Abstract
Inhibition of cholesterol synthesis by 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoAR) inhibitors has been associated with an increase in intestinal cholesterol absorption. This study examined how HMG-CoAR inhibition by atorvastatin modulates expression of key genes involved in intestinal cholesterol metabolism. A crossover study was conducted in which 22 hyperlipidemic men received atorvastatin, 40 mg/day, or placebo, each for 12 weeks. Gene expression was assessed by real-time PCR using duodenal biopsy samples obtained at the end of each phase of treatment. Treatment with atorvastatin was associated with a 76% reduction in lathosterol and significant increases in sitosterol (70%). Atorvastatin significantly increased intestinal mRNA levels of HMG-CoAR (59%), LDL receptor (LDLR) (52%), PCSK9 (187%), SREBP-2 (44%), and HNF-4α (13%). Furthermore, atorvastatin significantly increased intestinal mRNA levels of NPC1L1 by 19% and decreased mRNA levels of both ABCG5 and ABCG8 by 14%. Positive correlations were observed between changes in SREBP-2 and HNF-4α expression and concurrent changes in the intestinal mRNA levels of HMG-CoAR, LDLR, and NPC1L1. These results indicate that HMG-CoAR inhibition with atorvastatin stimulates the intestinal expression of NPC1L1, LDLR, and PCSK9; increases cholesterol absorption; and reduces expression of ABCG5/8; these effects are most likely mediated by upregulation of the transcription factors SREBP-2 and HNF-4α.
Keywords: Niemann-Pick C1-like 1, cholesterol metabolism, 3-hydroxy-3-methylglutaryl CoA reductase, intestine, low density lipoprotein receptor
The relationship between elevated plasma levels of LDL-cholesterol (LDL-C) and the risk of atherosclerosis has been very well established (1–3). Clinical trials have shown that reduction of LDL-C constitutes a primary strategy for the prevention and regression of coronary heart disease (4). Plasma cholesterol levels are regulated by feedback mechanisms including exogenous cholesterol absorption through the gastrointestinal tract and endogenous cholesterol synthesis by various tissues. Several studies have shown that the amount of dietary cholesterol absorbed also influences endogenous cholesterol synthesis (5–7). The newly identified Niemann-Pick C1-like 1 (NPC1L1) protein expressed at the apical membrane of enterocytes has been shown to play a crucial role in the absorption of cholesterol and plant sterol (8). Several physiological determinants and pharmacological agents modulate cholesterol homeostasis, including genetic factors, body weight, ezetimibe therapy, and 3-hydroxy-3-methylglutaryl CoA reductase (HMG-CoAR) inhibitors (statins) therapy, the rate-limiting step in the cholesterol biosynthesis pathway (9). For instance, obese subjects show an increase in cholesterol synthesis with an associated decrease in cholesterol absorption (10, 11). Ezetimibe therapy has been shown to reduce intestinal cholesterol absorption while reciprocally elevating synthesis (12). These findings suggest the presence of a reciprocal relationship between cholesterol absorption and synthesis, as a change in one vector results in a compensatory and opposing change in the other. Although recent data suggest that statin therapy is associated with a rise in intestinal cholesterol absorption (13), the impact of HMG-CoAR inhibitors on cholesterol absorption and the molecular mechanisms underlying this effect has not been fully characterized. Therefore, the primary objective of the present study was to gain further insight into this key physiological process by examining the impact of a 12-week regimen of atorvastatin therapy, 40 mg/day, on intestinal expression of the sterol transporter NPC1L1 in subjects with mixed hyperlipidemia. Furthermore, we examined the impact of atorvastatin therapy on intestinal expression of the key gene products involved in cholesterol metabolism, such as ATP-binding cassette transporter 5 (ABCG5) and ABCG8, HMG-CoAR, LDL receptor, sterol regulatory element binding transcription factor 2 (SREBP-2), hepatocyte nuclear factor 4 α (HNF-4α), proprotein convertase subtilisin kexin-9 (PCSK9), and microsomal triglyceride transfer protein (MTTP). Gene expression studies were undertaken using a human duodenal biopsy model, which we have recently developed.
MATERIALS AND METHODS
Subjects
Twenty-three men with plasma LDL-C levels above the 50th percentile for their age were recruited from the Quebec City area to participate in the study (14). One subject had to be withdrawn from analyses because of poor RNA quality. Subjects were excluded if they had persistent elevation of serum transaminases; monogenic hyperlipidemia such as familial hypercholesterolemia; plasma triglyceride (TG) levels >4.5 mmol/l; a recent history of alcohol or drug abuse; diabetes mellitus; or a history of cancer. Furthermore, all participants were unrelated at the first and second degree. All eligible subjects had to be withdrawn from lipid-lowering medications for at least 6 weeks before the beginning of the study. The study consisted of a 1 week screening period and a 4 week placebo run-in period, followed by two consecutive 12 week double-blind treatment periods with atorvastatin, 40 mg/day, or placebo in random order. Fasting blood samples and duodenal biopsies were performed following each phase of treatment. Participants were instructed to take one capsule at the time of their evening meal. Compliance was assessed by pill counting. Participants were asked not to change their dietary habits or use of alcohol and level of physical exercise during the study. The research protocol was approved by the Laval University Medical Center ethical review committee, and written informed consent was obtained from each subject.
Characterization of plasma lipids and lipoproteins
Twelve hour fasting venous blood samples were obtained from an antecubital vein and collected in Vacutainer tubes containing EDTA (0.1%, final concentration) at the end of each phase of treatment. Plasma was separated from blood cells by centrifugation at 3,000 rpm for 10 min at 4°C. Plasma cholesterol and TG concentrations were determined with an Analyzer RA-1000 (Technicon Instruments Corporation, Tarrytown, NY), as previously described (15). The LDL-C level was also calculated according to the equation described by Friedewald et al. (16): [LDL-C] = [total cholesterol] − [HDL-C] − [TG]/2.2, and HDL-cholesterol was measured as previously described (17). Plasma apolipoprotein B-48 (apoB-48) was assessed by ELISA (Shibayagi Co., Japan) (18). C-reactive protein (CRP) concentrations were measured with a highly sensitive commercial immunoassay (Dade Behring, Mississauga, ON, Canada) as described previously (19). Plasma concentrations of lathosterol, a precursor in the biosynthesis of cholesterol, and of the plant sterols campesterol and sitosterol, used as plasma surrogates of intestinal cholesterol absorption, were quantified at Laval University, using a gas chromatography method similar to that described previously (20). Coefficients of variation ranged between 3.9% and 9.9%. Because non-cholesterol sterols are transported in plasma by lipoproteins, their concentrations have been expressed relative to the concentration of total cholesterol to correct for differing numbers of lipoprotein acceptor particles. This method for quantifying cholesterol absorption has been validated relative to that of the continuous isotope feeding method (21), both in metabolic (22) and population settings (23).
Intestinal biopsies
Biopsy samples were obtained from the second portion of the duodenum during gastroduodenoscopy. Six biopsy samples were collected using multiple sample single-use biopsy forceps and immediately flash frozen in liquid nitrogen and stored at −80°C before RNA extraction.
Total RNA extraction
Intestinal biopsy tissue samples were homogenized in RLT buffer (Qiagen) using a Tissue-Tearor (BioSpec Products, Inc., Bartlesville, OK) and a 4.5 mm stainless steel probe. The RNA content from homogenized tissue samples were then extracted using an RNeasy fibrous tissue mini-kit (Qiagen). Tissue samples were also treated with an RNase-free DNase set to eliminate any contaminant DNA. Total RNA was then eluted into 100 µl RNase-free H2O and stored at −80°C.
RNA quantification and quantitative real-time PCR
RNA quality was assessed with a 2100 Bioanalyzer (Agilent Technologies, Inc.) as previously described (24). Data calculation and normalization were performed using the second derivative and double correction method as described in the study by Warrington et al. (25) and using the reference genes hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1), ATP synthase O subunit (Atp5o), glucose-6-phosphate dehydrogenase (G6PD), and 18S rRNA. The Hprt1, Atp5o, and G6PD genes have been shown to have stable expression levels from embryonic life through adulthood in various tissues. mRNA expression levels are expressed as the number of copies/µg total RNA, using a standard curve of crossing points versus the logarithm of the quantity. The standard curve was established by using known amounts of purified PCR products (10, 102, 103, 104, 105, and 106 copies) and LightCycler 480 version 1.5 software provided by the manufacturer (Roche Inc).
NPC1L1 protein analysis in duodenal biopsy samples
Duodenal tissue samples were homogenized, and total protein from each sample was subjected to SDS-PAGE and analyzed by Western blotting (8) with code 1801 polyclonal anti-NPC1L1 antibodies (26). NPC1L1 signal was normalized with the enterocyte-specific marker, villin (8).
Measurement of plasma PCSK9
Plasma PCSK9 was measured by ELISA using a polyclonal antibody against human PCSK9 (27).
Statistical analysis
Nonparametric Wilcoxon matched pair analyses were used to compare the effects of atorvastatin on the lipid/lipoprotein profile and on mRNA expression. Spearman correlation coefficients were determined to assess the significance of associations. Differences were considered significant at a P value of ≤0.05. All analyses were performed using JMP statistical software (version 8.0.1; SAS Institute, Cary, NC).
RESULTS
Characteristics of subjects
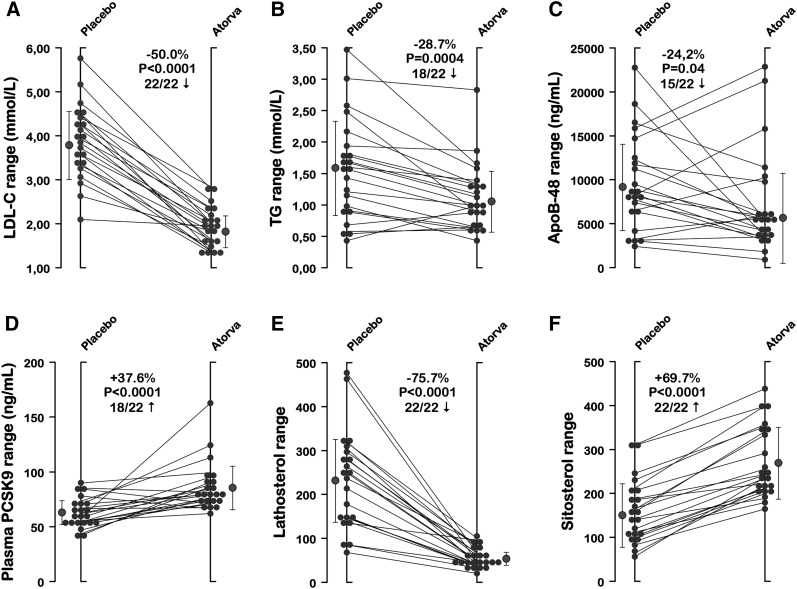
Participants’ mean ± SD age, body mass index, and waist circumference were 38.1 ± 9.8 years, 29.0 ± 4.0 kg/m2, and 100.1 ± 12.1 cm, respectively. Subjects maintained their weight throughout the study. One subject had to be withdrawn from the analyses because of poor RNA quality. Table 1 shows the lipid/lipoprotein profiles of the 22 subjects following a 12-week treatment with atorvastatin and placebo. Atorvastatin, 40 mg/day, significantly reduced levels of plasma cholesterol (−36.8%; P < 0.0001), LDL-C (−50.0%; P < 0.0001), TG (−28.7%; P = 0.0004), and apoB-48 (−24.2%; P = 0.04) but had no significant effect on plasma HDL-C concentrations and CRP levels. The impact of atorvastatin on plasma surrogates of cholesterol absorption (campesterol and sitosterol) and on synthesis (lathosterol) was also assessed. Compared with placebo, atorvastatin significantly increased plasma campesterol (+64.7%; P < 0.0001) and sitosterol (+69.7%; P < 0.0001) and was associated with a significant reduction in plasma lathosterol (−75.7%; P < 0.0001). The lathosterol/campesterol and lathosterol/sitosterol ratios, representing indexes of cholesterol homeostasis, were significantly reduced following therapy with atorvastatin. Atorvastatin significantly increased plasma levels of PCSK9 (+37.6%; P < 0.0001), a binding protein enhancing the degradation of the LDL receptor in endosomes/lysosomes (28). Interindividual variability in responses of plasma LDL-C, TG, apoB-48, plasma PCSK9, lathosterol, and sitosterol is illustrated in Fig. 1. Twenty-two of 22 participants showed changes in LDL-C, lathosterol, and sitosterol levels in the same direction, while atorvastatin-induced changes in plasma TG and apoB-48 were negative in 18 and 15 subjects, respectively.
TABLE 1.
Lipid/lipoprotein profiles for study subjects
| Mean ± SD expression level after treatment with |
||||
|---|---|---|---|---|
| Characteristic | Placebo | Atorvastatin | % of change | P |
| Age (years) | 38.1 ± 9.8 | 38.1 ± 9.8 | ||
| Body mass index (kg/m2) | 29.0 ± 4.0 | 29.0 ± 4.0 | ||
| Waist circumference (cm) | 100.1 ± 12.1 | 100.1 ± 12.1 | ||
| Plasma-cholesterol (mmol/l) | 5.72 ± 0.95 | 3.61 ± 0.48 | −36.8 | <0.0001 |
| Triglycerides (mmol/l) | 1.57 ± 0.81 | 1.12 ± 0.54 | −28.7 | 0.0004 |
| LDL-cholesterol (mmol/l) | 3.86 ± 0.84 | 1.93 ± 0.45 | −50.0 | <0.0001 |
| HDL-cholesterol (mmol/l) | 1.14 ± 0.24 | 1.17 ± 0.24 | +2.6 | 0.15 |
| Plasma apolipoprotein B-48 (ng/ml) | 9571 ± 5450 | 7252 ± 5876 | −24.2 | 0.04 |
| C-reactive protein (mg/l) | 2.50 ± 2.20 | 2.24 ± 2.18 | −10.4 | 0.31 |
| PCSK9 (ng/ml) | 62.8 ± 13.4 | 86.4 ± 22.7 | +37.6 | <0.0001 |
| Plasma lathosterol | 231.4 ± 110.5 | 56.3 ± 22.3 | −75.7 | <0.0001 |
| Plasma campesterol | 157.7 ± 73.6 | 259.8 ± 83.9 | +64.7 | <0.0001 |
| Plasma sitosterol | 158.6 ± 72.0 | 269.1 ± 80.6 | +69.7 | <0.0001 |
| Lathosterol/campesterol ratio | 1.94 ± 1.37 | 0.25 ± 0.15 | −87.1 | <0.0001 |
| Lathosterol/sitosterol ratio | 1.95 ± 1.44 | 0.23 ± 0.11 | −88.4 | <0.0001 |
Table shows lipid/lipoprotein profiles and surrogate markers of cholesterol synthesis and absorption from 22 subjects following 12-week treatment with atorvastatin, 40 mg/day. P values are the difference between baseline and treatment values. PCSK9, proprotein convertase subtilisin kexin-9.
Fig. 1.
Individual responses (placebo vs. atorvastatin treatment) of the 22 participants. Data for (A) plasma LDL-C, (B) TG, (C) apoB-48, (D) PCSK9, (E) lathosterol, and (F) sitosterol are shown along with means ± SD (gray symbols). The proportion of participants’ responses moving in the same direction following atorvastatin is also provided for each variable.
Intestinal mRNA levels
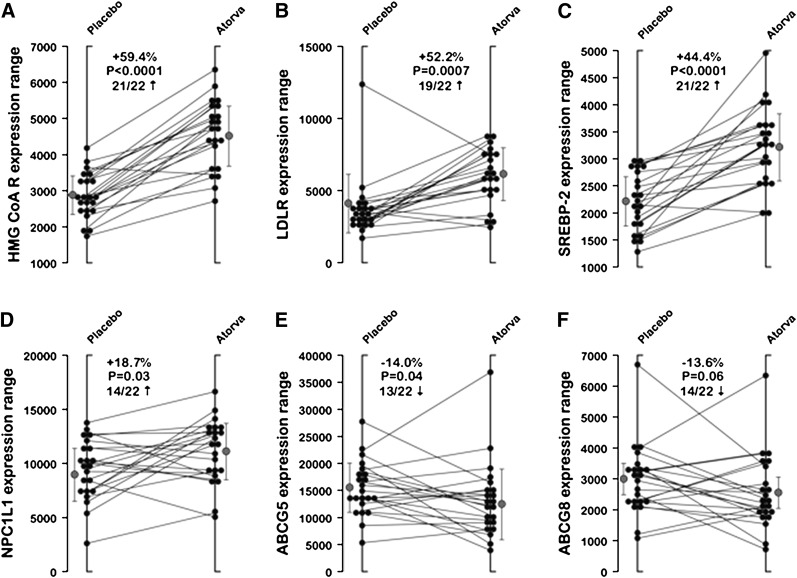
As shown in Table 2, studies of gene product expression revealed that atorvastatin significantly increased intestinal mRNA levels of HMG-CoAR (+59.4%; P < 0.0001), LDL receptor (+52.2%; P = 0.0007), acetyl-CoA acetyltransferase-2 (ACAT-2) (+64.5%; P < 0.0001), SREBP-2 (+44.4%; P < 0.0001), and HNF-4α (+13.4%; P = 0.02). Intestinal mRNA expression levels of PCSK9 (+186.6%; P < 0.0001) and NPC1L1 (+18.7%, P=0.03) were also significantly increased by atorvastatin. On the other hand, atorvastatin decreased mRNA expression levels of ABCG5 and ABCG8 by −14.0% (P = 0.04) and −13.6% (P = 0.06), respectively. Finally, treatment with atorvastatin had no significant impact on mRNA levels of apoB-48, fatty acid binding protein-2 (FABP-2), fatty acid transporter protein-4 (FATP-4), MTTP, and SREBP-1c. As shown in Fig. 2, the atorvastatin-induced changes in mRNA levels of HMG-CoAR, LDL receptor, and SREBP-2 were positive in at least 19 of 22 participants, while 14 participants exhibited changes in NPC1L1 and ABCG5/8 expression in the same direction.
TABLE 2.
Intestinal gene mRNA expression levels
| Mean no. of copies ± SD/100,000 copies of Atp5o control gene following |
||||
|---|---|---|---|---|
| Gene | Placebo | Atorvastatin | % of change | P |
| ABCG5 | 15,393 ± 4,959 | 13,241 ± 7,004 | −14.0 | 0.04 |
| ABCG8 | 2,959 ± 1,160 | 2,558 ± 1208 | −13.6 | 0.06 |
| ACAT-2 | 1,756 ± 454 | 2,888 ± 889 | +64.5 | <0.0001 |
| ApoB gene | 66,611 ± 30,686 | 75,654 ± 32,739 | +13.6 | 0.1 |
| FABP-2 | 21,250 ± 6,649 | 22,043 ± 7,126 | +3.7 | NS |
| FATP-4 | 12,475 ± 2,021 | 12,943 ± 2,125 | +3.8 | NS |
| HMG-CoAR | 2,866 ± 639 | 4,568 ± 952 | +59.4 | <0.0001 |
| HNF-4α | 9,558 ± 2,269 | 10,841 ± 2,402 | +13.4 | 0.02 |
| LDL receptor | 3,915 ± 2,293 | 5,959 ± 1875 | +52.2 | 0.0007 |
| MTTP | 134,810 ± 34,405 | 136,137 ± 34,707 | +1.0 | NS |
| NPC1L1 | 9,376 ± 2,781 | 11,127 ± 2,919 | +18.7 | 0.03 |
| PCSK9 | 238 ± 174 | 682 ± 298 | +186.6 | <0.0001 |
| SREBP-1c | 3,951 ± 1,000 | 3,806 ± 987 | −3.7 | NS |
| SREBP-2 | 2,215 ± 533 | 3,199 ± 745 | +44.4 | <0.0001 |
NS, not significant; ABCG5/8, ATP binding cassette; ACAT-2, acetyl-CoA acetyltransferase 2; FABP-2, fatty acid binding protein 2; FATP-4, fatty acid transporter protein 4; HMG-CoAR, 3-hydroxyl-3-methylglutaryl-CoA reductase; HNF-4α, hepatocyte nuclear factor-4α; MTTP, microsomal triglyceride transfer protein; NPC1L1, Niemann-Pick C1-like 1; PCSK9, proprotein convertase subtilisin kexin-9; SREBP-1c and -2, sterol regulatory element binding transcription factors 1c and 2.
Fig. 2.
Individual responses (placebo vs. atorvastatin treatment) of the 22 participants. Data for the expression of (A) HMG-CoAR, (B) LDL receptor, (C) SREBP-2, (D) NPC1L1, (E) ABCG5, and (F) ABCG8 are shown along with means ± SD (gray symbols). The proportion of participants’ responses moving in the same direction following atorvastatin is also provided for each variable.
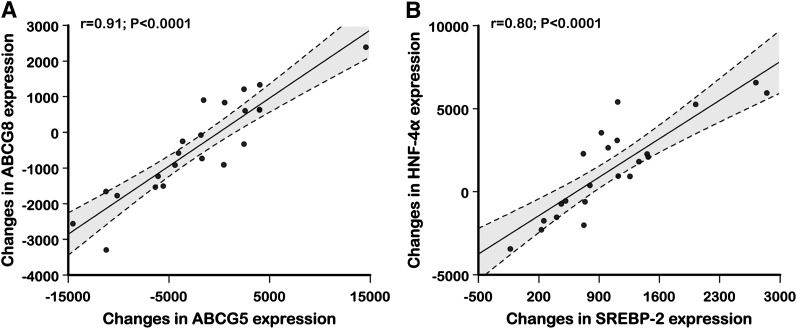
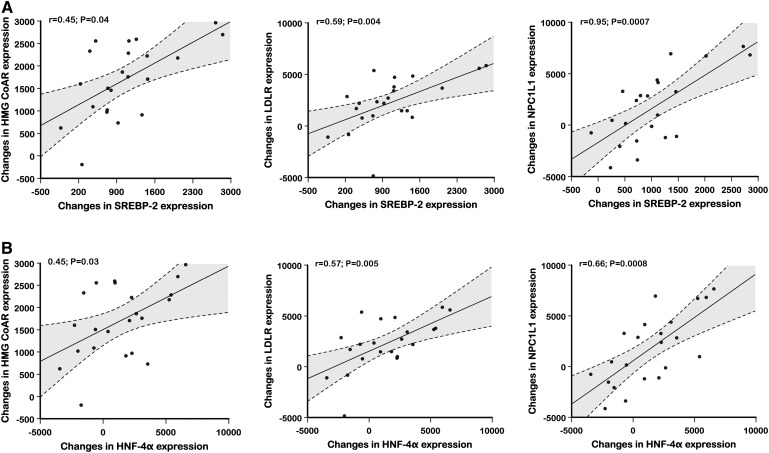
As shown in Fig. 3, changes in intestinal mRNA levels of ABCG5 were significantly correlated with changes in mRNA levels of ABCG8, while changes in HNF-4α expression were highly and positively correlated with changes in mRNA levels of SREBP-2. Fig. 4 shows positive correlations between changes in SREBP-2 mRNA levels and concurrent changes in mRNA levels of HMG-CoAR (r = 0.45; P = 0.04), LDL receptor (r = 0.59; P = 0.004), and NPC1L1 (r = 0.65; P = 0.0007). In addition, positive correlations were also observed between changes in HNF-4α mRNA levels and concurrent changes in mRNA levels of HMG-CoAR (r = 0.45; P = 0.03), LDL receptor (r = 0.57; P=0.005), and NPC1L1 (r = 0.66; P=0.0008).
Fig. 3.
Correlation between changes in intestinal ABCG5 and ABCG8 mRNA expression (A) and correlation between changes in HNF-4α and changes in intestinal SREBP-2 mRNA expression (B) following treatment with atorvastatin, 40 mg/day, versus placebo.
Fig. 4.
Correlation between changes in HMG-CoAR, LDL receptor, and intestinal NPC1L1 mRNA expression and changes in intestinal SREBP-2 (A) and HNF-4α (B) mRNA expression following treatment with atorvastatin, 40 mg/day, versus placebo are shown.
Intestinal protein levels of NPC1L1
Following treatment with atorvastatin, subjects’ mean percentage of changes in NPC1L1 protein expression in intestinal biopsy samples was increased by +33.5%, but this difference did not reach statistical significance due to a large variability in protein measurements. In addition, no significant correlation was observed between changes in NPC1L1 protein levels and changes in mRNA levels of NPC1L1 (data not shown).
Discussion
In the present study, the 12-week treatment with atorvastatin, 40 mg/day, resulted in a significant reduction in levels of plasma cholesterol (−37%), TG (−29%), LDL-C (−50%), and apolB-48 (−24%). Furthermore, atorvastatin significantly decreased plasma lathosterol (−76%), a marker of cholesterol synthesis, and significantly increased plasma campesterol (+65%) and sitosterol (+70%), two surrogates of cholesterol absorption. Finally, treatment with atorvastatin upregulated intestinal mRNA levels of NPC1L1 (+19%), HMG-CoAR (+59%), LDL receptor (52%), ACAT-2 (+65%), SREBP-2 (+44%), HNF-4α (+13%), and PCSK9 (+187%) and reduced ABCG5/8 expression by 14%.
The homeostasis of circulating cholesterol levels is modulated primarily by cholesterol absorption and synthesis (29, 30). Several factors have been shown to influence cholesterol homeostasis including genetic factors, circadian rhythm, body weight, and various therapeutic agents such as ezetimibe, statins, and plant sterols (9). Recent data have suggested that the downregulation of cholesterol synthesis by statin therapy is compensated by a rise in intestinal cholesterol absorption (11). Our study is consistent with this concept (31, 32), having shown a reduction in plasma lathosterol levels (synthesis) compensated by an increase in both campesterol and sitosterol (absorption) following treatment with atorvastatin.
In the present study, treatment with atorvastatin significantly increased intestinal mRNA levels of NPC1L1, which was paralleled by a nonsignificant increase in NPC1L1 protein expression. In agreement with our findings, a study with miniature pigs showed that NPC1L1 expression was increased incrementally in both the jejunum and the liver by combination therapy with ezetimibe and simvastatin (33). Activation of the nuclear transcription factor SREBP-2 is known to be negatively regulated by sterols and was recently reported to activate NPC1L1 transcription (34). Similarly, HNF-4α, a key modulator of lipid and glucose metabolism, has also been reported to interact synergistically with SREBP-2 in the regulation of NPC1L1 expression (35). Our results showed a positive correlation between changes in NPC1L1 and changes in both SREBP-2 and HNF-4α mRNA expression, a finding that supports the notion that these transcription factors stimulate intestinal NPC1L1 expression. Therefore, it is likely that the increase in mRNA expression of NPC1L1 reflects cholesterol depletion within the enterocyte, consistent with transcriptional regulation by sterols via sterol-regulated elements within the NPC1L1 promoter (8).
The present study demonstrated a significant increase in intestinal mRNA expression of the LDL receptor following treatment with atorvastatin. Previous kinetic studies have already suggested an increased fractional catabolic rate of LDL apoB-100 following statin therapy, an effect most likely mediated by activation of the LDL receptor gene expression (36, 37). Our data are consistent with results from previous animal and human studies showing an increase in the LDL receptor gene expression following statin therapy (38–41). Under physiological conditions, HMG-CoAR and LDL receptor mRNAs were closely coregulated, probably because of their common transcriptional activation by SREBP-2 and HNF-4α (42–44). Indeed, changes in intestinal mRNA levels of SREBP-2 and HNF-4α were positively correlated with intestinal mRNA levels of the LDL receptor and HMG-CoAR, providing a mechanism for the increased expression of both of these sterol-responsive gene products.
PCSK9 is expressed mainly in the liver, small intestine, and kidney (45) and is thought to accelerate the degradation of LDL receptor in endosomes/lysosomes (46). Recent studies have demonstrated that PCSK9 mRNA expression was upregulated to a greater extent than that of the LDL receptor in human hepatocytes in primary culture (45, 47). Our results support and extend these previous findings by showing that treatment with atorvastatin had greater impact on PCSK9 than on LDL receptor mRNA expression in human enterocytes as well. Our findings also support the role of SREBP-2 as a transcriptional regulator of both the LDL receptor and PCSK9 in human enterocytes.
ABCG5/8 are heterodimers involved in the transport of cholesterol from hepatocytes into the bile and from enterocytes into the intestinal lumen (48–50). In animal models, hepatic expression of ABCG5/8 has been shown to be increased following statin therapy, which was associated with an increase in biliary cholesterol concentration (51, 52). Lally et al. (53) reported a significant increase in intestinal ABGC5/8 mRNA expression in diabetic patients following treatment with statins. These observations contrast with our findings showing that intestinal mRNA expression of ABCG5/8 was decreased following atorvastatin therapy. Therefore, ABCG5/8 may work in different ways in the enterocyte in order to prevent intracellular cholesterol depletion associated with HMG-CoAR inhibition. Further studies are needed to clarify this issue. Although there was a large discrepancy between expression of the two transporters, the high correlation between atorvastatin-induced changes in ABCG5 and ABCG8 expression observed in the present study supports the concept that these two transporters are obligate heterodimers and suggests that dimerization of ABCG5 and ABCG8 could be regulated at the posttranslational level. Finally, the variability among individual responses of LDL-C, lathosterol, and surrogates of cholesterol absorption was smaller than that of NPC1L1 and ABCG5/8 expression, suggesting that other determinants, either constitutional or environmental, play an important role in cholesterol metabolism.
In conclusion, these results indicate that HMG-CoAR inhibition with atorvastatin stimulates intestinal expression of NPC1L1 and PCSK9, increases cholesterol absorption, and reduces ABCG5/8 expression; these effects are mediated most likely by stimulation of the transcription factors SREBP-2 and HNF-4α.
Supplementary Material
Acknowledgments
The authors thank the subjects for excellent collaboration and the staff of the Institute of Nutraceutical and Functional Food for their dedication. We also thank D. Aubin, Marjolaine Lapointe, and Johanne Marin and Pascal Dubé for dedicated work.
Footnotes
Abbreviations:
- ABCG
- ATP-binding cassette G
- ACAT-2
- acetyl-CoA acetyltransferase-2
- apoB-48
- apolipoprotein B-48
- CRP
- C-reactive protein
- FABP-2
- fatty acid binding protein-2
- FATP-4
- fatty acid transporter protein-4
- HMG-CoAR
- HMG-CoA reductase
- HNF-4α
- hepatocyte nuclear factor-4α
- LDL-C
- LDL-cholesterol
- MTTP
- microsomal triglyceride transfer protein
- NPC1L1
- Niemann-Pick C1-like 1
- PCSK9
- proprotein convertase subtilisin kexin-9 (PCSK9)
- SREBP-2
- sterol regulatory element binding transcription factor 2
- TG
- triglyceride
This work was supported by an unrestricted research grant from Merck Frosst/Schering Company. P. Couture is the recipient of a scholarship from the FRSQ. B. Lamarche is the recipient of the Canada Research Chair in Nutrition and Cardiovascular Health. N. G. Seidah is a recipient of the Canada Research Chair in Precursor Proteolysis (No. RCHS0164).
REFERENCES
- 1.Coronary heart disease in seven countries. Circulation. 1970. 41(Suppl I): I1–I211. [PubMed] [Google Scholar]
- 2.Castelli W. P. 1984. Epidemiology of coronary heart disease: the Framingham study. Am. J. Med. 76: 4–12. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J., Wentworth D., Neaton J. D. 1986. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 256: 2823–2828. [PubMed] [Google Scholar]
- 4.Genest J., McPherson R., Frohlich J., Anderson T., Campbell N., Carpentier A., Couture P., Dufour R., Fodor G., Francis G. A., et al. 2009. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can. J. Cardiol. 25: 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilvis R. S., Miettinen T. A. 1986. Serum plant sterols and their relation to cholesterol absorption. Am. J. Clin. Nutr. 43: 92–97. [DOI] [PubMed] [Google Scholar]
- 6.Matthan N. R., Raeini-Sarjaz M., Lichtenstein A. H., Ausman L. M., Jones P. J. 2000. Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids. 35: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 7.Rajaratnam R. A., Gylling H., Miettinen T. A. 2000. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J. Am. Coll. Cardiol. 35: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 8.Altmann S. W., Davis H. R., Jr., Zhu L. J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P., Maguire M., Golovko A., Zeng M., et al. 2004. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 9.Dietschy J. M., Turley S. D., Spady D. K. 1993. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 34: 1637–1659. [PubMed] [Google Scholar]
- 10.Stahlberg D., Rudling M., Angelin B., Bjorkhem I., Forsell P., Nilsell K., Einarsson K. 1997. Hepatic cholesterol metabolism in human obesity. Hepatology. 25: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen T. A., Gylling H. 2000. Cholesterol absorption efficiency and sterol metabolism in obesity. Atherosclerosis. 153: 241–248. [DOI] [PubMed] [Google Scholar]
- 12.Sudhop T., Lutjohann D., Kodal A., Igel M., Tribble D. L., Shah S., Perevozskaya I., von Bergmann K. 2002. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 106: 1943–1948. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen T. A., Strandberg T. E., Gylling H. 2000. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler. Thromb. Vasc. Biol. 20: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 14.Connelly P. W., MacLean D. R., Horlick L., O'Connor B., Petrasovits A., Little J. A. 1992. Plasma lipids and lipoproteins and the prevalence of risk for coronary heart disease in Canadian adults. Canadian Heart Health Surveys Research Group. CMAJ. 146: 1977–1987. [PMC free article] [PubMed] [Google Scholar]
- 15.Moorjani S., Dupont A., Labrie F., Lupien P. J., Brun D., Gagné C., Giguère M., Bélanger A. 1987. Increase in plasma high-density lipoprotein concentration following complete androgen blockage in men with prostatic carcinoma. Metabolism. 36: 244–250. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 17.Albers J. J., Warnick G. R., Wiebe D., King P., Steiner P., Smith L., Breckenridge C., Chow A., Kuba K., Weidman S., et al. 1978. Multi-laboratory comparison of three heparin-Mn2+ precipitation procedures for estimating cholesterol in high-density lipoprotein. Clin. Chem. 24: 853–856. [PubMed] [Google Scholar]
- 18.Kinoshita M., Kojima M., Matsushima T., Teramoto T. 2005. Determination of apolipoprotein B-48 in serum by a sandwich ELISA. Clin. Chim. Acta. 351: 115–120. [DOI] [PubMed] [Google Scholar]
- 19.Pirro M., Bergeron J., Dagenais G. R., Bernard P. M., Cantin B., Despres J. P., Lamarche B. 2001. Age and duration of follow-up as modulators of the risk for ischemic heart disease associated with high plasma C-reactive protein levels in men. Arch. Intern. Med. 161: 2474–2480. [DOI] [PubMed] [Google Scholar]
- 20.Matthan N. R., Giovanni A., Schaefer E. J., Brown B. G., Lichtenstein A. H. 2003. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J. Lipid Res. 44: 800–806. [DOI] [PubMed] [Google Scholar]
- 21.Crouse J. R., Grundy S. M. 1978. Evaluation of a continuous isotope feeding method for measurement of cholesterol absorption in man. J. Lipid Res. 19: 967–971. [PubMed] [Google Scholar]
- 22.Gylling H., Miettinen T. A. 2002. Baseline intestinal absorption and synthesis of cholesterol regulate its response to hypolipidaemic treatments in coronary patients. Atherosclerosis. 160: 477–481. [DOI] [PubMed] [Google Scholar]
- 23.Miettinen T. A., Tilvis R. S., Kesaniemi Y. A. 1990. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 131: 20–31. [DOI] [PubMed] [Google Scholar]
- 24.Luu-The V., Paquet N., Calvo E., Cumps J. 2005. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques. 38: 287–293. [DOI] [PubMed] [Google Scholar]
- 25.Warrington J. A., Nair A., Mahadevappa M., Tsyganskaya M. 2000. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol. Genomics. 2: 143–147. [DOI] [PubMed] [Google Scholar]
- 26.Iyer S. P., Yao X., Crona J. H., Hoos L. M., Tetzloff G., Davis H. R., Jr., Graziano M. P., Altmann S. W. 2005. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochim. Biophys. Acta. 1722: 282–292. [DOI] [PubMed] [Google Scholar]
- 27.Dubuc G., Tremblay M., Pare G., Jacques H., Hamelin J., Benjannet S., Boulet L., Genest J., Bernier L., Seidah N. G., et al. 2010. A new method for measurement of total plasma PCSK9: clinical applications. J. Lipid Res. 51: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidah N. G. 2009. PCSK9 as a therapeutic target of dyslipidemia. Expert Opin. Ther. Targets. 13: 19–28. [DOI] [PubMed] [Google Scholar]
- 29.Dietschy J. M., Siperstein M. D. 1967. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J. Lipid Res. 8: 97–104. [PubMed] [Google Scholar]
- 30.Spady D. K., Dietschy J. M. 1983. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J. Lipid Res. 24: 303–315. [PubMed] [Google Scholar]
- 31.Ntanios F. Y., Jones P. J., Frohlich J. J. 1999. Effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor on sterol absorption in hypercholesterolemic subjects. Metabolism. 48: 68–73. [DOI] [PubMed] [Google Scholar]
- 32.Miettinen T. A., Gylling H. 2003. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur. J. Clin. Invest. 33: 976–982. [DOI] [PubMed] [Google Scholar]
- 33.Telford D. E., Sutherland B. G., Edwards J. Y., Andrews J. D., Barrett P. H., Huff M. W. 2007. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J. Lipid Res. 48: 699–708. [DOI] [PubMed] [Google Scholar]
- 34.Alrefai W. A., Annaba F., Sarwar Z., Dwivedi A., Saksena S., Singla A., Dudeja P. K., Gill R. K. 2007. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: Role of sterol regulatory element binding protein 2. Am. J. Physiol. Gastrointest. Liver Physiol. 292: G369–G376. [DOI] [PubMed] [Google Scholar]
- 35.Iwayanagi Y., Takada T., Suzuki H. 2008. HNF4alpha is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm. Res. 25: 1134–1141. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay A. J., Lamarche B., Hogue J. C., Couture P. 2009. Effects of ezetimibe and simvastatin on apolipoprotein B metabolism in males with mixed hyperlipidemia. J. Lipid Res. 50: 1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamon-Fava S., Diffenderfer M. R., Barrett P. H., Buchsbaum A., Matthan N. R., Lichtenstein A. H., Dolnikowski G. G., Horvath K., Asztalos B. F., Zago V., et al. 2007. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J. Lipid Res. 48: 1746–1753. [DOI] [PubMed] [Google Scholar]
- 38.Gouni-Berthold I., Berthold H. K., Chamberland J. P., Krone W., Mantzoros C. S. 2008. Short-term treatment with ezetimibe, simvastatin or their combination does not alter circulating adiponectin, resistin or leptin levels in healthy men. Clin. Endocrinol. (Oxf.). 68: 536–541. [DOI] [PubMed] [Google Scholar]
- 39.Pocathikorn A., Taylor R. R., Mamotte C. D. 2010. Atorvastatin increases expression of low-density lipoprotein receptor mRNA in human circulating mononuclear cells. Clin. Exp. Pharmacol. Physiol. 37: 471–476. [DOI] [PubMed] [Google Scholar]
- 40.Ness G. C., Chambers C. M. 2000. Feedback and hormonal regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase: the concept of cholesterol buffering capacity. Proc. Soc. Exp. Biol. Med. 224: 8–19. [DOI] [PubMed] [Google Scholar]
- 41.Rudling M., Angelin B., Stahle L., Reihner E., Sahlin S., Olivecrona H., Bjorkhem I., Einarsson C. 2002. Regulation of hepatic low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and cholesterol 7alpha-hydroxylase mRNAs in human liver. J. Clin. Endocrinol. Metab. 87: 4307–4313. [DOI] [PubMed] [Google Scholar]
- 42.Horton J. D., Shimomura I., Brown M. S., Hammer R. E., Goldstein J. L., Shimano H. 1998. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J. Clin. Invest. 101: 2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown M. S., Goldstein J. L. 1999. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. U S A. 96: 11041–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misawa K., Horiba T., Arimura N., Hirano Y., Inoue J., Emoto N., Shimano H., Shimizu M., Sato R. 2003. Sterol regulatory element-binding protein-2 interacts with hepatocyte nuclear factor-4 to enhance sterol isomerase gene expression in hepatocytes. J. Biol. Chem. 278: 36176–36182. [DOI] [PubMed] [Google Scholar]
- 45.Seidah N. G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S. B., Stifani S., Basak A., Prat A., Chretien M. 2003. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. U S A. 100: 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nassoury N., Blasiole D. A., Tebon Oler A., Benjannet S., Hamelin J., Poupon V., McPherson P. S., Attie A. D., Prat A., Seidah N. G. 2007. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic. 8: 718–732. [DOI] [PubMed] [Google Scholar]
- 47.Dubuc G., Chamberland A., Wassef H., Davignon J., Seidah N. G., Bernier L., Prat A. 2004. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 24: 1454–1459. [DOI] [PubMed] [Google Scholar]
- 48.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2002. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. U S A. 99: 16237–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., Hobbs H. H. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graf G. A., Yu L., Li W. P., Gerard R., Tuma P. L., Cohen J. C., Hobbs H. H. 2003. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 278: 48275–48282. [DOI] [PubMed] [Google Scholar]
- 51.Tang W., Ma Y., Yu L. 2006. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 44: 1259–1266. [DOI] [PubMed] [Google Scholar]
- 52.Kamisako T., Ogawa H. 2004. Effects of pravastatin and bezafibrate on biliary lipid excretion and hepatic expression of Abcg5 and Abcg8 in the rat. J. Gastroenterol. Hepatol. 19: 879–883. [DOI] [PubMed] [Google Scholar]
- 53.Lally S., Tan C. Y., Owens D., Tomkin G. H. 2006. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: the role of Niemann-Pick C1-like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia. 49: 1008–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.