Abstract
AIM: To reveal the manner of hepatocellular carcinoma (HCC) development in patients with nonalcoholic steatohepatitis (NASH) focusing on multicentric occurrence (MO) of HCC.
METHODS: We compared clinicopathological characteristics between patients with and without MO of HCC arising from NASH background. The clinical features were implicated with reference to the literature available.
RESULTS: MO of HCC was identified with histological proof in 4 out of 12 patients with NASH-related HCC (2 males and 2 females). One patient had synchronous MO; an advanced HCC, two well-differentiated HCCs and a dysplastic nodule, followed by the development of metachronous MO of HCC. The other three patients had multiple advanced HCCs accompanied by a well-differentiated HCC or a dysplastic nodule. Of these three patients, one had synchronous MO, one had metachronous MO and the other had both synchronous and metachronous MO. There were no obvious differences between the patients with or without MO in terms of liver function tests, tumor markers and anatomical extent of HCC. On the other hand, all four patients with MO of HCC were older than 70 years old and had the comorbidities of obesity, type 2 diabetes mellitus (T2DM), hypertension and cirrhosis. Although these conditions were not limited to MO of HCC, all the conditions were met in only one of eight patients without MO of HCC. Thus, concurrence of these conditions may be a predisposing situation to synchronous MO of HCC. In particular, old age, T2DM and cirrhosis were suggested to be prerequisite for MO because these factors were depicted in common among two other cases with MO of HCC under NASH in the literature.
CONCLUSION: The putative predisposing factors and necessary preconditions for synchronous MO of HCC in NASH were suggested in this study. Further investigations are required to clarify the accurate prevalence and predictors of MO to establish better strategies for treatment and prevention leading to the prognostic improvement in NASH.
Keywords: Nonalcoholic steatohepatitis, Hepatocellular carcinoma, Multicentric occurrence
INTRODUCTION
With the increasing prevalence of obesity and type 2 diabetes mellitus (T2DM), nonalcoholic fatty liver disease (NAFLD) has become pandemic, particularly in developed countries, causing public health problems. Within the broad spectrum of the pathophysiology of NAFLD, nonalcoholic steatohepatitis (NASH) is the most serious form because of its propensity to progress toward a fatal event. Although NASH was previously thought to be often indolent[1,2], the following analyses revealed that NASH leads to fibrosis of the liver, cirrhosis and eventually hepatocellular carcinoma (HCC) in a substantial number of patients[3-7]. HCC is currently regarded as a late complication of NASH according to a number of recent reports[3-7]. In addition, it was recently shown that the development of HCC is associated with mortality in cirrhotic NASH in a prospective study in Japanese cohort[5]. Thus, it is very important to elucidate the natural course of NASH in terms of the development of HCC for better management.
Multicentric occurrence (MO) and intrahepatic metastasis (IM) are characteristic in the development of HCC with chronic liver diseases that are caused by hepatitis B virus (HBV) or hepatitis C virus (HCV)[8-13]. It is very important to distinguish MO from IM so that appropriate treatment options may be selected in a variety of clinical settings. Unfortunately, there have been few studies that focus on the characterization of MO in NASH-related HCC despite the increasing number of case reports on HCC based on NASH.
In this report, characteristic features of NASH cases that developed HCC with MO are discussed.
MATERIALS AND METHODS
Patients
From July 2002 to March 2010, we diagnosed and treated 40 adult patients with NASH at the Division of Gastroenterology and Hepatology, Niigata University Medical and Dental Hospital. Of these, HCC was observed in 12 cases including 4 with MO of HCC. The proportion of HCC patients is high in our series with NASH because our hospital is a tertiary referral center and most of the NASH patients who are referred to us are complicated cases.
Definitions
NASH was defined to satisfy all of the following requirements: (1) an absence of clinically significant alcohol intake (less than 20 g/d of ethanol consumption); (2) histological features showing steatosis with various combinations of ballooning liver cells, inflammatory infiltrate of neutrophils, pericellular fibrosis and Mallory bodies; and (3) no other liver diseases. All non-tumorous specimens were histologically scored according to the classification by Brunt et al[14].
MO of HCC was pathologically determined according to the classification of the Liver Cancer Study Group of Japan[15] as follows: 2 or more separate lesions including an early HCC with a dysplastic nodule or no substantial destruction of the preexisting hepatic framework, or moderately and/or poorly differentiated HCCs with a margin of well-differentiated HCC. When histological specimens of HCC could not be obtained, MO was identified by particular findings that correspond to MO on imaging studies[16,17].
Advanced HCCs were defined when they had a vascular pattern that was consistent with contrast enhancement in the arterial phase followed by rapid washout in the portal and/or equilibrium phases on dynamic computed tomography (CT) and/or dynamic magnetic resonance (MR) imaging. A nodule was also diagnosed with an advanced HCC when the lesion was depicted as a defect on CT during arterial portography (CTAP) and as a hyperattenuated lesion in the first phase of double-phase CT during hepatic arteriography (CTHA) followed by corona-like enhancement in the second phase[16,17]. A nodule-in-nodule appearance on dynamic CT, dynamic MR imaging or CTHA was regarded as a specific finding that indicated the emergence of a dedifferentiated component (moderately or poorly differentiated HCC) within a well-differentiated HCC, even without histological proof. Otherwise, well-differentiated HCCs or dysplastic nodules were diagnosed based on histological examinations.
The clinical stage of HCC was stratified according to the TNM classification of the Liver Cancer Study Group of Japan[18].
Obesity was defined as body mass index (BMI) ≥ 25 kg/m2 according to the criteria proposed by the Japan Society for the Study of Obesity[19]. The definition was based on the fact that obesity-related diseases increase with BMI ≥ 25 kg/m2 in the Japanese population. T2DM was diagnosed according to the criteria advocated by the Japan Diabetes Society as follows: fasting plasma glucose ≥ 126 mg/dL, random plasma glucose ≥ 200 mg/dL or hemoglobin A1c ≥ 6.5% on two separate occasions. The diagnosis of hypertension was made if the patient was on antihypertensive medication or had blood pressure ≥ 140/90 mmHg on at least two separate occasions. Dyslipidemia was defined as total cholesterol level ≥ 220 mg/dL and/or fasting triglyceride level ≥ 150 mg/dL on at least two separate occasions or continuously receiving lipid-lowering agents.
Laboratory examinations
The following laboratory tests were recorded at diagnosis of HCC in all patients: aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltranspeptidase (γ-GTP), total bilirubin, albumin, prothrombin time, platelet count, Child-Pugh score, hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), HCV antibody (anti-HCV), alpha-fetoprotein (AFP) and des-γ-carboxy-prothrombin (DCP).
RESULTS
The clinical and laboratory data from 12 patients with NASH-related HCC are shown in Table 1. Of these, MO of HCC was observed in cases 1 to 4. Regarding common characteristics, all 4 patients with MO were over 70 years old, obese and had T2DM, hypertension and cirrhosis. Although these conditions were not limited to MO of HCC, all the conditions were met in only one (case 9) of eight patients without MO of HCC. There were no obvious differences between the patients with or without MO in terms of liver function tests, tumor markers and stages of tumor development. Although a few patients had positive anti-HBc, the titer of the antibody was low and no attribution of HBV infection to background liver disease was histologically ascertained.
Table 1.
Clinical characteristics of patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma with or without multicentric occurrence
|
HCC with MO |
HCC without MO |
|||||||||||
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Age at Dx of NASH | 73 | 81 | 78 | 71 | 68 | 75 | 71 | 80 | 75 | 78 | 81 | 66 |
| Gender | F | M | M | F | M | F | M | F | F | M | M | M |
| History of BTF | - | - | - | - | - | - | - | + | - | - | - | - |
| BMI (kg/m2) | 31.0 | 26.3 | 25.5 | 32.0 | 26.8 | 23.0 | 27.0 | 32.1 | 34.0 | 23.1 | 24.5 | 25.2 |
| T2DM | + | + | + | + | - | + | + | - | + | - | - | - |
| Hypertension | + | + | + | + | - | - | - | + | + | - | + | + |
| Dyslipidemia | - | - | - | + | - | + | + | + | - | + | + | - |
| Varices | - | - | - | - | + | - | + | - | + | - | - | - |
| Ascites | - | - | - | - | - | - | - | - | - | - | - | - |
| AST (IU/L) | 91 | 67 | 43 | 33 | 34 | 43 | 36 | 34 | 43 | 25 | 83 | 78 |
| ALT (IU/L) | 46 | 48 | 49 | 21 | 17 | 32 | 22 | 33 | 40 | 20 | 37 | 75 |
| γ-GTP (IU/L) | 122 | 129 | 418 | 72 | 30 | 222 | 46 | 67 | 73 | 104 | 360 | 279 |
| Total bilirubin (mg/dL) | 1.0 | 0.7 | 0.8 | 1.1 | 1.4 | 1.1 | 1.5 | 0.6 | 1.2 | 0.6 | 0.6 | 0.8 |
| Albumin (g/dL) | 3.8 | 3.7 | 3.4 | 2.9 | 3.3 | 4.0 | 2.9 | 4.1 | 3.7 | 3.8 | 3.4 | 4.2 |
| Prothrombin time (%) | 53 | 72 | 97 | 62 | 69 | NAa | 59 | 69 | 86 | 75 | 84 | NAa |
| Platelet (× 104/μL) | 21.1 | 12.3 | 10.4 | 11.2 | 4.5 | 14.5 | 6.4 | 31.6 | 11.7 | 12.1 | 26.4 | 11.4 |
| Child-Pugh score | A | A | A | B | B | -a | B | A | A | A | A | -a |
| HBsAg | - | - | - | - | - | - | - | - | - | - | - | - |
| Anti-HBc | + | - | - | + | - | - | - | - | + | + | - | + |
| Anti-HCV | - | - | - | - | - | - | - | - | - | - | - | - |
| Histological features (Classification by Brunt) | ||||||||||||
| Grade | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 2 |
| Stage | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 2 | 4 |
| Type of cirrhosis | Mixed | Mixed | Macro | Mixed | Micro | Mixed | Mixed | Mixed | Mixed | - | - | Macro |
| AFP (ng/mL) | 11 | 26 | 15 | 25 | 7 | 9 | 3 | 8 757 | 12 | 2 963 | 900 100 | 8 |
| DCP (mAU/mL) | 18 | 24 | 20 | 116 | 24 | NAa | 14 | 35 | 907 | 131 | 10 700 | NAa |
| Maximum size of HCC (mm) | 35 | 50 | 40 | 28 | 22 | 17 | 50 | 26 | 40 | 40 | 110 | 70 |
| Number of HCC | Mul | Mul | Mul | Mul | Mul | Sol | Mul | Sol | Mul | Mul | Mul | Sol |
| TNM stage | III | III | III | III | IVA | I | III | II | III | IVB | III | II |
| Initial treatment | Ope | TACE | Ope | TACE | TAI | RFA | TACE | TACE | Ope | Chemo | Ope | TAI |
| + RFA | + RFA | + Ope | + RFA | + Ope | ||||||||
| Outcome | Alive | Dead | Dead | Alive | Alive | Alive | Alive | Alive | Dead | Dead | Alive | Alive |
| Cause of death | - | LR | NLR | - | - | - | - | - | LR | LR | - | - |
| Follow-up period after Dx of HCC (days) | 2 599 | 1 706 | 288 | 1 140 | 280 | 1 011 | 687 | 2 198 | 1 525 | 489 | 162 | 330 |
HCC: hepatocellular carcinoma; MO: multicentric occurrence; Dx: diagnosis; BTF: blood transfusion; BMI: body mass index; M: Male; F: Female; T2DM: type 2 diabetes mellitus; AST: aspartate aminotransferase; ALT: alanine aminotransferase; γ-GTP: γ-glutamyltranspeptidase; NA: not available; AFP: alpha-fetoprotein; DCP: des-γ-carboxy-prothrombin; Mixed: mixed nodular cirrhosis; Macro: macronodular cirrhosis; Micro: micronodular cirrhosis; Mul: multiple; Sol: solitary; TACE: transcatheter arterial chemoembolization; TAI: transcatheter arterial infusion chemotherapy; RFA: radiofrequency ablation; Ope: operation; LR: liver-related death; NLR: non-liver-related death;
An oral administration of warfarin prevented the evaluation.
The clinical courses of the 4 patients with MO of HCC are detailed below.
Case 1
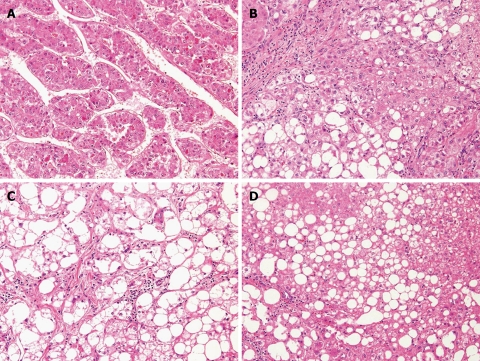
A 73 year old female had been diagnosed with hypertension at age 40 and T2DM at age 70. After the diagnoses, she was treated with an angiotensin II receptor blocker and an oral anti-hyperglycemic agent at our affiliated hospital. A liver tumor measuring 35 mm in diameter in segment 6 was found with a nodule-in-nodule appearance on a surveillance dynamic CT in January 2003. The finding was consistently shown on dynamic MR imaging, CTAP and CTHA. Near the tumor, another nodule 8 mm in diameter was depicted as a hyperechoic nodule on ultrasonography (US) and as a perfusion defect on CTAP without early enhancement on CTHA. A well-differentiated HCC or a dysplastic nodule was suspected. The patient was referred to our hospital and underwent surgical resection of segment 6. During the operation, multiple hyperechoic nodules measuring less than 10 mm in diameter in addition to the main tumor were detected in segment 6 and segment 8 by intraoperative US which led to an additional resection of segment 8. The largest tumor in segment 6 was histologically diagnosed with a moderately differentiated HCC (Figure 1A). In addition, two well-differentiated HCCs and one dysplastic nodule were observed among the other small nodules (Figure 1B, C and D). All of these tumors were considered to occur in a multicentric manner.
Figure 1.
Histological examination of resected specimen in Case 1 showing four liver tumors of multicentric origin (HE stain, × 200). A: A moderately differentiated hepatocellular carcinoma (HCC); B: A well-differentiated HCC; C: A well-differentiated HCC; D: A dysplastic nodule.
In October 2008, recurrence of HCC was found in segment 8 on dynamic CT with early enhancement and the patient underwent transcatheter arterial chemoembolization. In March 2009, one and two nodules were detected in segments 8 and 3, respectively, as hypovascular tumors measuring less than 15 mm in diameter on dynamic MR imaging that were suspicious for well-differentiated HCCs or dysplastic nodules. The tumors subsequently increased in diameter and were compatible with well-differentiated HCCs on imaging studies which suggested metachronous MO. The patient was treated with percutaneous ablation therapy and has had no recurrence of HCC after the therapy.
Case 2
An 81 year old male had been diagnosed with hypertension at age 40 and T2DM at age 75. After the diagnoses, he was treated with a calcium channel blocker and an oral anti-hyperglycemic agent. When he was admitted to the Division of Dermatology at our hospital for the treatment of psoriasis vulgaris, dynamic CT and MR imaging depicted multiple tumors in the bilateral lobes of the liver. The classical pattern of contrast enhancement that indicated advanced HCCs was observed in those tumors with the exception of a nodule measuring 10 mm in diameter in segment 3. This distinct nodule was recognized as hypoattenuation in the arterial and equilibrium phases on dynamic CT. Dynamic MR imaging demonstrated hypointensity only in the equilibrium phase but isointensity in the arterial phase and other sequences. The nodule was also shown as isoattenuation on CTAP and CTHA. We regarded this nodule as an equivocal lesion and decided to follow it closely. The combination therapy with transcatheter arterial infusion chemotherapy and percutaneous radiofrequency ablation was repeated four times for the advanced HCCs. Approximately 1 year later, the follow-up study with dynamic MR imaging showed an altered appearance of the nodule in segment 3; it was hypointense in the arterial and equilibrium phases and on T1- and T2-weighed images (WIs) and was slightly enlarged, 18 mm in diameter. This nodule was finally diagnosed with a well-differentiated HCC by tumor biopsy and the surrounding nontumorous parenchyma was histologically defined as cirrhosis derived from NASH. Thus, the development of this well-differentiated HCC was considered MO and the tumor was treated with local ablation therapy. Afterward, multiple HCCs recurred via intrahepatic metastases and transcatheter arterial chemoembolization therapies were repeated. Unfortunately, the patient died of hepatic failure with marked extension of HCCs at age 86.
Case 3
A 78 year old male was referred to our hospital for further workup and treatment of liver tumors detected on CT. Abnormal liver function tests had been found for 8 years. The patient had a 2 year history of treatment for hypertension and T2DM with oral drugs. Two tumors, 40 mm in diameter in the posterior segment and 20 mm in diameter in segment 8, respectively, were discovered on US, performed as part of the regular screening. Other imaging studies, including dynamic CT, MR imaging, CTAP and CTHA, indicated advanced HCCs. Other than those tumors, a nodule measuring 8 mm in diameter with different characteristics was detected in segment 5. The nodule was slightly hypointense in the arterial and equilibrium phases and isointense on T1- and T2-WIs of dynamic MR imaging. It was exhibited as isoattenuation on CTAP and hypoattenuation in both phases of CTHA. On the basis of these results, the tumor was suspicious for a well-differentiated HCC or a dysplastic nodule. The patient underwent surgical resection of the right hepatic lobe. The histological examination revealed cirrhosis of a nontumorous liver caused by NASH. The advanced tumors in the posterior segment and segment 8 were diagnosed with moderately differentiated HCCs and the distinct nodule in segment 5 was confirmed as a well-differentiated HCC which implied MO. After the surgery, the patient suffered from pneumonia, renal insufficiency and subsequent hepatic failure and he finally died of multiple organ failure approximately 2 mo later.
Case 4
A 71 year old female had received medical treatment for hypertension, dyslipidemia and T2DM at a clinic 7 years prior to presentation. At age 66, abnormal liver function tests were revealed on a routine examination and the patient was diagnosed with cryptogenic cirrhosis by liver biopsy at another hospital. Multiple liver tumors were detected on periodic US and the patient was referred to our hospital for further management. The definitive diagnosis of NASH was made as the cause of cirrhosis by a pathologist at our hospital with reevaluation of the previously obtained liver specimen. Two tumors in segment 8 and one tumor in segment 3, both less than 30 mm in diameter, were demonstrated as advanced HCCs on dynamic CT, MR imaging and CTHA. CTAP was not informative because of insufficient portal perfusion due to hepatofugal portal flow through a gastrorenal shunt. Meanwhile, a nodule in segment 1 with a diameter of 28 mm was shown as a nodule-in-nodule appearance on dynamic MR imaging. Therefore, this tumor was considered multicentric in origin. The patient was treated with transcatheter arterial chemoembolization and/or percutaneous ablation therapy. Furthermore, 18 mo after the treatment, another well-differentiated histologically confirmed HCC emerged in segment 7 and was treated with RFA. Thereafter, the patient remains alive without recurrence of HCC.
DISCUSSION
HCC occurs frequently through multicentric carcinogenesis in chronic liver diseases caused by HBV or HCV infection. In the present study, we found the prevalent occurrence of HCC with multicentric manner in patients with NASH as well. According to the previous studies based on pathological examinations, frequencies of synchronous MO in patients with HBV- or HCV-related HCC were 3.7%-16.5% and 11.9%-34.1%, respectively[10-12]. As for metachronous MO, it was reported that the 1 year, 3 year and 5 year MO rates were also as high as 5.3%, 28.9% and 50.8%, respectively, in HCC patients, most of whom were associated with HCV infection[9]. HCV infection that causes persistent active inflammation in the liver is believed to be one of the most important factors for MO of HCC[8-13]. Contrary to the abundant clinical data on MO in HBV- and HCV-related HCC, there is no epidemiological study addressing MO in NASH-related HCC. However, informative observations were reported by Oikawa et al[12]. They evaluated the liver specimens surgically obtained from 94 cases with nonB-nonC HCC and detected synchronous MO of HCC in 12 cases (12.8%). They speculated that these cases might have occult HBV infection or other undefined hepatitis virus infection. Although the etiology of the underlying chronic liver disease in this group could not be further estimated because of the lack of clinical profiles and histological findings of noncancerous liver tissues, it is not difficult to suppose a causal attribution to NASH in some cases. In addition, Tokushige et al[20] revealed a high recurrence rate of HCC with NASH after two years or more of curative treatment. Those recurrent tumors in the later follow-up years were found in 9 of 16 patients (56.3%) and some of them were presumed to be of multicentric origin. Therefore, even in our cases without synchronous MO of HCC, it is possible that HCC may develop metachronously in the future. MO may be a frequent manner of HCC development in NASH as well and it is crucial to clarify the exact prevalence of MO with further studies.
Clinical features of patients with MO of HCC related to NASH are also quite obscure. There are only two case reports that have documented MO of HCC in NASH with histological proof. Zen et al[6] reported a 72 year old female with HCC arising multicentrically from cirrhotic NASH. The patient was diagnosed with T2DM. Three tumors developed synchronously and metachronously and were histologically defined as a moderately differentiated HCC, a well-differentiated HCC and a dysplastic nodule, respectively. Sasaki et al[7] described a 73 year old female with MO of HCC based on cirrhotic NASH. The patient was complicated by T2DM and obesity. Two liver tumors were detected metachronously and both were diagnosed with well-differentiated HCCs.
In the present report, the clinical profiles of old age, obesity, T2DM, hypertension and cirrhosis are identified as common characteristics of the 4 patients with MO of HCC (Table 1); these may be predisposing factors to MO of HCC. Moreover, of these characteristics, old age, T2DM and cirrhosis are recognized as common conditions among the 2 patients with MO of HCC in previous reports (Table 2). Although these conditions are also observed in some patients with solitary HCC which is unaccompanied by MO as described in the present report and previous literature (Table 3)[21-36], they do not always satisfy these conditions. A literature search shows that the proportion of patients with age older than 70 years, T2DM and cirrhosis with solitary HCC is 46.4% (26 of 56 cases), 67.9% (36 of 53 cases) and 64.3% (36 of 56 cases), respectively. Thus, it is appropriate to consider that those conditions (old age, T2DM and cirrhosis) are necessary preconditions of synchronous MO.
Table 2.
Clinical characteristics of patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma with multicentric occurrence in previous and present reports
| Author | Cases | Age older than 70 yr |
Gender |
Metabolic diseases |
Histological features |
|||||
| M | F | OB | DM | DL | HT | LC | Non-LC | |||
| Zen et al[6] | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Sasaki et al[7] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Present authors | 4 | 4 | 2 | 2 | 4 | 4 | 1 | 4 | 4 | 0 |
| Total | 6 | 6 | 2 | 4 | 5 | 6 | 1 | 4 | 6 | 0 |
| (%) | - | (100) | (33.3) | (66.7) | (83.3) | (100) | (16.7) | (66.7) | (100) | (0) |
M: male; F: female; OB: obesity; DM: type 2 diabetes mellitus; DL: dyslipidemia; HT: hypertension; LC: liver cirrhosis; Non-LC: non-liver cirrhosis. All values represent number of cases.
Table 3.
Clinical characteristics of patients with solitary hepatocellular carcinoma related to nonalcoholic steatohepatitis in previous and present reports
| Author | Cases | Age older than 70 yr |
Gender |
Metabolic diseases |
Histological features |
|||||
| M | F | OB | DM | DL | HT | LC | Non-LC | |||
| Cotrim et al[21] | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Orikasa et al[22] | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Shimada et al[23] | 4 | 1 | 2 | 2 | 3 | 2 | 1 | 2 | 4 | 0 |
| Mori et al[24] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Bullock et al[25] | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 2 |
| Cuadrado et al[26] | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 0 | 1 | 1 |
| Sato et al[27] | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Ichikawa et al[28] | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 2 |
| Ikeda et al[29] | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| Hai et al[30] | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | 1 |
| Hashizume et al[31] | 7 | 5 | 4 | 3 | 5 | 5 | 6 | 5 | 5 | 2 |
| Maeda et al[32] | 3 | 0 | 2 | 1 | NA | NA | NA | NA | 3 | 0 |
| Kawada et al[33] | 6 | 4 | 3 | 3 | 2 | 3 | 1 | 4 | 0 | 6 |
| Malik et al[34] | 8 | 2 | 6 | 2 | 5 | 6 | NA | 5 | 8 | 0 |
| Chagas et al[35] | 4 | 3 | 2 | 2 | 4 | 3 | 1 | NA | 4 | 0 |
| Takuma et al[36] | 8 | 5 | 4 | 4 | 4 | 6 | 3 | 6 | 3 | 5 |
| Present authors | 3 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 3 | 0 |
| Total | 56 | 26 | 35 | 21 | 36 | 36 | 16 | 29 | 36 | 20 |
| (%) | - | (46.4) | (62.5) | (37.5) | (67.9) | (67.9) | (35.6) | (59.2) | (64.3) | (35.7) |
M: male; F: female; OB: obesity; DM: type 2 diabetes mellitus; DL: dyslipidemia; HT: hypertension; NA: not available; LC: liver cirrhosis; Non-LC: non-liver cirrhosis. All values represent number of cases.
The role of each factor in the pathogenesis of MO of HCC is not understood. Old age and cirrhosis have been shown to be the strongest risk factors for the development of HCC in NASH with a prospective cohort study[37]. Obesity has been confirmed as an independent risk factor for the development of HCC in several studies[38-42], as has T2DM[43-45]. With regard to the association of T2DM, it was postulated that chronic hyperinsulinemia and insulin-like growth factor 1 might be involved in carcinogenesis[46]. Although MO of HCC may be attributable to independent factors, a concurrence of the factors of old age, metabolic abnormalities and cirrhosis perhaps significantly raises the malignant potential in the liver and probability of subsequent synchronous and multifocal development of HCCs. The exact mechanism of MO of HCC under those conditions needs to be elucidated.
It is unclear whether the same conditions can be adapted to cases with metachronous MO because it may not be long enough to observe metachronous MO of HCC in the present study. Even in patients without MO of HCC at diagnosis, HCC of multicentric origin might occur metachronously after longer periods of follow up. Moreover, the number of subjects is too small to draw a clear conclusion. These are limitations of this study and warrant further exploration with a larger scale and longer duration.
Discrimination of MO from IM is critical for selecting an appropriate method of treatment for HCC caused by HCV infection. The prognosis of HCV-related HCC patients with MO is thought to be better than that with IM because of the low rate of recurrence by IM in the MO group[13]. Therefore, it is reasonable to adopt locoregional therapy such as surgical removal or radiofrequency ablation which is more effective than transcatheter arterial chemoembolization or systemic chemotherapy, even in cases with multiple HCCs when they are considered of multicentric origin. However, whether the same strategy can be adapted to NASH-related HCC remains unclear because outcomes of HCC with or without MO in NASH have not been clarified. To establish an adequate strategy against HCC with NASH, their outcomes should be analyzed with stratification on the basis of not only tumor staging, hepatic reserve and kinds of treatment but also the manner of HCC development.
Furthermore, it is important to prevent MO of HCC. In patients with HCV-related HCC, eradication of HCV by interferon therapy after curative treatment for HCC provided promising effects on the prevention of HCC recurrence in several reports[47-49]. Adjuvant therapy using interferon reduced late recurrence of HCC after two or more years of curative treatment. This fact may be attributable to the suppression of multicentric carcinogenesis through the inhibition of chronic inflammation. Even in NASH, it would be possible that anti-inflammation or anti-fibrosis therapy for the liver leads to the prevention of HCC development with multicentric manner. Based on this, it may be worth attempting to intensely treat underlying liver disease as well as HCC to produce consequent improvement in the prognosis of NASH.
In summary, synchronous and/or metachronous MO was recognized in 4 of 12 patients with NASH-related HCC. MO may be frequently provoked in NASH-related HCC as well as in chronic liver diseases caused by HBV or HCV infection. The clinical status of age older than 70 years, obesity, T2DM, hypertension and cirrhosis was identified as common characteristics of the patients with MO of HCC and they might be predisposing factors to at least synchronous MO. Of these characteristics, old age, T2DM and cirrhosis are also common features of the other 2 patients from the previous case reports so these confined conditions may be necessary preconditions for synchronous MO. Adequate methods of treatment and prevention for MO of HCC are necessary and may lead to a consequent improvement in the prognosis of NASH.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is an often fatal event of nonalcoholic steatohepatitis (NASH). Although multicentric occurrence (MO) is frequently observed characteristics in the development of HCC caused by HBV or HCV infection, the manner of HCC development in NASH remains unclear.
Research frontiers
Although many clinicopathological and epidemiological studies on NASH have been conducted to define the incidence of HCC and the predisposing factors to HCC, there has been no study that focuses on MO of HCC with NASH.
Innovations and breakthroughs
The authors demonstrated that MO of HCC was found in 4 of 12 patients with NASH-related HCC. The common characteristics among the patients with MO of HCC were old age, obesity, type 2 diabetes mellitus (T2DM), hypertension and cirrhosis, suggesting putative predisposing factors to synchronous MO of HCC with NASH. Of these characteristics, old age, T2DM and cirrhosis are also common features of the other 2 patients from previous case reports so these confined conditions may be necessary preconditions for synchronous MO.
Applications
Understanding the manner of HCC development in NASH may be helpful for developing an adequate treatment strategy for HCC.
Terminology
MO of HCC means development of multiple HCCs with independent origins. When multiple HCCs include an early HCC with a dysplastic nodule or a moderately and/or poorly differentiated HCC with a margin of well-differentiated HCC, they are referred to as multicentric in origin.
Peer review
The present manuscript reports four cases of NASH patients that developed HCC with multicentric occurrence. The results showed are potentially interesting, and the conclusions are, in general, adequately supported by the experimental findings. All these considerations plus the interest of the data reported make the paper to be considered worth publishing.
Footnotes
Peer reviewers: Sonia Ramos, PhD, Department of Metabolism and Nutrition, Instituto del Frio (CSIC), Jose Antonio Novais, Madrid 28040, Spain; Stefan Rose-John, Director, Department of Biochemistry, Christian-Albrechts-Universität zu Kiel, Medical Faculty, Olshausenstraäe 40, Kiel D24098, Germany; Johanna Kassiani Delladetsima, Associate Professor, Department of Pathology, Medical School, University of Athens, Goudi, Athens 11527, Greece
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Heuman D, Coterrell A, Fisher RA, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 5.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 6.Zen Y, Katayanagi K, Tsuneyama K, Harada K, Araki I, Nakanuma Y. Hepatocellular carcinoma arising in non-alcoholic steatohepatitis. Pathol Int. 2001;51:127–131. doi: 10.1046/j.1440-1827.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki M, Itatsu K, Minato H, Takamura H, Ohta T, Nakanuma Y. Flare-up of nonalcoholic steatohepatitis after hepatectomy resulted in hepatic failure in a patient with type 2 diabetes mellitus. Dig Dis Sci. 2007;52:3473–3476. doi: 10.1007/s10620-006-9662-7. [DOI] [PubMed] [Google Scholar]
- 8.Takenaka K, Adachi E, Nishizaki T, Hiroshige K, Ikeda T, Tsuneyoshi M, Sugimachi K. Possible multicentric occurrence of hepatocellular carcinoma: a clinicopathological study. Hepatology. 1994;19:889–894. [PubMed] [Google Scholar]
- 9.Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. doi: 10.1053/jhep.1997.v25.pm0008985270. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima O, Kojiro M. Recurrence of hepatocellular carcinoma: multicentric occurrence or intrahepatic metastasis? A viewpoint in terms of pathology. J Hepatobiliary Pancreat Surg. 2001;8:404–409. doi: 10.1007/s005340100001. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto Y, Fujii H, Matsuda M, Kono H. Multicentric occurrence of hepatocellular carcinoma: diagnosis and clinical significance. J Hepatobiliary Pancreat Surg. 2001;8:435–440. doi: 10.1007/s005340100006. [DOI] [PubMed] [Google Scholar]
- 12.Oikawa T, Ojima H, Yamasaki S, Takayama T, Hirohashi S, Sakamoto M. Multistep and multicentric development of hepatocellular carcinoma: histological analysis of 980 resected nodules. J Hepatol. 2005;42:225–229. doi: 10.1016/j.jhep.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Shimada M, Hamatsu T, Yamashita Y, Rikimaru T, Taguchi K, Utsunomiya T, Shirabe K, Sugimachi K. Characteristics of multicentric hepatocellular carcinomas: comparison with intrahepatic metastasis. World J Surg. 2001;25:991–995. doi: 10.1007/s00268-001-0068-6. [DOI] [PubMed] [Google Scholar]
- 14.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 15.Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer [in Japanese]. 5th ed. Tokyo: Kanehara; 2008. p. 43. [Google Scholar]
- 16.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Nonomura A, Nakanuma Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR. 1999;172:969–976. doi: 10.2214/ajr.172.4.10587130. [DOI] [PubMed] [Google Scholar]
- 17.Takayasu K, Muramatsu Y, Mizuguchi Y, Moriyama N, Ojima H. Imaging of early hepatocellular carcinoma and adenomatous hyperplasia (dysplastic nodules) with dynamic ct and a combination of CT and angiography: experience with resected liver specimens. Intervirology. 2004;47:199–208. doi: 10.1159/000078473. [DOI] [PubMed] [Google Scholar]
- 18.Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909–922. doi: 10.1097/01.sla.0000254368.65878.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 20.Tokushige K, Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Shiratori K. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol. 2010;45:960–967. doi: 10.1007/s00535-010-0237-1. [DOI] [PubMed] [Google Scholar]
- 21.Cotrim HP, Paraná R, Braga E, Lyra L. Nonalcoholic steatohepatitis and hepatocellular carcinoma: natural history? Am J Gastroenterol. 2000;95:3018–3019. doi: 10.1111/j.1572-0241.2000.03241.x. [DOI] [PubMed] [Google Scholar]
- 22.Orikasa H, Ohyama R, Tsuka N, Eyden BP, Yamazaki K. Lipid-rich clear-cell hepatocellular carcinoma arising in non-alcoholic steatohepatitis in a patient with diabetes mellitus. J Submicrosc Cytol Pathol. 2001;33:195–200. [PubMed] [Google Scholar]
- 23.Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, Takasaki K, Ludwig J. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154–160. doi: 10.1016/s0168-8278(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 24.Mori S, Yamasaki T, Sakaida I, Takami T, Sakaguchi E, Kimura T, Kurokawa F, Maeyama S, Okita K. Hepatocellular carcinoma with nonalcoholic steatohepatitis. J Gastroenterol. 2004;39:391–396. doi: 10.1007/s00535-003-1308-3. [DOI] [PubMed] [Google Scholar]
- 25.Bullock RE, Zaitoun AM, Aithal GP, Ryder SD, Beckingham IJ, Lobo DN. Association of non-alcoholic steatohepatitis without significant fibrosis with hepatocellular carcinoma. J Hepatol. 2004;41:685–686. doi: 10.1016/j.jhep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Cuadrado A, Orive A, García-Suárez C, Domínguez A, Fernández-Escalante JC, Crespo J, Pons-Romero F. Non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma. Obes Surg. 2005;15:442–446. doi: 10.1381/0960892053576596. [DOI] [PubMed] [Google Scholar]
- 27.Sato K, Ueda Y, Ueno K, Okamoto K, Iizuka H, Katsuda S. Hepatocellular carcinoma and nonalcoholic steatohepatitis developing during long-term administration of valproic acid. Virchows Arch. 2005;447:996–999. doi: 10.1007/s00428-005-0042-z. [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa T, Yanagi K, Motoyoshi Y, Hamasaki K, Nakao K, Toriyama K, Eguchi K. Two cases of non-alcoholic steatohepatitis with development of hepatocellular carcinoma without cirrhosis. J Gastroenterol Hepatol. 2006;21:1865–1866. doi: 10.1111/j.1440-1746.2006.04282.x. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda H, Suzuki M, Takahashi H, Kobayashi M, Okuse N, Moriya H, Koike J, Maeyama S, Yotsuyanagi H, Itoh F. Hepatocellular carcinoma with silent and cirrhotic non-alcoholic steatohepatitis, accompanying ectopic liver tissue attached to gallbladder. Pathol Int. 2006;56:40–45. doi: 10.1111/j.1440-1827.2006.01916.x. [DOI] [PubMed] [Google Scholar]
- 30.Hai S, Kubo S, Shuto T, Tanaka H, Takemura S, Yamamoto T, Kanazawa A, Ogawa M, Hirohashi K, Wakasa K. Hepatocellular carcinoma arising from nonalcoholic steatohepatitis: report of two cases. Surg Today. 2006;36:390–394. doi: 10.1007/s00595-005-3167-4. [DOI] [PubMed] [Google Scholar]
- 31.Hashizume H, Sato K, Takagi H, Hirokawa T, Kojima A, Sohara N, Kakizaki S, Mochida Y, Shimura T, Sunose Y, et al. Primary liver cancers with nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2007;19:827–834. doi: 10.1097/MEG.0b013e3282748ef2. [DOI] [PubMed] [Google Scholar]
- 32.Maeda T, Hashimoto K, Kihara Y, Ikegami T, Ishida T, Aimitsu S, Fujiwara M. Surgically resected hepatocellular carcinomas in patients with non-alcoholic steatohepatitis. Hepatogastroenterology. 2008;55:1404–1406. [PubMed] [Google Scholar]
- 33.Kawada N, Imanaka K, Kawaguchi T, Tamai C, Ishihara R, Matsunaga T, Gotoh K, Yamada T, Tomita Y. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 34.Malik SM, Gupte PA, de Vera ME, Ahmad J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:800–806. doi: 10.1016/j.cgh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Chagas AL, Kikuchi LO, Oliveira CP, Vezozzo DC, Mello ES, Oliveira AC, Cella LC, Herman P, Bachella T, Caldwell SH, et al. Does hepatocellular carcinoma in non-alcoholic steatohepatitis exist in cirrhotic and non-cirrhotic patients? Braz J Med Biol Res. 2009;42:958–962. doi: 10.1590/s0100-879x2009005000019. [DOI] [PubMed] [Google Scholar]
- 36.Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol. 2010;16:1436–1441. doi: 10.3748/wjg.v16.i12.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 38.Møller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 39.Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, Adam HO. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 40.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 41.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 42.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 43.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 44.Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, Degos F, Farges O, Belghiti J. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69–S73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 45.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9:291–293. [PubMed] [Google Scholar]
- 47.Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Yamazaki O, Shiomi S, Tamori A, Oka H, Igawa S, et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134:963–967. doi: 10.7326/0003-4819-134-10-200105150-00010. [DOI] [PubMed] [Google Scholar]
- 48.Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418–422. doi: 10.1046/j.0007-1323.2001.02054.x. [DOI] [PubMed] [Google Scholar]
- 49.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]