Abstract
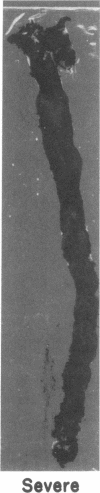
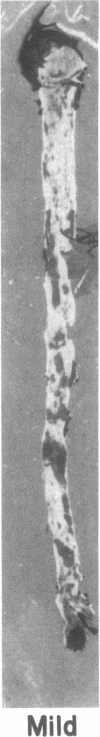
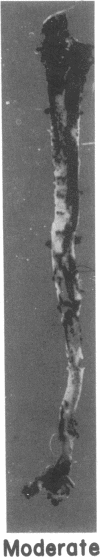
Dehydroepiandrosterone (DHEA) is an endogenous steroid that blocks carcinogenesis, retards aging, and exerts antiproliferative properties. In vitro, it is a potent inhibitor of glucose-6-phosphate dehydrogenase, the first committed step of the pentose phosphate pathway. In man, serum levels of DHEA and its sulfate peak in early adulthood and drop markedly with age. Epidemiologic evidence indicates that low levels of DHEA or its sulfate conjugate are linked to an increased risk of developing cancer or of death from cardiovascular disease. Like cancer, atherosclerosis is a proliferative process characterized by both initiation and promotion phases. This similarity provided a framework in which to study the antiatherogenic effects of DHEA. Rabbits were randomly assigned to four groups. Two groups of rabbits received aortic endothelial injury by balloon catheter and were fed a 2% cholesterol diet for 12 wk. DHEA, 0.5%, was incorporated into the diet of one group receiving the 2% cholesterol diet and endothelial injury and also into the diet of one of the control groups. Animals were killed after 12 wk and aortas, hearts, and livers were studied. Plasma samples were analyzed for total cholesterol, VLDL, LDL, HDL, triglycerides, DHEA, and DHEA-sulfate levels. The atherogenic insult resulted in severe atherosclerosis in animals not treated with DHEA. In those receiving DHEA there was an almost 50% reduction in plaque size (P = 0.006), inversely related to the serum level of DHEA attained. Fatty infiltration of the heart and liver were also markedly reduced. These beneficial actions were not attributable to differences in body weight gain, food intake, total plasma cholesterol or distribution of cholesterol among the VLDL, LDL, or HDL fractions. The results show that high levels of plasma DHEA inhibit the development of atherosclerosis and they provide an important experimental link to the epidemiologic studies correlating low DHEA-sulfate plasma levels with an enhanced risk of cardiovascular mortality.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Vanderlaan M., Burns F. J., Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977 Jul;37(7 Pt 1):2232–2235. [PubMed] [Google Scholar]
- Barrett-Connor E., Khaw K. T., Yen S. S. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986 Dec 11;315(24):1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R. The role of blood flow in platelet adhesion, fibrin deposition, and formation of mural thrombi. Microvasc Res. 1973 Mar;5(2):167–179. doi: 10.1016/0026-2862(73)90069-1. [DOI] [PubMed] [Google Scholar]
- Ben-David M., Dikstein S., Bismuth G., Sulman F. G. Anti-hypercholesterolemic effect of dehydroepiandrosterone in rats. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1136–1140. doi: 10.3181/00379727-125-32297. [DOI] [PubMed] [Google Scholar]
- Brownsey B., Cameron E. H., Griffiths K., Gleave E. N., Forrest A. P., Campbell H. Plasma dehydroepiandrosterone sulphate levels in patients with benign and malignant breast disease. Eur J Cancer. 1972 Feb;8(1):131–137. doi: 10.1016/0014-2964(72)90094-1. [DOI] [PubMed] [Google Scholar]
- Buja L. M., Kita T., Goldstein J. L., Watanabe Y., Brown M. S. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arteriosclerosis. 1983 Jan-Feb;3(1):87–101. doi: 10.1161/01.atv.3.1.87. [DOI] [PubMed] [Google Scholar]
- Chubb C., Ewing L. L. Steroid secretion by in vitro perfused testes: secretions of rabbit and rat testes. Am J Physiol. 1979 Sep;237(3):E231–E238. doi: 10.1152/ajpendo.1979.237.3.E231. [DOI] [PubMed] [Google Scholar]
- Clarkson T. B. Animal models of atherosclerosis. Adv Vet Sci Comp Med. 1972;16:151–173. [PubMed] [Google Scholar]
- Goldstein J. L., Kita T., Brown M. S. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med. 1983 Aug 4;309(5):288–296. doi: 10.1056/NEJM198308043090507. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Newitt J. A., Shantz L. M., Weng D. E., Talalay P. Inhibition of the conversion of 3T3 fibroblast clones to adipocytes by dehydroepiandrosterone and related anticarcinogenic steroids. Cancer Res. 1986 Jul;46(7):3389–3395. [PubMed] [Google Scholar]
- Gordon G. B., Shantz L. M., Talalay P. Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv Enzyme Regul. 1987;26:355–382. doi: 10.1016/0065-2571(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Hough J. L., Zilversmit D. B. Effect of 17 beta estradiol on aortic cholesterol content and metabolism in cholesterol-fed rabbits. Arteriosclerosis. 1986 Jan-Feb;6(1):57–63. doi: 10.1161/01.atv.6.1.57. [DOI] [PubMed] [Google Scholar]
- KASK E. 17-Ketosteroids and arteriosclerosis. Angiology. 1959 Oct;10:358–368. doi: 10.1177/000331975901000504. [DOI] [PubMed] [Google Scholar]
- Kushwaha R. S., Hazzard W. R. Exogenous estrogens attenuate dietary hypercholesterolemia and atherosclerosis in the rabbit. Metabolism. 1981 Apr;30(4):359–366. doi: 10.1016/0026-0495(81)90116-5. [DOI] [PubMed] [Google Scholar]
- Levy H. R. Glucose-6-phosphate dehydrogenases. Adv Enzymol Relat Areas Mol Biol. 1979;48:97–192. doi: 10.1002/9780470122938.ch3. [DOI] [PubMed] [Google Scholar]
- Lopez-S A., Wingo C., Hebert J. A. Total serum cholesterol and urinary dehydroepiandrosterone in humans. Atherosclerosis. 1976 Sep;24(3):471–481. doi: 10.1016/0021-9150(76)90139-8. [DOI] [PubMed] [Google Scholar]
- MIGEON C. J., KELLER A. R., LAWRENCE B., SHEPARD T. H., 2nd Dehydroepiandrosterone and androsterone levels in human plasma: effect of age and sex; day-to-day and diurnal variations. J Clin Endocrinol Metab. 1957 Sep;17(9):1051–1062. doi: 10.1210/jcem-17-9-1051. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Banks J. INHIBITION OF MAMMALIAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE BY STEROIDS. Proc Natl Acad Sci U S A. 1960 Apr;46(4):447–452. doi: 10.1073/pnas.46.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieschlag E., Loriaux D. L., Ruder H. J., Zucker I. R., Kirschner M. A., Lipsett M. B. The secretion of dehydroepiandrosterone and dehydroepiandrosterone sulphate in man. J Endocrinol. 1973 Apr;57(1):123–134. doi: 10.1677/joe.0.0570123. [DOI] [PubMed] [Google Scholar]
- Nowaczynski W., Fragachan F., Silah J., Millette B., Genest J. Further evidence of altered adrenocortical function in hypertension. Dehydroepiandrosterone excretion rate. Can J Biochem. 1968 Sep;46(9):1031–1038. doi: 10.1139/o68-155. [DOI] [PubMed] [Google Scholar]
- Orentreich N., Brind J. L., Rizer R. L., Vogelman J. H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984 Sep;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Paigen B., Holmes P. A., Morrow A., Mitchell D. Effect of 3-methylcholanthrene on atherosclerosis in two congenic strains of mice with different susceptibilities to methylcholanthrene-induced tumors. Cancer Res. 1986 Jul;46(7):3321–3324. [PubMed] [Google Scholar]
- Pashko L. L., Rovito R. J., Williams J. R., Sobel E. L., Schwartz A. G. Dehydroepiandrosterone (DHEA) and 3 beta-methylandrost-5-en-17-one: inhibitors of 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)-promoted skin papilloma formation in mice. Carcinogenesis. 1984 Apr;5(4):463–466. doi: 10.1093/carcin/5.4.463. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J., Williams K. J., Wolff J. A., Adams R., Solez K., Heptinstall R. H., Malmros H., Sternby N. Clonal characteristics of experimentally induced "atherosclerotic" lesions in the hybrid hare. Science. 1979 Dec 21;206(4425):1423–1425. doi: 10.1126/science.505016. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi M., Leask J. T., Waites G. M. In vitro metabolism of androgens by mammalian cauda epididymal spermatozoa. Steroids. 1978 Jun;31(6):747–760. doi: 10.1016/s0039-128x(78)80040-3. [DOI] [PubMed] [Google Scholar]
- Rogers K. A., Hoover R. L., Castellot J. J., Jr, Robinson J. M., Karnovsky M. J. Dietary cholesterol-induced changes in macrophage characteristics. Relationship to atherosclerosis. Am J Pathol. 1986 Nov;125(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Tsukada T., Gown A. M., Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987 Jan-Feb;7(1):9–23. doi: 10.1161/01.atv.7.1.9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Tsukada T., Gown A. M., Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987 Jan-Feb;7(1):9–23. doi: 10.1161/01.atv.7.1.9. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Sonka J., Fassati M., Fassati P., Gregorová I., Picek K. Serum lipids and dehydroepiandrosterone excretion in normal subjects. J Lipid Res. 1968 Nov;9(6):769–772. [PubMed] [Google Scholar]
- Sporn M. B., Harris E. D., Jr Proliferative diseases. Am J Med. 1981 Jun;70(6):1231–1235. doi: 10.1016/0002-9343(81)90832-9. [DOI] [PubMed] [Google Scholar]
- TANNENBAUM A., SILVERSTONE H. Nutrition in relation to cancer. Adv Cancer Res. 1953;1:451–501. doi: 10.1016/s0065-230x(08)60009-3. [DOI] [PubMed] [Google Scholar]
- Tilton G. D., Buja L. M., Bilheimer D. W., Apprill P., Ashton J., McNatt J., Kita T., Willerson J. T. Failure of a slow channel calcium antagonist, verapamil, to retard atherosclerosis in the Watanabe heritable hyperlipidemic rabbit: an animal model of familial hypercholesterolemia. J Am Coll Cardiol. 1985 Jul;6(1):141–144. doi: 10.1016/s0735-1097(85)80265-5. [DOI] [PubMed] [Google Scholar]
- VANDEWIELE R. L., MACDONALD P. C., GURPIDE E., LIEBERMAN S. STUDIES ON THE SECRETION AND INTERCONVERSION OF THE ANDROGENS. Recent Prog Horm Res. 1963;19:275–310. [PubMed] [Google Scholar]
- Wissler R. W., Vesselinovitch D. Experimental models of human atherosclerosis. Ann N Y Acad Sci. 1968 Nov 21;149(2):907–922. [PubMed] [Google Scholar]
- Zumoff B., Levin J., Rosenfeld R. S., Markham M., Strain G. W., Fukushima D. K. Abnormal 24-hr mean plasma concentrations of dehydroisoandrosterone and dehydroisoandrosterone sulfate in women with primary operable breast cancer. Cancer Res. 1981 Sep;41(9 Pt 1):3360–3363. [PubMed] [Google Scholar]
- Zumoff B., Rosenfeld R. S., Strain G. W., Levin J., Fukushima D. K. Sex differences in the twenty-four-hour mean plasma concentrations of dehydroisoandrosterone (DHA) and dehydroisoandrosterone sulfate (DHAS) and the DHA to DHAS ratio in normal adults. J Clin Endocrinol Metab. 1980 Aug;51(2):330–333. doi: 10.1210/jcem-51-2-330. [DOI] [PubMed] [Google Scholar]
- Zumoff B., Troxler R. G., O'Connor J., Rosenfeld R. S., Kream J., Levin J., Hickman J. R., Sloan A. M., Walker W., Cook R. L. Abnormal hormone levels in men with coronary artery disease. Arteriosclerosis. 1982 Jan-Feb;2(1):58–67. doi: 10.1161/01.atv.2.1.58. [DOI] [PubMed] [Google Scholar]