Abstract
Hepatic lipase (HL) plays a role in the catabolism of apolipoprotein (apo)B-containing lipoproteins through its lipolytic and ligand-binding properties. We describe a potential intracellular role of HL in the assembly and secretion of VLDL. Transient or stable expression of HL in McA-RH7777 cells resulted in decreased (by 40%) incorporation of [3H]glycerol into cell-associated and secreted triacylglycerol (TAG) relative to control cells. However, incorporation of [35S]methionine/cysteine into cell and medium apoB-100 was not decreased by HL expression. The decreased 3H-TAG synthesis/secretion in HL expressing cells was not attributable to decreased expression of genes involved in lipogenesis. Fractionation of medium revealed that the decreased [3H]TAG from HL expressing cells was mainly attributable to decreased VLDL. Expression of catalytically-inactive HL (HLSG) (Ser-145 at the catalytic site was substituted with Gly) in the cells also resulted in decreased secretion of VLDL-[3H]TAG. Examination of lumenal contents of microsomes showed a 40% decrease in [3H]TAG associated with lumenal lipid droplets in HL or HLSG expressing cells as compared with control. The microsomal membrane-associated [3H]TAG was decreased by 50% in HL expressing cells but not in HLSG expressing cells. Thus, expression of HL, irrespective of its lipolytic function, impairs formation of VLDL precursor [3H]TAG in the form of lumenal lipid droplets. These results suggest that HL expression in McA-RH7777 cells result in secretion of [3H]TAG-poor VLDL.
Keywords: apolipoprotein B-100, heparin sulfate proteoglycans, lumenal lipid droplet, microsomal triglyceride transfer protein, triacylglycerol, very low density lipoprotein
HL is a 65 kDa glycoprotein that is synthesized and secreted primarily from parenchymal cells of the liver (1). Once secreted, HL is bound, via heparan sulfate proteoglycans (HSPG), to the surfaces of hepatic sinusoids, the external surfaces of hepatocyte microvilli in the space of Disse and in interhepatocyte spaces (2). As a member of the triglyceride lipase gene family that includes LPL and endothelial lipase (EL), HL plays a central role in lipoprotein metabolism and atherosclerosis. Like LPL and EL, HL has a number of functional domains including the serine-aspartic acid-histidine catalytic triad, a lipid-binding surface loop shielding the catalytic pocket, and heparin binding domains (3, 4). Two major functions have been ascribed to HL, namely i) a hydrolase that catalyzes hydrolysis of triacylglycerol (TAG) and phospholipids (PLs) present in circulating lipoproteins, and ii) a ligand that facilitates binding and uptake of lipoproteins (5, 6). Both functions of HL contribute to the catabolism and clearance of lipids and lipoproteins from the circulation.
In humans, HL deficiency is associated with increased plasma concentrations of HDL as well as TAG-rich apolipoprotein (apo)B-containing lipoproteins, and also an increased prevalence of premature atherosclerosis (7–11). In mice, HL-deficiency results in mild dyslipidemia as compared with wild-type littermates (12, 13). Studies with genetically modified mice, either through overexpression or inactivation of the human HL gene (LIPC), have also provided evidence for HL action in apoB-containing lipoprotein metabolism. Introducing HL deficiency into apoE-null or LDL receptor (LDLR)-null background results in increased plasma concentrations of apoB-containing lipoproteins, TAG, PL, and cholesterol (14, 15). On the other hand, overexpression of human HL in HL-null/LDLR-null background results in ∼60–70% reduction of plasma VLDL-associated TAG, PL, and cholesterol under chow or Western diet (16, 17). Liver-specific expression of human HL in HL-null/apoE-null mice also results in ∼40% reduction in plasma levels of TAG, PL, and cholesterol (18). These in vivo animal studies have firmly established a role for HL in regulating plasma apoB-containing lipoproteins; however, it remains unclear whether the HL action increases or decreases the risk of developing atherosclerosis (6).
Although the majority of the data describes the extracellular role of HL in the catabolism of circulating lipoproteins, some studies have suggested that HL may possess lipolytic activity intracellularly (19–21). The intracellular activity of HL has been detected within the endoplasmic reticulum (ER)/Golgi secretory pathway in transfected Chinese hamster ovary cells (20). In the present study, we tested the hypothesis that expression of HL in hepatic cells may attenuate the assembly and secretion of lipid-rich VLDL. The process of VLDL assembly is initiated during translation and translocation of apoB-100 across the ER membrane (22). The nascent VLDL particle is further enlarged in TAG content through a “second step” lipidation process, where bulk TAG is incorporated to form mature VLDL. In McA-RH7777 cells, maturation of TAG-rich VLDL is achieved in post-ER compartments (23), and the TAG utilized for VLDL maturation is present in the microsomal lumen in the form of lipid droplets (24, 25). Formation of these lumenal lipid droplets (LLD) appears to require the activity of microsomal triglyceride-transfer protein (MTP) (26–28). Recent experimental evidence has suggested that formation of LLD under lipid-rich conditions may also require apoC-III (24, 25). However, how LLD-TAG is incorporated into the nascent VLDL precursor is not known, nor is it clear about lipid or protein factors that regulate the formation of LLD. Data obtained from the present transfection studies suggested that transient or stable expression of HL, regardless of its catalytic activity, exerted a negative impact on the formation of LLD and, consequently, the assembly and secretion of TAG-rich lipoproteins under lipid-rich conditions.
MATERIALS AND METHODS
Materials
DMEM, methionine/cysteine-free DMEM, FBS, horse serum, Lipofectamine, Geneticin® (G418), and TriZol® Reagent were purchased from Invitrogen Canada (Burlington, ON, Canada). Heparin, tributyrin, nonconjugated oleate, and fumed silica were purchased from Sigma-Aldrich (Oakville, ON, Canada). [35S]methionine/cysteine was obtained from MP Biochemicals (Solon, OH). [1-14C]tributyrin and [2-3H]glycerol were obtained from American Radiolabeled Chemicals (St. Louis, MO). Protease inhibitor cocktail and chemiluminescent substrates were purchased from Roche Diagnostics (Laval, PQ, Canada). Antibodies against human HL and actin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and Millipore (Temecula, CA), respectively. The antiserum against rat apoB-100 or apoA-I was generated in our laboratory. The QuantiTect Reverse Transcription Kit and the iQ SYBR Green Supermix were purchased from Qiagen (Mississauga, ON, Canada) and BioRad (Mississauga, ON, Canada), respectively.
Construction of expression plasmids
The expression plasmid encoding human HL (pCMV5-HL) was generated as previously described (29). The plasmid encoding the catalytic inactive HLSG (pCMV5-HLSG), in which the active site Ser-145 was mutated into Gly (S145G), was generated by site-specific mutagenesis using the QuikChange mutagenesis kit (Stratagene). The sense oligonucleotide used for mutagenesis was 5′-CCTAATTGGGTACAGCCTGGGTGC-ACACGTGTCAGG-3′. The adenovirus vector encoding HL was prepared using the AdEasy adenoviral system (Q-Biogene, Carlsbad, CA) according to manufacturer's instructions.
Cell culture and transfection
McA-RH7777 cells were obtained from the American Type Culture Collection and cultured in DMEM containing 10% FBS and 10% horse serum. Stable cell lines were generated by cotransfection with pSV2neo together with either pCMV5-HL or pCMV5HLSG using Lipofectamine (Invitrogen) or calcium phosphate precipitation (30) methods, respectively. The stable clones were selected with G418 (400 μg/ml). After screening for HL expression, the stable clones were maintained in G418 (200 μg/ml). For adenovirus mediated HL expression, cells were incubated with HL-encoding adenovirus (40 pfu per cell) in a minimal amount of serum-free DMEM (1 ml per 60 mm dish) for 1 h. After infection, the cells were cultured in normal media for an additional 36 h prior to experiments.
HL activity assay
Stable cell lines expressing HL or HLSG were cultured for 4 h with serum-free DMEM supplemented with heparin (100 U/ml). The conditioned medium was used as the enzyme source to determine HL activity using [14C]tributyrin as a substrate according to described protocols (31). The amount of [14C]butyrate released into the aqueous phase was quantified by scintillation counting.
Immunoblot analysis
The cells were incubated with serum-free DMEM medium ± heparin (100 U/ml) for 4 h. The cells were harvested in sample loading buffer (SLB: 10 mM Tris, pH 8.0, 8 M urea, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, and bromophenol blue), and the secreted HL proteins were adsorbed onto hydrated fumed silica (Cab-o-sil) as previously described (32) and eluted in SLB. The cell and medium protein samples were loaded onto polyacrylamide gels based on equal cell protein levels and transferred to nitrocellulose membrane for immunoblot analysis with appropriate antibodies against HL and actin.
Metabolic labeling of lipids and proteins
For lipid labeling, cells were labeled for the indicated times with [3H]glycerol (5 μCi/ml) in DMEM containing 20% FBS and 0.4 mM oleate and either with or without 100 U/ml heparin. Total lipids were extracted from cells and media and resolved by TLC as previously described (33). The radioactivity associated with [3H]TAG and [3H]phosphatidylcholine (PC) was quantified by scintillation counting. For protein labeling, cells were pretreated with methionine/cysteine-free media for 30 min prior to incubation with [35S]methionine/cysteine (100 μCi/ml) in the same media for indicated times. Both pretreatment and labeling media were supplemented with 20% FBS and 0.4 mM oleate. In some experiments, heparin (100 U/ml) was included in the labeling media. The respective 35S-labeled proteins were immunoprecipitated from the cells and media and resolved by SDS-PAGE (5% gel for apoB; 12% gel for albumin and actin). Radioactivity associated with the 35S-labeled proteins was quantified by scintillation counting.
Subcellular fractionation
Cells were labeled with [3H]glycerol for the indicated times, homogenized, and fractionated as previously described (23). The postnuclear supernatant was subjected to 165,000 × g for 30 min to isolate the microsomal pellet. The membranous and lumenal fractions of the microsome were separated as previously described (23). Lipids were extracted from all the fractions and analyzed by TLC as described above.
Density fractionation of lipoproteins by cumulative rate flotation ultracentrifugation
Cells were labeled with [3H]glycerol or [35S]methionine/cysteine for the indicated times as described above. Lipoproteins, either present in the media or within the microsomal lumen, from metabolically labeled cells were fractionated into VLDL1 (Sf >100), VLDL2 (Sf 20-100), and other lipoproteins as described previously (27). Lipids were extracted from each fraction and analyzed by TLC as described above.
Real-time RT-PCR
Total RNA was extracted using TriZol reagent following the manufacturer's instructions. Reverse transcription was performed using QuantiTect Reverse Transcription Kit according to manufacturer's directions. PCR was performed on the iCycler (BioRad) using iQ SYBR Green Supermix following manufacturer's instructions. The cycle threshold values were normalized to cyclophilin A (Ppia). Sequences of the PCR primers are listed in supplementary Table I.
Statistical analysis
Values were expressed as mean ± SD. The significance of differences between control and HL expressing cells or between control and HLSG expressing cells were analyzed using Student's t-test.
RESULTS
Expression of hepatic lipase resulted in decreased synthesis and secretion of [3H]TAG
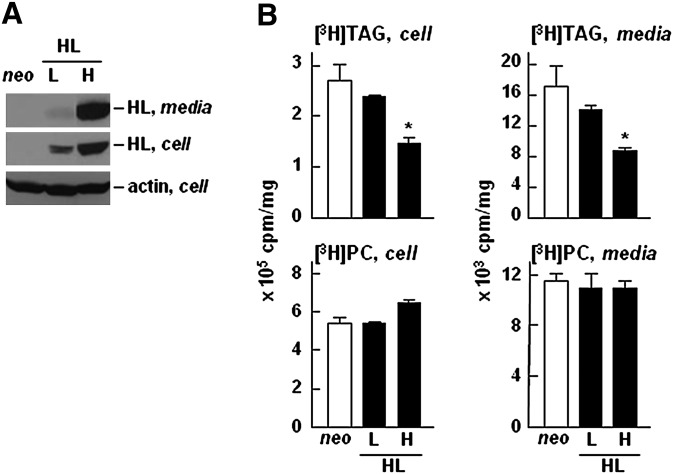
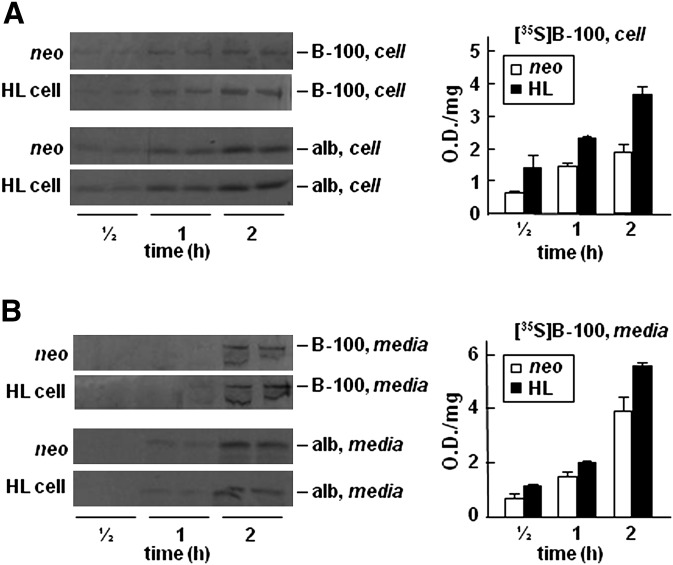
In the current studies, the potential role of HL on hepatic TAG-rich lipoprotein assembly and secretion was determined by transient or stable expression of wild-type or the catalytically-inactive form of HL in McA-RH7777 cells. Among stable clones that expressed different levels of HL (supplementary Fig. I A), the TAG hydrolysis activity (assayed using tributyrin as a substrate) was readily detectable in heparin-treated media (supplementary Fig. I B), confirming that the recombinant enzyme was catalytically active. A very low amount of HL was detectable in the conditioned media containing no heparin (data not shown), suggesting that the recombinant HL, as expected, was mainly bound to extracellular matrix (29). With increasing levels of HL expression in the stable clones (Fig. 1A), the synthesis and secretion of [3H]TAG, as determined by incorporation of [3H]glycerol under lipid-rich conditions (i.e., in the presence of 20% serum and 0.4 mM oleate), were decreased (Fig. 1B, top panels). On the other hand, synthesis or secretion of [3H]PC was not affected by HL expression (Fig. 1B, bottom panels), indicating that the reduced [3H]TAG synthesis in HL cells was not due to impaired [3H]glycerol uptake. Labeling cells with [3H]glycerol for as short as 15 and 30 min also showed a decrease in [3H]glycerol incorporation into cell and medium [3H]TAG in HL expressing cells (supplementary Fig. II), even though the decreased medium [3H]TAG did not reach statistical significance. The decreased incorporation of [3H]glycerol into cell and medium [3H]TAG was also observed in McA-RH7777 cells transiently infected with adenovirus encoding HL (supplementary Fig. III), suggesting that the observed decrease in [3H]TAG in stable HL clones was not due to an adaptation of chronic HL expression. These data combined provide strong evidence that expression of HL resulted in decreased synthesis and secretion of [3H]TAG in McA-RH7777. Unlike that of [3H]TAG, expression of HL did not result in decreased synthesis or secretion of [35S]apoB-100. Metabolic labeling experiments showed that incorporation of 35S-label into cell-associated or medium apoB-100 was not diminished and, rather, was increased in HL expressing cells, although the increase in [35S]apoB-100 did not reach statistical significance (Fig. 2A). Synthesis or secretion of [35S]albumin, another hepatic protein used as a control, was unaffected by HL expression (Fig. 2B). The decreased [3H]TAG secretion without accompanying decrease in [35S]apoB-100 secretion provide the first hint for secretion of lipid-poor VLDL particles upon HL expression.
Fig. 1.
Expression of HL resulted in decreased synthesis and secretion of [3H]TAG. A: Western blots of recombinant HL expressed in cells and secreted into media (collected from 4 h serum-free DMEM containing 100 U/ml heparin). Actin was used as a loading control. L and H denote low and high levels of HL expressing cells. B: Cells were labeled with [3H]glycerol for 2 h in DMEM containing 20% FBS and 0.4 mM oleate. At the end of the labeling period, lipids were extracted from cells (left panels) and media (right panels), respectively, and separated by TLC. Radioactivity associated with [3H]TAG (top panels) and [3H]PC (bottom panels) was quantified by scintillation counting. The values are expressed as cpm/mg cell protein. * p < 0.05 (Student's t -test of HL vs. neo samples, n = 3).
Fig. 2.
Expression of HL does not decrease synthesis or secretion of [35S]apoB-100. Cells were labeled with [35S]methionine/cysteine for 1/2, 1, and 2 h in the presence of 20% FBS and 0.4 mM oleate. At indicated times, apoB-100 and albumin (alb) were recovered from the cells (A) and media (B), respectively, by immunoprecipitation, resolved by SDS-PAGE, and visualized by fluorography (left panels). The intensity of [35S]apoB-100 bands was quantified by scanning densitometry (right panels). Data are presented as the average of two samples (densitometry units/mg cell protein). Repetition of the experiment yielded similar results.
Decreased [3H]TAG secretion as VLDL upon HL expression was independent of the catalytic function of HL
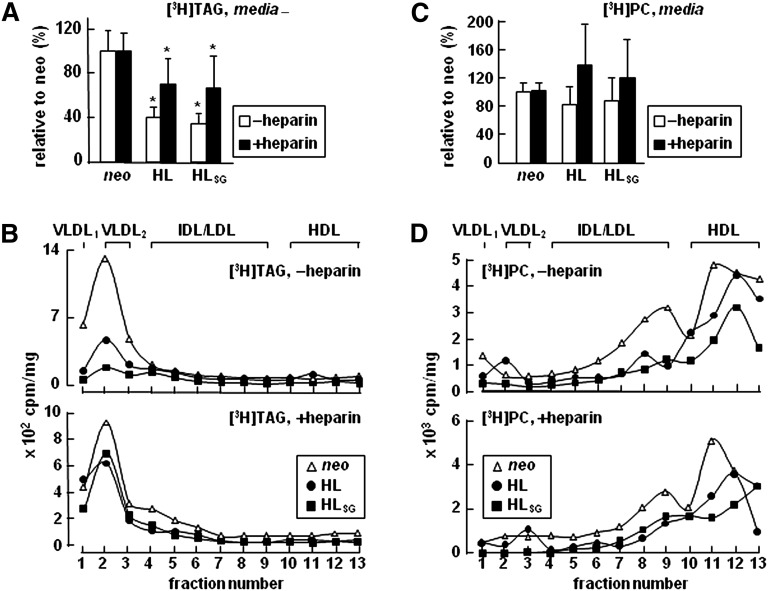
We next determined whether or not the decreased [3H]TAG secretion upon HL expression was attributable to the catalytic activity of the enzyme. To this end, we prepared stable cell lines expressing a mutant HL in which Ser-145 at the catalytic site was substituted with Gly (designated HLSG) (supplementary Fig. IV A). As expected, the mutant HLSG secreted from the transfected cells lost its TAG hydrolysis activity (supplementary Fig. IV B). However, metabolic labeling experiments showed that expression of the mutant HLSG also resulted in decreased [3H]TAG secretion (by >50%), the effect similar to that of wild-type HL (Fig. 3A, open bars). Fractionation of lipoproteins showed that the decreased [3H]TAG secretion from HL and HLSG cells was mainly attributable to decreased VLDL1 and VLDL2 (by ∼75% and ∼60%, respectively) relative to control cells (Fig. 3B, top panel). These results indicate that expression of HL, regardless of its catalytic activity, decreased VLDL-TAG secretion. Secretion of [3H]PC as VLDL1 or VLDL2 from HL or HLSG cells was unchanged as compared with control cells (Fig. 3C, open bars; Fig. 3D, top panel).
Fig. 3.
Decreased secretion of [3H]TAG as VLDL upon HL expression, irrespective of HL catalytic activity. A: Cells were labeled with [3H]glycerol for 2 h in DMEM containing 20% FBS and 0.4 mM oleate ± heparin (100 U/ml). At the end of labeling, lipids were extracted from the media and resolved by TLC. Radioactivity associated with [3H]TAG, quantified by scintillation counting, and was expressed as cpm/mg cell protein. Data presented are converted to [3H]TAG secretion from HL or HLSG cells relative to that from neo control cells (set to 100%). * p < 0.05 (Student's t-test of HL or HLSG vs. neo samples; n = 3). B: The 2 h conditioned labeling media were fractionated by cumulative rate flotation into VLDL1, VLDL2, and other lipoproteins. Lipids were extracted from each fraction, resolved by TLC, and quantified for [3H]TAG. Results are presented as cpm per fraction (normalized with cell proteins). C, D: The experiment was performed the same as in A and B, except that [3H]PC associated with cells (C) and fractionated lipoproteins (D) was quantified.
It has been suggested previously (34–36) that HSPG-anchored HL can capture the secreted TAG-rich lipoproteins on the cell surface. To determine whether or not the decreased VLDL-[3H]TAG from HL or HLSG cells was attributable to surface-anchored HL, we performed metabolic labeling in the presence of heparin to prevent HL cell surface binding. Under this condition, the amount of [3H]TAG recovered from the HL or HLSG media was increased as compared with heparin-free conditions (Fig. 3A, closed bars). This result indicated that a significant amount of newly secreted VLDL particles was indeed captured on the surface of cells expressing either HL or HLSG. However, as compared with neo control, the released [3H]TAG from HL or HLSG cells was still significantly decreased (Fig. 3A, closed bars). Fractionation of lipoproteins secreted under heparin-supplemented conditions also demonstrated reduced secretion of VLDL-[3H]TAG (by 30%) from HL or HLSG cells as compared with neo control (Fig. 3B, bottom panel). Secretion of VLDL-[3H]PC from HL or HLSG cells under heparin supplemented conditions was unchanged as compared with control cells (Fig. 3C, closed bars; Fig. 3D, bottom panel). The presence of heparin in the labeling media did not have an effect on incorporation of [3H]glycerol into cell-associated TAG or PC (results not shown). These data together demonstrated that the reduced secretion of VLDL-TAG upon HL expression in McA-RH7777 cells cultured under lipid-rich conditions was independent of the catalytic activity of HL, and that the capture of newly secreted TAG-rich by cell surface-anchored HL did not completely account for the reduced recovery of VLDL-[3H]TAG in the media. Moreover, the reduced secretion of VLDL-[3H]TAG from HL expressing cells was also observed under lipid-poor conditions (i.e., in the absence of oleate). Under these conditions, although incorporation of [3H]glycerol into TAG and PC was low (supplementary Figs. V A and B), a 50% decrease in VLDL-[3H]TAG secretion from HL expression cells was detected (supplementary Fig. V C) with no changes in [3H]PC secretion (supplementary Fig. V D). Thus, HL expression impairs VLDL-[3H]TAG secretion from McA-RH7777 cells under both lipid-rich and basal conditions.
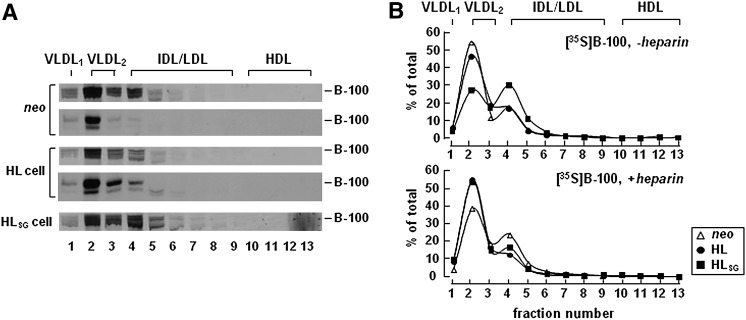
The effect of HL expression on VLDL secretion was also determined by analysis of metabolically labeled [35S]apoB-100. As shown in Fig. 4, distribution of [35S]apoB-100 among lipoproteins was similar between HL expressing cells (middle two panels) and neo controls (top two panels).
Fig. 4.
Expression of HL did not impair [35S]apoB-100 secretion. A: Cells were labeled with [35S]methionine/cysteine for 2 h in the presence of 20% FBS, 0.4 mM oleate, and ± heparin (100 U/ml). The 2 h conditioned labeling media were fractionated by cumulative rate flotation to separate VLDL1, VLDL2, and other lipoproteins. [35S]apoB-100 in each fraction was recovered by immunoprecipitation, resolved by SDS-PAGE, and subjected to fluorography (Fluorograms for fractional [35S]apoB-100 under plus heparin conditions are not shown). Two independent experiments were performed with neo and HL expressing cells. Experiment with HLSG cells was performed once. B: Radioactivity associated with [35S]apoB-100 in each fraction was quantified by scintillation counting. Data are presented as fractional values as percent of total lumenal [35S]apoB-100 under plus and minus heparin conditions.
Likewise, lipoprotein profile of [35S]apoB-100 secreted from HLSG cells (bottom panel) was nearly identical to that from neo controls. Moreover, between heparin-supplemented and heparin-free conditions, the lipoprotein profiles of [35S]apoB-100 secreted from HL, HLSG, and neo control cells are also similar (Fig. 4B). These data, in agreement with what was observed in Fig. 2, indicate that HL expression had minimal effect on [35S]apoB-100 secretion even though the secretion of VLDL-[3H]TAG was markedly decreased.
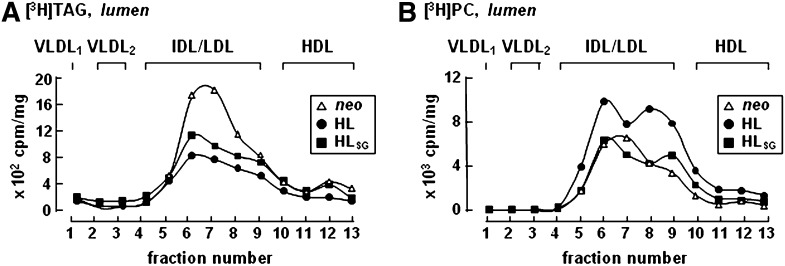
Expression of HL, irrespective of catalytic activity, results in attenuated formation of lumenal lipid droplets rich in [3H]TAG
The above data suggest that HL expression might have exerted an effect on VLDL assembly and secretion. To gain an insight into mechanisms underlying the HL action intracellularly, we determined the effect of HL expression on the formation of LLD that are precursors for TAG-rich VLDL maturation during the second step lipidation (24, 25). Metabolic labeling experiments showed that after 1 h incubation with [3H]glycerol, the association of [3H]TAG with LLD [the fractions with buoyant density resembling that of intermediate density lipoprotein (IDL)/LDL] was readily detectable in neo control cells (Fig. 5A). However, accumulation of [3H]TAG in LLD was reduced (by ∼40%) in cells expressing HL or HLSG. These results show that the expression of HL, regardless of its catalytic activity, resulted in attenuated formation of LLD rich in [3H]TAG. On the other hand, accumulation of [3H]PC in LLD was unaffected by HLSG expression and rather, increased by HL expression (Fig. 5B).
Fig. 5.
Decreased formation of [3H]TAG-rich lumenal lipid droplets upon HL expression, irrespective of HL catalytic activity. Cells were labeled with [3H]glycerol for 1 h in the presence of 20% FBS and 0.4 mM oleate. Cells were homogenized and microsomal lumenal contents were obtained as described under Materials and Methods. The lumenal contents were fractionated by cumulative rate flotation to separate VLDL1, VLDL2, and other lipoproteins, and the lipids were extracted from each fraction and separated by TLC. The radioactivity associated with [3H]TAG (A) and [3H]PC (B) in each fraction was quantified by scintillation counting. Results are presented as cpm per fraction (normalized with cell proteins).
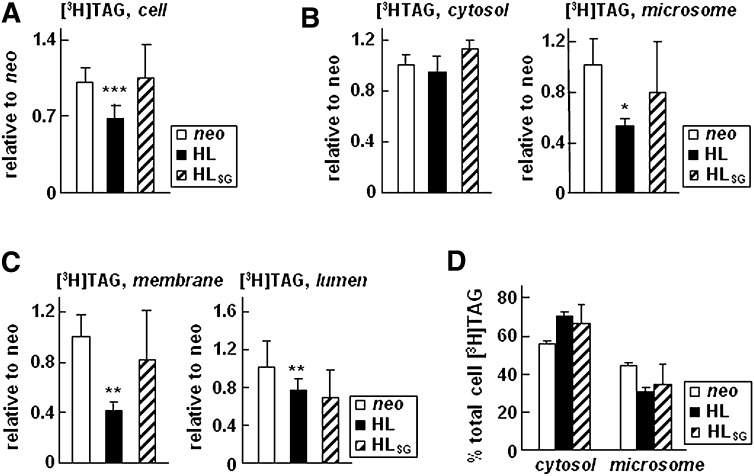
As mentioned earlier (Fig. 1B), cells expressing HL exhibited decrease in incorporation of [3H]glycerol into cell-associated [3H]TAG, suggesting impaired TAG synthesis. We thus determined whether or not expression of HLSG exerted similar effect and contrasted TAG synthesis between HL and HLSG cells. As shown in Fig. 6A, although HL expression decreased the amount of [3H]TAG in cells as compared with that in neo control, expression of the catalytically inactive HLSG had no effect on [3H]glycerol incorporation into TAG. These results, in conjunction with the above observation of reduced formation of [3H]TAG-rich LLD (Fig. 5A) in HLSG cells, suggest that i) the catalytic activity of HL probably was responsible for the decreased incorporation of [3H]glycerol into cell [3H]TAG, and ii) mobilization of newly synthesized [3H]TAG into microsomal lumen was impaired upon HL expression, irrespective of catalytic activity of the enzyme.
Fig. 6.
Catalytic function of HL is required to decrease the microsomal membrane-associated [3H]TAG. Cell expressing HL, HLSG, or neo control cells were metabolically labeled for 1 or 2 h in DMEM containing 20% FBS and 0.4 mM oleate. A: Total cell-associated [3H]TAG at the end of 2 h labeling. B: [3H]TAG presented in cytosol and microsomes at the end of 1 h labeling. C: [3H]TAG presented between microsomal membrane and microsomal lumen at the end of 1 h labeling. The radioactivity associated with [3H]TAG in A–C were converted to cpm/mg of cell protein, and data are presented as relative values with respect to that of neo control (set to 1). D: Distribution of [3H]TAG (at the end of 1 h labeling) between cytosol and total microsomes. Data are presented as percent of total [3H]TAG. *** p < 0.001; ** p < 0.01; * p < 0.05 (Student's t-test of HL or HLSG vs. neo control samples; n = 4 for neo and HL, and n = 2 for HLSG).
To confirm that HL expression results in impaired partitioning of [3H]TAG into microsomes, we determined subcellular localization of newly synthesized [3H]TAG between cytosol and microsomes (Fig. 6B), as well as between microsomal membranes and microsomal lumen (Fig. 6C). At the end of 1 h labeling, partitioning of [3H]TAG into microsomes in HL expressing cells was decreased (by 50%) as compared with neo control, whereas cytosolic fraction-associated [3H]TAG was comparable (Fig. 6B). The decreased microsomal [3H]TAG in HL expressing cells occurred in both microsomal membrane and microsomal lumen fractions (Fig. 6C). In HLSG expressing cells, the only significant decrease in [3H]TAG occurred in the microsomal lumen, whereas changes in [3H]TAG associated with total microsomes or microsomal membranes did not reach statistical significance (Figs. 6B, C). The decreased lumenal [3H]TAG corroborate the observed decrease in LLD-associated [3H]TAG in HL and HLSG expressing cells (see Fig. 5A). These data suggest that the reduced formation of [3H]TAG-rich LLD is related to expression of the HL protein, not the HL activity. Subcellular distribution of newly synthesized [3H]PC between cytosol, microsomal membranes, and microsomal lumen was little affected by expressing of either HL or HLSG (data not shown). The overt decrease in lumenal [3H]TAG in cells expressing HL or HLSG (Fig. 6C) suggested that HL might play a role in TAG partitioning between microsomes and cytosol. Indeed, the relative distribution of [3H]TAG showed pronounced presentation in cytosol and reduced association with microsomes in HL or HLSG expressing cells relative to neo controls (Fig. 6D).
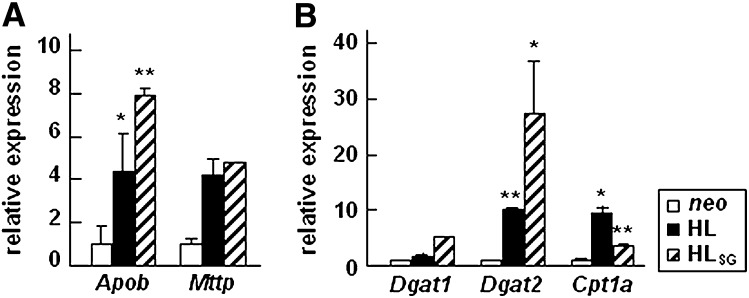
Altered expression of lipogenesis genes in cells expressing HL or HLSG
Finally, we determined expression of genes involved in VLDL assembly/secretion (e.g., Apob and Mttp), lipogenesis (e.g., Dgat1 and Dgat2), and β-oxidation (e.g., Cpt1a) in cells expressing HL or HLSG. As shown in Fig. 7A, expression of HL or HLSG did not impair, but rather stimulated, the apoB and Mttp mRNA. These results are in accord with the metabolic labeling data indicating uncompromised apoB synthesis and secretion in cells expressing HL or HLSG (Figs. 2, 4). The level of Dgat1 mRNA was unchanged and Dgat2 mRNA increased significantly in HL or HLSG expressing cells as compared with that in neo controls (Fig. 7B). The relative expression level of Cpt1a, the gene encoding carnitine palmitoyl transferase involved in β-oxidation, was also increased significantly as compared with neo controls (Fig. 7B). Thus, expression of HL or HLSG in McA-RH7777 cells did not compromise the TAG biosynthesis pathway and might promote β-oxidation.
Fig. 7.
Relative abundance of mRNA of genes involved in VLDL synthesis or β-oxidation. Total RNAs were extracted from indicated cells and real-time RT PCR analysis was performed on genes encoding apoB-100 (Apob) and MTP (Mttp) (A), DGAT1 (Dgat1), DGAT2 (Dgat2), and carnitine palmitoyl transferase (Cpt1a) (B). Each value is normalized to cyclophilin A (Ppia) and expressed as relative to the level in neo cells. ** p < 0.01; * p < 0.05 (Student's t -test of HL or HLSG vs. neo samples; n = 3).
DISCUSSION
The present results provide in vitro evidence that expression of the HL protein, not necessarily the HL activity, results in the secretion of TAG-poor VLDL particles (Fig. 3) with no effect on apoB-100 (Fig. 4). Secretion of the TAG-poor VLDL particles from cells expressing HL (regardless of the catalytic activity) is most likely the consequence of impaired second step lipidation of VLDL particles, which results from the diminished formation of TAG precursors in the form of LLD (24, 25) (Fig. 5A). The decreased TAG precursor pool within the microsomal lumen in HL expressing cells is not entirely attributable to impaired TAG synthesis. Rather, expression of HL (irrespective of catalytic activity) resulted in attenuated partitioning of newly synthesized TAG into the microsomes, particularly into the microsomal lumen (Fig. 6). These data add another level of complexity to the multifunctional HL in hepatic lipoprotein metabolism. Thus, in addition to the well-documented extracellular role of HL in catalyzing TAG/PL hydrolysis and facilitating lipoprotein clearance through receptor- or HSPG-mediated endocytosis, HL may also play an intracellular role in the assembly and secretion of TAG-rich VLDL.
The mechanism by which HL, a secretory hydrolytic enzyme that traverses through the ER/Golgi pathway, affects TAG metabolism intracellularly, thereby influencing VLDL assembly and secretion remains to be defined. However, many microsome-resident protein/enzyme factors have been identified that play a role in mobilization and utilization of TAG for VLDL assembly and secretion (37–39). Expression of these microsome-associated proteins/enzymes has been shown to exert different impacts on the metabolism of hepatic TAG and apoB-100 associated with VLDL. For instance, cells expressing triacylglycerol hydrolase exhibited increased depletion of storage TAG (with little effect on [3H]TAG synthesis) and accompanied increase in secreted VLDL-TAG mass and apoB-100 (37). Thus, expression of triacylglycerol hydrolase promotes TAG-rich VLDL secretion through increased TAG substrate availability, which is probably achieved via the process termed hydrolysis/re-esterification. Recently, evidence has been obtained for another ER-associated protein, namely arylacetamide deacetylase, whose expression resulted in decreased [3H]TAG in the cell and an accompanying decrease in [3H]TAG and apoB-100 secretion (38). On the other hand, expression of Esterase-x had no effect on [3H]TAG or apoB-100 secretion, even though TAG synthesis was impaired (39). The present study is the first demonstration that HL, a secretory lipase, also negatively impacts TAG-rich VLDL assembly/secretion by attenuating the availability of TAG substrates without affecting apoB-100 secretion. Decreased secretion of TAG with no concomitant decrease in apoB-100 has also been observed in vivo in animal models such as liver specific inactivation of the transcription factor XBP1 in mice (40).
Transfection studies have shown that HL possessed catalytic activity in the ER (20) and Golgi (19), albeit the intracellular activity was 10 times lower than that of the secreted enzyme. Intracellular maturation of HL involves conversion of catalytically inactive mass into functional, dimeric conformation prior to secretion (20), a slow process that occurs in the ER and takes hours to accomplish (41). The prolonged residence time of HL within the ER/Golgi secretory pathway may exert a negative effect on mobilization of TAG into microsomal lumen for VLDL assembly. The mechanism by which the HL traversing the ER/Golgi secretory pathway affects TAG partitioning into microsomes is currently unclear. However, the present mutagenesis study indicates that the catalytic activity of HL is not absolutely required for this effect. A group of HL-interacting proteins have been identified that may play a role in intracellular HL maturation process, none of which, however, appear to be involved in lipid binding or partitioning (41). Lipid binding regions are present within HL molecules (42). It has been shown recently that the fully functional homodimer of HL is associated with a microsomal membrane protein (43). Thus, it is tempting to speculate that microsomal traversing HL may interact with the membranes directly or indirectly (via other lumenal proteins) thereby affecting partitioning of newly synthesized TAG. Early studies have shown that partitioning of TAG into microsomal lumen for VLDL assembly/secretion requires the activity of MTP (26). Although it is unclear whether or not HL would affect the MTP activity, determination of the Mttp mRNA suggested that its abundance was not diminished in HL expressing cells.
Several hepatic proteins, including apoE and HL, have been shown to play a role in the process termed secretion-recapture that regulates, extracellularly, the net output of apoB-containing lipoproteins from the liver (36). The present study confirmed that the recombinant HL expressed in McA-RH7777 was vividly bound to the cell surface in a heparin-sensitive manner. By comparing the respective levels and lipoprotein distribution of [3H]TAG and [35S]apoB-100 in the presence or absence of heparin, we have shown that the HSPG-anchored HL indeed captures newly synthesized lipoprotein particles on the cells surface, and that capturing of the newly secreted lipoproteins by HL is independent of its catalytic function (Figs. 3A, B). Thus, consideration was given to the possibility that the apparent reduction in VLDL-[3H]TAG “secretion” from HL expressing cells was attributable to enhanced cell surface association. However, quantitative determination of metabolically labeled [3H]TAG in cells and media revealed that the HL-mediated cell surface binding of newly secreted VLDL could not entirely account for the decrease in [3H]TAG accumulation in the media. Rather, the decreased [3H]TAG in the media was the result of impaired assembly and secretion of TAG-rich VLDL particles.
Another point of interest of the current study is decreased amount of cell-associated [3H]TAG, but not [3H]PC, in cells transfected with HL, either transiently or stably. Currently, we are unable to ascertain whether the low cell [3H]TAG during metabolic labeling was attributable to decreased synthesis, rapid turnover, or both. Reduced incorporation of [3H]glycerol into cell TAG was observed as early as 15 or 30 min labeling (supplementary Fig. II A), ruling out rapid turnover being a likely reason for reduction in cell-associated [3H]TAG. However, a decrease in cell [3H]TAG was not observed in cells expressing the catalytically inactive HLSG, suggesting a possible role that HL hydrolytic activity may play in lowering cell [3H]TAG (Fig. 6A). On the other hand, real-time RT-PCR analysis indicated that expression of genes involved in TAG synthesis was undiminished (Fig. 3B), suggesting mechanisms other than synthesis might be involved. Enhanced expression of Cpt1a may suggest a role for β-oxidation (Fig. 3B), but how HL expression could lead to enhanced β-oxidation is unclear. What remains to be determined is whether the intracellular catalytic activity of HL can be regulated by other protein factors. A potential regulation of extracellular HL activity by angiopoietin-like proteins, a family of secreted glycoproteins, has been reported (44).
In summary, the present cell culture studies have revealed an intracellular role of HL expression in attenuating the assembly and secretion of TAG-rich lipoproteins, and the mode of action of HL is independent of its hydrolytic activity.
Supplementary Material
Acknowledgments
The authors thank Dr. Yuwei Wang for helpful discussions throughout the project, Dr. David Bickel for invaluable advice on statistical analysis, and Dr. Wen Qin and Mr. Sanjay Manhas for technical assistance.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- EL
- endothelial lipase
- ER
- endoplasmic reticulum
- HLSG
- catalytically-inactive HL
- HSPG
- heparin sulfate proteoglycan
- LDLR
- low density lipoprotein receptor
- LLD
- lumenal lipid droplet
- MTP
- microsomal triglyceride transfer protein
- PL
- phospholipid
- TAG
- triacylglycerol
This work is supported by the research grant (Grant # T6903) awarded to Z.Y. by the Heart and Stroke Foundation of Ontario.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table and five figures.
REFERENCES
- 1.Martin G. A., Busch S. J., Meredith G. D., Cardin A. D., Blankenship D. T., Mao S. J., Rechtin A. E., Woods C. W., Racke M. M., Schafer M. P., et al. 1988. Isolation and cDNA sequence of human postheparin plasma hepatic triglyceride lipase. J. Biol. Chem. 263: 10907–10914. [PubMed] [Google Scholar]
- 2.Sanan D. A., Fan J., Bensadoun A., Taylor J. M. 1997. Hepatic lipase is abundant on both hepatocyte and endothelial cell surfaces in the liver. J. Lipid Res. 38: 1002–1013. [PubMed] [Google Scholar]
- 3.Yu W., Hill J. S. 2006. Mapping the heparin-binding domain of human hepatic lipase. Biochem. Biophys. Res. Commun. 343: 659–665. [DOI] [PubMed] [Google Scholar]
- 4.Stahnke G., Sprengel R., Augustin J., Will H. 1987. Human hepatic triglyceride lipase: cDNA cloning, amino acid sequence and expression in a cultured cell line. Differentiation. 35: 45–52. [DOI] [PubMed] [Google Scholar]
- 5.McCoy M. G., Sun G. S., Marchadier D., Maugeais C., Glick J. M., Rader D. J. 2002. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 43: 921–929. [PubMed] [Google Scholar]
- 6.Santamarina-Fojo S., Gonzalez-Navarro H., Freeman L., Wagner E., Nong Z. 2004. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 24: 1750–1754. [DOI] [PubMed] [Google Scholar]
- 7.Breckenridge W. C., Little J. A., Alaupovic P., Wang C. S., Kuksis A., Kakis G., Lindgren F., Gardiner G. 1982. Lipoprotein abnormalities associated with a familial deficiency of hepatic lipase. Atherosclerosis. 45: 161–179. [DOI] [PubMed] [Google Scholar]
- 8.Hegele R. A., Little J. A., Vezina C., Maguire G. F., Tu L., Wolever T. S., Jenkins D. J., Connelly P. W. 1993. Hepatic lipase deficiency. Clinical, biochemical, and molecular genetic characteristics. Arterioscler. Thromb. 13: 720–728. [DOI] [PubMed] [Google Scholar]
- 9.Ruel I. L., Couture P., Cohn J. S., Bensadoun A., Marcil M., Lamarche B. 2004. Evidence that hepatic lipase deficiency in humans is not associated with proatherogenic changes in HDL composition and metabolism. J. Lipid Res. 45: 1528–1537. [DOI] [PubMed] [Google Scholar]
- 10.Ruel I. L., Couture P., Cohn J. S., Lamarche B. 2005. Plasma metabolism of apoB-containing lipoproteins in patients with hepatic lipase deficiency. Atherosclerosis. 180: 355–366. [DOI] [PubMed] [Google Scholar]
- 11.Zambon A., Bertocco S., Vitturi N., Polentarutti V., Vianello D., Crepaldi G. 2003. Relevance of hepatic lipase to the metabolism of triacylglycerol-rich lipoproteins. Biochem. Soc. Trans. 31: 1070–1074. [DOI] [PubMed] [Google Scholar]
- 12.Homanics G. E., de Silva H. V., Osada J., Zhang S. H., Wong H., Borensztajn J., Maeda N. 1995. Mild dyslipidemia in mice following targeted inactivation of the hepatic lipase gene. J. Biol. Chem. 270: 2974–2980. [DOI] [PubMed] [Google Scholar]
- 13.Qiu S., Bergeron N., Kotite L., Krauss R. M., Bensadoun A., Havel R. J. 1998. Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice. J. Lipid Res. 39: 1661–1668. [PubMed] [Google Scholar]
- 14.Mezdour H., Jones R., Dengremont C., Castro G., Maeda N. 1997. Hepatic lipase deficiency increases plasma cholesterol but reduces susceptibility to atherosclerosis in apolipoprotein E-deficient mice. J. Biol. Chem. 272: 13570–13575. [DOI] [PubMed] [Google Scholar]
- 15.Barcat D., Amadio A., Palos-Pinto A., Daret D., Benlian P., Darmon M., Berard A. M. 2006. Combined hyperlipidemia/hyperalphalipoproteinemia associated with premature spontaneous atherosclerosis in mice lacking hepatic lipase and low density lipoprotein receptor. Atherosclerosis. 188: 347–355. [DOI] [PubMed] [Google Scholar]
- 16.Dichek H. L., Qian K., Agrawal N. 2004. Divergent effects of the catalytic and bridging functions of hepatic lipase on atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24: 1696–1702. [DOI] [PubMed] [Google Scholar]
- 17.Freeman L., Amar M. J., Shamburek R., Paigen B., Brewer H. B., Jr, Santamarina-Fojo S., Gonzalez-Navarro H. 2007. Lipolytic and ligand-binding functions of hepatic lipase protect against atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 48: 104–113. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Navarro H., Nong Z., Amar M. J., Shamburek R. D., Najib-Fruchart J., Paigen B. J., Brewer H. B., Jr., Santamarina-Fojo S. 2004. The ligand-binding function of hepatic lipase modulates the development of atherosclerosis in transgenic mice. J. Biol. Chem. 279: 45312–45321. [DOI] [PubMed] [Google Scholar]
- 19.Verhoeven A. J., Neve B. P., Jansen H. 2000. Intracellular activation of rat hepatic lipase requires transport to the Golgi compartment and is associated with a decrease in sedimentation velocity. J. Biol. Chem. 275: 9332–9339. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Zeev O., Doolittle M. H. 2004. Maturation of hepatic lipase. Formation of functional enzyme in the endoplasmic reticulum is the rate-limiting step in its secretion. J. Biol. Chem. 279: 6171–6181. [DOI] [PubMed] [Google Scholar]
- 21.Peterfy M., Ben-Zeev O., Mao H. Z., Weissglas-Volkov D., Aouizerat B. E., Pullinger C. R., Frost P. H., Kane J. P., Malloy M. J., Reue K., et al. 2007. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat. Genet. 39: 1483–1487. [DOI] [PubMed] [Google Scholar]
- 22.Sundaram M., Yao Z. 2010. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr. Metab. (Lond). 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran K., Thorne-Tjomsland G., DeLong C. J., Cui Z., Shan J., Burton L., Jamieson J. C., Yao Z. 2002. Intracellular assembly of very low density lipoproteins containing apolipoprotein B100 in rat hepatoma McA-RH7777 cells. J. Biol. Chem. 277: 31187–31200. [DOI] [PubMed] [Google Scholar]
- 24.Sundaram M., Zhong S., Khalil M. B., Zhou H., Jiang Z. G., Zhao Y., Iqbal J., Hussain M. M., Figeys D., Wang Y., et al. 2010. Functional analysis of the missense APOC3 mutation Ala23Thr associated with human hypotriglyceridemia. J. Lipid Res. 51: 1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundaram M., Zhong S., Khalil M. B., Links P. H., Zhao Y., Iqbal J., Hussain M. M., Parks R. J., Wang Y., Yao Z. 2010. Expression of apolipoprotein C–III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 51: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Tran K., Yao Z. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J. Biol. Chem. 274: 27793–27800. [DOI] [PubMed] [Google Scholar]
- 28.Kulinski A., Rustaeus S., Vance J. E. 2002. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J. Biol. Chem. 277: 31516–31525. [DOI] [PubMed] [Google Scholar]
- 29.Brown R. J., Schultz J. R., Ko K. W., Hill J. S., Ramsamy T. A., White A. L., Sparks D. L., Yao Z. 2003. The amino acid sequences of the carboxyl termini of human and mouse hepatic lipase influence cell surface association. J. Lipid Res. 44: 1306–1314. [DOI] [PubMed] [Google Scholar]
- 30.Blackhart B. D., Yao Z. M., McCarthy B. J. 1990. An expression system for human apolipoprotein B100 in a rat hepatoma cell line. J. Biol. Chem. 265: 8358–8360. [PubMed] [Google Scholar]
- 31.Shirai K., Matsuoka N., Saito Y., Yoshida S. 1984. Post-heparin plasma hepatic triacylglycerol lipase-catalyzed hydrolysis of tributyrin. Effect of lipid interface. Biochim. Biophys. Acta. 795: 1–8. [PubMed] [Google Scholar]
- 32.Vance D. E., Weinstein D. B., Steinberg D. 1984. Isolation and analysis of lipoproteins secreted by rat liver hepatocytes. Biochim. Biophys. Acta. 792: 39–47. [DOI] [PubMed] [Google Scholar]
- 33.Tran K., Wang Y., DeLong C. J., Cui Z., Yao Z. 2000. The assembly of very low density lipoproteins in rat hepatoma McA-RH7777 cells is inhibited by phospholipase A2 antagonists. J. Biol. Chem. 275: 25023–25030. [DOI] [PubMed] [Google Scholar]
- 34.Choi S. Y., Goldberg I. J., Curtiss L. K., Cooper A. D. 1998. Interaction between ApoB and hepatic lipase mediates the uptake of ApoB-containing lipoproteins. J. Biol. Chem. 273: 20456–20462. [DOI] [PubMed] [Google Scholar]
- 35.Ji Z. S., Lauer S. J., Fazio S., Bensadoun A., Taylor J. M., Mahley R. W. 1994. Enhanced binding and uptake of remnant lipoproteins by hepatic lipase-secreting hepatoma cells in culture. J. Biol. Chem. 269: 13429–13436. [PubMed] [Google Scholar]
- 36.Mahley R. W., Huang Y. 2007. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J. Clin. Invest. 117: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehner R., Vance D. E. 1999. Cloning and expression of a cDNA encoding a hepatic microsomal lipase that mobilizes stored triacylglycerol. Biochem. J. 343 Pt 1: 1–10. [PMC free article] [PubMed] [Google Scholar]
- 38.Lo V., Erickson B., Thomason-Hughes M., Ko K. W., Dolinsky V. W., Nelson R., Lehner R. 2010. Arylacetamide deacetylase attenuates fatty-acid-induced triacylglycerol accumulation in rat hepatoma cells. J. Lipid Res. 51: 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko K. W., Erickson B., Lehner R. 2009. Es-x/Ces1 prevents triacylglycerol accumulation in McArdle-RH7777 hepatocytes. Biochim. Biophys. Acta. 1791: 1133–1143. [DOI] [PubMed] [Google Scholar]
- 40.Lee A. H., Scapa E. F., Cohen D. E., Glimcher L. H. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 320: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doolittle M. H., Ben-Zeev O., Bassilian S., Whitelegge J. P., Peterfy M., Wong H. 2009. Hepatic lipase maturation: a partial proteome of interacting factors. J. Lipid Res. 50: 1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keiper T., Schneider J. G., Dugi K. A. 2001. Novel site in lipoprotein lipase (LPL415;-438) essential for substrate interaction and dimer stability. J. Lipid Res. 42: 1180–1186. [PubMed] [Google Scholar]
- 43.Doolittle M. H., Peterfy M. 2010. Mechanisms of lipase maturation. Clin. Lipidol. 5: 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichtenstein L., Berbee J. F., van Dijk S. J., van Dijk K. W., Bensadoun A., Kema I. P., Voshol P. J., Muller M., Rensen P. C., Kersten S. 2007. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arterioscler. Thromb. Vasc. Biol. 27: 2420–2427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.