed] [Google Scholar]
- 69.van Domburg P. H., Willemsen M. A., Rotteveel J. J., de Jong J. G., Thijssen H. O., Heerschap A., Cruysberg J. R., Wanders R. J., Gabreels F. J., Steijlen P. M. 1999. Sjogren-Larsson syndrome: clinical and MRI/MRS findings in FALDH-deficient patients. Neurology. 52: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 70.Wierzbicki A. S., Lloyd M. D., Schofield C. J., Feher M. D., Gibberd F. B. 2002. Refsum's disease: a peroxisomal disorder affecting phytanic acid alpha-oxidation. J. Neurochem. 80: 727–735. [DOI] [PubMed] [Google Scholar]
- 71.Steinberg S. J., Dodt G., Raymond G. V., Braverman N. E., Moser A. B., Moser H. W. 2006. Peroxisome biogenesis disorders. Biochim. Biophys. Acta. 1763: 1733–1748. [DOI] [PubMed] [Google Scholar]
- 72.Mayatepek E., Baumann M., Meissner T., Hanefeld F., Korenke G. C. 2003. Role of leukotrienes as indicators of the inflammatory demyelinating reaction in x–linked cerebral adrenoleukodystrophy. J. Neurol. 250: 1259–1260. [DOI] [PubMed] [Google Scholar]
- 73.Kassmann C. M., Nave K. A. 2008. Oligodendroglial impact on axonal function and survival - a hypothesis. Curr. Opin. Neurol. 21: 235–241. [DOI] [PubMed] [Google Scholar]
- 74.Van Veldhoven P. P. 2010. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 51: 2863–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imgrund S., Hartmann D., Farwanah H., Eckhardt M., Sandhoff R., Degen J., Gieselmann V., Sandhoff K., Willecke K. 2009. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 284: 33549–33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brites P., Mooyer P. A., El Mrabet L., Waterham H. R., Wanders R. J. 2009. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain. 132: 482–492. [DOI] [PubMed] [Google Scholar]
- 77.Brites P., Motley A. M., Gressens P., Mooyer P. A., Ploegaert I., Everts V., Evrard P., Carmeliet P., Dewerchin M., Schoonjans L., et al. 2003. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: a model for rhizomelic chondrodysplasia punctata. Hum. Mol. Genet. 12: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 78.Maxwell M., Bjorkman J., Nguyen T., Sharp P., Finnie J., Paterson C., Tonks I., Paton B. C., Kay G. F., Crane D. I. 2003. Pex13 inactivation in the mouse disrupts peroxisome biogenesis and leads to a Zellweger syndrome phenotype. Mol. Cell. Biol. 23: 5947–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faust P. L., Hatten M. E. 1997. Targeted deletion of the PEX2 peroxisome assembly gene in mice provides a model for Zellweger syndrome, a human neuronal migration disorder. J. Cell Biol. 139: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baes M., Aubourg P. 2009. Peroxisomes, myelination, and axonal integrity in the CNS. Neuroscientist. 15: 367–379. [DOI] [PubMed] [Google Scholar]
- 81.Baes M., Gressens P., Baumgart E., Carmeliet P., Casteels M., Fransen M., Evrard P., Fahimi D., Declercq P. E., Collen D., et al. 1997. A mouse model for Zellweger syndrome. Nat. Genet. 17: 49–57. [DOI] [PubMed] [Google Scholar]
- 82.Kassmann C. M., Lappe-Siefke C., Baes M., Brugger B., Mildner A., Werner H. B., Natt O., Michaelis T., Prinz M., Frahm J., et al. 2007. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 39: 969–976. [DOI] [PubMed] [Google Scholar]
- 83.Powers J. M., Liu Y., Moser A. B., Moser H. W. 1992. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J. Neuropathol. Exp. Neurol. 51: 630–643. [DOI] [PubMed] [Google Scholar]
- 84.Ito M., Blumberg B. M., Mock D. J., Goodman A. D., Moser A. B., Moser H. W., Smith K. D., Powers J. M. 2001. Potential environmental and host participants in the early white matter lesion of adreno-leukodystrophy: morphologic evidence for CD8 cytotoxic T cells, cytolysis of oligodendrocytes, and CD1-mediated lipid antigen presentation. J. Neuropathol. Exp. Neurol. 60: 1004–1019. [DOI] [PubMed] [Google Scholar]
- 85.elMasry M. N., Rich R. R. 1989. Prostaglandin E2 selectively increases interferon gamma receptor expression on human CD8+ lymphocytes. J. Clin. Invest. 83: 1436–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jozefowski S., Biedron R., Bobek M., Marcinkiewicz J. 2005. Leukotrienes modulate cytokine release from dendritic cells. Immunology. 116: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schepers L., Casteels M., Vamecq J., Parmentier G., Van Veldhoven P. P., Mannaerts G. P. 1988. Beta-oxidation of the carboxyl side chain of prostaglandin E2 in rat liver peroxisomes and mitochondria. J. Biol. Chem. 263: 2724–2731. [PubMed] [Google Scholar]
- 88.Ferdinandusse S., Meissner T., Wanders R. J., Mayatepek E. 2002. Identification of the peroxisomal beta-oxidation enzymes involved in the degradation of leukotrienes. Biochem. Biophys. Res. Commun. 293: 269–273. [DOI] [PubMed] [Google Scholar]
- 89.Rodemer C., Thai T. P., Brugger B., Kaercher T., Werner H., Nave K. A., Wieland F., Gorgas K., Just W. W. 2003. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 12: 1881–1895. [DOI] [PubMed] [Google Scholar]
- 90.Teigler A., Komljenovic D., Draguhn A., Gorgas K., Just W. W. 2009. Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum. Hum. Mol. Genet. 18: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tamby J. P., Reinaud P., Charpigny G. 1996. Preferential esterification of arachidonic acid into ethanolamine phospholipids in epithelial cells from ovine endometrium. J. Reprod. Fertil. 107: 23–30. [DOI] [PubMed] [Google Scholar]
- 92.Brites P., Waterham H. R., Wanders R. J. 2004. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta. 1636: 219–231. [DOI] [PubMed] [Google Scholar]
- 93.Marin-Valencia I., Roe C. R., Pascual J. M. 2010. Pyruvate carboxylase deficiency: mechanisms, mimics and anaplerosis. Mol. Genet. Metab. 101: 9–17. [DOI] [PubMed] [Google Scholar]
- 94.Schiff M., Levrat V., Acquaviva C., Vianey-Saban C., Rolland M. O., Guffon N. 2006. A case of pyruvate carboxylase deficiency with atypical clinical and neuroradiological presentation. Mol. Genet. Metab. 87: 175–177. [DOI] [PubMed] [Google Scholar]
- 95.Cesar M., Hamprecht B. 1995. Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J. Neurochem. 64: 2312–2318. [DOI] [PubMed] [Google Scholar]
- 96.Shank R. P., Bennett G. S., Freytag S. O., Campbell G. L. 1985. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 329: 364–367. [DOI] [PubMed] [Google Scholar]
- 97.Murin R., Cesar M., Kowtharapu B. S., Verleysdonk S., Hamprecht B. 2009. Expression of pyruvate carboxylase in cultured oligodendroglial, microglial and ependymal cells. Neurochem. Res. 34: 480–489. [DOI] [PubMed] [Google Scholar]
- 98.Namboodiri A. M., Peethambaran A., Mathew R., Sambhu P. A., Hershfield J., Moffett J. R., Madhavarao C. N. 2006. Canavan disease and the role of N-acetylaspartate in myelin synthesis. Mol. Cell. Endocrinol. 252: 216–223. [DOI] [PubMed] [Google Scholar]
- 99.Madhavarao C. N., Moffett J. R., Moore R. A., Viola R. E., Namboodiri M. A., Jacobowitz D. M. 2004. Immunohistochemical localization of aspartoacylase in the rat central nervous system. J. Comp. Neurol. 472: 318–329. [DOI] [PubMed] [Google Scholar]
- 100.Adachi M., Schneck L., Cara J., Volk B. W. 1973. Spongy degeneration of the central nervous system (van Bogaert and Bertrand type; Canavan's disease). A review. Hum. Pathol. 4: 331–347. [DOI] [PubMed] [Google Scholar]
- 101.Gascon G. G., Ozand P. T., Mahdi A., Jamil A., Haider A., Brismar J., al-Nasser M. 1990. Infantile CNS spongy degeneration–14 cases: clinical update. Neurology. 40: 1876–1882. [DOI] [PubMed] [Google Scholar]
- 102.Matalon R. M., Michals-Matalon K. 2000. Spongy degeneration of the brain, Canavan disease: biochemical and molecular findings. Front. Biosci. 5: D307–D311. [DOI] [PubMed] [Google Scholar]
- 103.Traka M., Wollmann R. L., Cerda S. R., Dugas J., Barres B. A., Popko B. 2008. Nur7 is a nonsense mutation in the mouse aspartoacylase gene that causes spongy degeneration of the CNS. J. Neurosci. 28: 11537–11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madhavarao C. N., Arun P., Moffett J. R., Szucs S., Surendran S., Matalon R., Garbern J., Hristova D., Johnson A., Jiang W., et al. 2005. Defective N-acetylaspartate catabolism reduces brain acetate levels and myelin lipid synthesis in Canavan's disease. Proc. Natl. Acad. Sci. USA. 102: 5221–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ledeen R. W., Wang J., Wu G., Lu Z. H., Chakraborty G., Meyenhofer M., Tyring S. K., Matalon R. 2006. Physiological role of N-acetylaspartate: contribution to myelinogenesis. Adv. Exp. Med. Biol. 576: 131–143; discussion 361–133. [DOI] [PubMed] [Google Scholar]
- 106.Namboodiri A. M., Moffett J. R., Arun P., Mathew R., Namboodiri S., Potti A., Hershfield J., Kirmani B., Jacobowitz D. M., Madhavarao C. N. 2006. Defective myelin lipid synthesis as a pathogenic mechanism of Canavan disease. Adv. Exp. Med. Biol. 576: 145–163; discussion 361–143. [DOI] [PubMed] [Google Scholar]
- 107.Chakraborty G., Mekala P., Yahya D., Wu G., Ledeen R. W. 2001. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J. Neurochem. 78: 736–745. [DOI] [PubMed] [Google Scholar]
- 108.Kumar S., Biancotti J. C., Matalon R., de Vellis J. 2009. Lack of aspartoacylase activity disrupts survival and differentiation of neural progenitors and oligodendrocytes in a mouse model of Canavan disease. J. Neurosci. Res. 87: 3415–3427. [DOI] [PubMed] [Google Scholar]
- 109.Baslow M. H., Guilfoyle D. N. 2009. Are astrocytes the missing link between lack of brain aspartoacylase activity and the spongiform leukodystrophy in Canavan disease? Neurochem. Res. 34: 1523–1534. [DOI] [PubMed] [Google Scholar]
- 110.Verheijen M. H., Camargo N., Verdier V., Nadra K., de Preux Charles A. S., Medard J. J., Luoma A., Crowther M., Inouye H., Shimano H., et al. 2009. SCAP is required for timely and proper myelin membrane synthesis. Proc. Natl. Acad. Sci. USA. 106: 21383–21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee A. G. 2003. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 1612: 1–40. [DOI] [PubMed] [Google Scholar]
- 112.Simons K., Vaz W. L. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33: 269–295. [DOI] [PubMed] [Google Scholar]
- 113.Sankaram M. B., Thompson T. E. 1990. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 29: 10676–10684. [DOI] [PubMed] [Google Scholar]
- 114.Huang J., Feigenson G. W. 1999. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76: 2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kakorin S., Brinkmann U., Neumann E. 2005. Cholesterol reduces membrane electroporation and electric deformation of small bilayer vesicles. Biophys. Chem. 117: 155–171. [DOI] [PubMed] [Google Scholar]
- 116.Pan J., Tristram-Nagle S., Nagle J. F. 2009. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 80: 021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hofsass C., Lindahl E., Edholm O. 2003. Molecular dynamics simulations of phospholipid bilayers with cholesterol. Biophys. J. 84: 2192–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang T. Y., Silvius J. R. 2003. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys. J. 84: 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kulkarni K., Snyder D. S., McIntosh T. J. 1999. Adhesion between cerebroside bilayers. Biochemistry. 38: 15264–15271. [DOI] [PubMed] [Google Scholar]
- 120.Ruocco M. J., Atkinson D., Small D. M., Skarjune R. P., Oldfield E., Shipley G. G. 1981. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 20: 5957–5966. [DOI] [PubMed] [Google Scholar]
- 121.Bosio A., Binczek E., Haupt W. F., Stoffel W. 1998. Composition and biophysical properties of myelin lipid define the neurological defects in galactocerebroside- and sulfatide-deficient mice. J. Neurochem. 70: 308–315. [DOI] [PubMed] [Google Scholar]
- 122.van Meer G., Voelker D. R., Feigenson G. W. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Glaser P. E., Gross R. W. 1994. Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry. 33: 5805–5812. [DOI] [PubMed] [Google Scholar]
- 124.Glaser P. E., Gross R. W. 1995. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry. 34: 12193–12203. [DOI] [PubMed] [Google Scholar]
- 125.Sastry P. S. 1985. Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res. 24: 69–176. [DOI] [PubMed] [Google Scholar]
- 126.Hu Y., Doudevski I., Wood D., Moscarello M., Husted C., Genain C., Zasadzinski J. A., Israelachvili J. 2004. Synergistic interactions of lipids and myelin basic protein. Proc. Natl. Acad. Sci. USA. 101: 13466–13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao S., Hu X., Park J., Zhu Y., Zhu Q., Li H., Luo C., Han R., Cooper N., Qiu M. 2007. Selective expression of LDLR and VLDLR in myelinating oligodendrocytes. Dev. Dyn. 236: 2708–2712. [DOI] [PubMed] [Google Scholar]
- 128.Goodrum J. F., Fowler K. A., Hostettler J. D., Toews A. D. 2000. Peripheral nerve regeneration and cholesterol reutilization are normal in the low-density lipoprotein receptor knockout mouse. J. Neurosci. Res. 59: 581–586. [DOI] [PubMed] [Google Scholar]
- 129.Simons M., Kramer E. M., Thiele C., Stoffel W., Trotter J. 2000. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J. Cell Biol. 151: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kramer-Albers E. M., Gehrig-Burger K., Thiele C., Trotter J., Nave K. A. 2006. Perturbed interactions of mutant proteolipid protein/DM20 with cholesterol and lipid rafts in oligodendroglia: implications for dysmyelination in spastic paraplegia. J. Neurosci. 26: 11743–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bansal R., Winkler S., Bheddah S. 1999. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J. Neurosci. 19: 7913–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hirahara Y., Bansal R., Honke K., Ikenaka K., Wada Y. 2004. Sulfatide is a negative regulator of oligodendrocyte differentiation: development in sulfatide-null mice. Glia. 45: 269–277. [DOI] [PubMed] [Google Scholar]
- 133.Nadra K., de Preux Charles A. S., Medard J. J., Hendriks W. T., Han G. S., Gres S., Carman G. M., Saulnier-Blache J. S., Verheijen M. H., Chrast R. 2008. Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 22: 1647–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huether G., Klapproth M., Neuhoff V. 1986. Fatty acid composition of myelin lipids from developing rat forebrain and spinal cord: influence of experimental hyperphenylalaninaemia. Neurochem. Res. 11: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 135.Nave K. A. 2010. Myelination and the trophic support of long axons. Natl. Rev. 11: 275–283. [DOI] [PubMed] [Google Scholar]
- 136.Chrast R., Verheijen M. H., Lemke G. 2004. Complement factors in adult peripheral nerve: a potential role in energy metabolism. Neurochem. Int. 45: 353–359. [DOI] [PubMed] [Google Scholar]
- 137.de Preux A. S., Goosen K., Zhang W., Sima A. A., Shimano H., Ouwens D. M., Diamant M., Hillebrands J. L., Rozing J., Lemke G., et al. 2007. SREBP-1c expression in Schwann cells is affected by diabetes and nutritional status. Mol. Cell. Neurosci. 35: 525–534. [DOI] [PubMed] [Google Scholar]
- 138.Verrips A., Wevers R. A., Van Engelen B. G., Keyser A., Wolthers B. G., Barkhof F., Stalenhoef A., De Graaf R., Janssen-Zijlstra F., Van Spreeken A., et al. 1999. Effect of simvastatin in addition to chenodeoxycholic acid in patients with cerebrotendinous xanthomatosis. Metabolism. 48: 233–238. [DOI] [PubMed] [Google Scholar]
- 139.Saheki A., Terasaki T., Tamai I., Tsuji A. 1994. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm. Res. 11: 305–311. [DOI] [PubMed] [Google Scholar]
- 140.Paintlia A. S., Paintlia M. K., Khan M., Vollmer T., Singh A. K., Singh I. 2005. HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. Faseb. J. 19: 1407–1421. [DOI] [PubMed] [Google Scholar]
- 141.Paintlia A. S., Paintlia M. K., Singh A. K., Singh I. 2008. Inhibition of rho family functions by lovastatin promotes myelin repair in ameliorating experimental autoimmune encephalomyelitis. Mol. Pharmacol. 73: 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stanislaus R., Gilg A. G., Singh A. K., Singh I. 2002. Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by Lovastatin. Neurosci. Lett. 333: 167–170. [DOI] [PubMed] [Google Scholar]
- 143.Youssef S., Stuve O., Patarroyo J. C., Ruiz P. J., Radosevich J. L., Hur E. M., Bravo M., Mitchell D. J., Sobel R. A., Steinman L., Zamvil S. S. 2002. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 420: 78–84. [DOI] [PubMed] [Google Scholar]
- 144.Sena A., Pedrosa R., Graca Morais M. 2003. Therapeutic potential of lovastatin in multiple sclerosis. J. Neurol. 250: 754–755. [DOI] [PubMed] [Google Scholar]
- 145.Vollmer T., Key L., Durkalski V., Tyor W., Corboy J., Markovic-Plese S., Preiningerova J., Rizzo M., Singh I. 2004. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 363: 1607–1608. [DOI] [PubMed] [Google Scholar]
- 146.Maier O., De Jonge J., Nomden A., Hoekstra D., Baron W. 2009. Lovastatin induces the formation of abnormal myelin-like membrane sheets in primary oligodendrocytes. Glia. 57: 402–413. [DOI] [PubMed] [Google Scholar]
- 147.Gaist D., Jeppesen U., Andersen M., Garcia Rodriguez L. A., Hallas J., Sindrup S. H. 2002. Statins and risk of polyneuropathy: a case-control study. Neurology. 58: 1333–1337. [DOI] [PubMed] [Google Scholar]
- 148.Law M., Rudnicka A. R. 2006. Statin safety: a systematic review. Am. J. Cardiol. 97: 52C–60C. [DOI] [PubMed] [Google Scholar]
- 149.Brown W. V. 2008. Safety of statins. Curr. Opin. Lipidol. 19: 558–562. [DOI] [PubMed] [Google Scholar]
- 150.Verhave P. S., Vanwersch R. A., van Helden H. P., Smit A. B., Philippens I. H. 2009. Two new test methods to quantify motor deficits in a marmoset model for Parkinson's disease. Behav. Brain Res. 200: 214–219. [DOI] [PubMed] [Google Scholar]
- 151.Jurevics H. A., Morell P. 1994. Sources of cholesterol for kidney and nerve during development. J. Lipid Res. 35: 112–120. [PubMed] [Google Scholar]
- 152.Morell P., Jurevics H. 1996. Origin of cholesterol in myelin. Neurochem. Res. 21: 463–470. [DOI] [PubMed] [Google Scholar]
- 153.Kelley R. I. 2000. Inborn errors of cholesterol biosynthesis. Adv. Pediatr. 47: 1–53. [PubMed] [Google Scholar]
- 154.Ullrich K., Koch H. G., Meschede D., Flotmann U., Seedorf U. 1996. Smith-Lemli-Opitz syndrome: treatment with cholesterol and bile acids. Neuropediatrics. 27: 111–112. [DOI] [PubMed] [Google Scholar]
- 155.Arun P., Madhavarao C. N., Moffett J. R., Hamilton K., Grunberg N. E., Ariyannur P. S., Gahl W. A., Anikster Y., Mog S., Hallows W. C., et al. 2010. Metabolic acetate therapy improves phenotype in the tremor rat model of Canavan disease. J. Inherit. Metab. Dis. 33: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Das A. K., Holmes R. D., Wilson G. N., Hajra A. K. 1992. Dietary ether lipid incorporation into tissue plasmalogens of humans and rodents. Lipids. 27: 401–405. [DOI] [PubMed] [Google Scholar]
- 157.Saito K., Dubreuil V., Arai Y., Wilsch-Brauninger M., Schwudke D., Saher G., Miyata T., Breier G., Thiele C., Shevchenko A., et al. 2009. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc. Natl. Acad. Sci. USA. 106: 8350–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karasinska J. M., Rinninger F., Lutjohann D., Ruddle P., Franciosi S., Kruit J. K., Singaraja R. R., Hirsch-Reinshagen V., Fan J., Brunham L. R., et al. 2009. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 29: 3579–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spencer B. J., Verma I. M. 2007. Targeted delivery of proteins across the blood-brain barrier. Proc. Natl. Acad. Sci. USA. 104: 7594–7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garbay B., Boiron-Sargueil F., Shy M., Chbihi T., Jiang H., Kamholz J., Cassagne C. 1998. Regulation of oleoyl-CoA synthesis in the peripheral nervous system: demonstration of a link with myelin synthesis. J. Neurochem. 71: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 161.Yao J. K., Holman R. T., Lubozynski M. F., Dyck P. J. 1980. Changes in fatty acid composition of peripheral nerve myelin in essential fatty acid deficiency. Arch. Biochem. Biophys. 204: 175–180. [DOI] [PubMed] [Google Scholar]
- 162.Bourre J. M., Youyou A., Durand G., Pascal G. 1987. Slow recovery of the fatty acid composition of sciatic nerve in rats fed a diet initially low in n-3 fatty acids. Lipids. 22: 535–538. [DOI] [PubMed] [Google Scholar]
- 163.Retra K., Bleijerveld O. B., van Gestel R. A., Tielens A. G., van Hellemond J. J., Brouwers J. F. 2008. A simple and universal method for the separation and identification of phospholipid molecular species. Rapid Commun. Mass Spectrom. 22: 1853–1862. [DOI] [PubMed] [Google Scholar]
- 164.Dod B. J., Gray G. M. 1968. The lipid composition of rat-liver plasma membranes. Biochim. Biophys. Acta. 150: 397–404. [DOI] [PubMed] [Google Scholar]
- 165.Ray T. K., Skipski V. P., Barclay M., Essner E., Archibald F. M. 1969. Lipid composition of rat liver plasma membranes. J. Biol. Chem. 244: 5528–5536. [PubMed] [Google Scholar]
- 166.Lorincz M. T., Rainier S., Thomas D., Fink J. K. 2005. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch. Neurol. 62: 1459–1463. [DOI] [PubMed] [Google Scholar]
- 167.Brunham L. R., Singaraja R. R., Hayden M. R. 2006. Variations on a gene: rare and common variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis. Annu. Rev. Nutr. 26: 105–129. [DOI] [PubMed] [Google Scholar]
- 168.Heim P., Claussen M., Hoffmann B., Conzelmann E., Gartner J., Harzer K., Hunneman D. H., Kohler W., Kurlemann G., Kohlschutter A. 1997. Leukodystrophy incidence in Germany. Am. J. Med. Genet. 71: 475–478. [PubMed] [Google Scholar]
- 169.Gustavson K. H., Hagberg B. 1971. The incidence and genetics of metachromatic leucodystrophy in northern Sweden. Acta Paediatr. Scand. 60: 585–590. [DOI] [PubMed] [Google Scholar]
- 170.Schuchman E. H. 2007. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease. J. Inherit. Metab. Dis. 30: 654–663. [DOI] [PubMed] [Google Scholar]
- 171.Jagell S., Gustavson K. H., Holmgren G. 1981. Sjogren-Larsson syndrome in Sweden. A clinical, genetic and epidemiological study. Clin. Genet. 19: 233–256. [DOI] [PubMed] [Google Scholar]
- 172.Lazarow P. B., Moser H. W. 1989. Disorders of peroxisome biogenesis. The Metabolic Basis of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., McGraw-Hill, New York: 1479–1509. [Google Scholar]
- 173.Berger J., Gartner J. 2006. X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim. Biophys. Acta. 1763: 1721–1732. [DOI] [PubMed] [Google Scholar]
- 174.Feigenbaum A., Moore R., Clarke J., Hewson S., Chitayat D., Ray P. N., Stockley T. L. 2004. Canavan disease: carrier-frequency determination in the Ashkenazi Jewish population and development of a novel molecular diagnostic assay. Am. J. Med. Genet. A. 124A: 142–147. [DOI] [PubMed] [Google Scholar]
- 175.Brun N., Robitaille Y., Grignon A., Robinson B. H., Mitchell G. A., Lambert M. 1999. Pyruvate carboxylase deficiency: prenatal onset of ischemia-like brain lesions in two sibs with the acute neonatal form. Am. J. Med. Genet. 84: 94–101. [PubMed] [Google Scholar]