Abstract
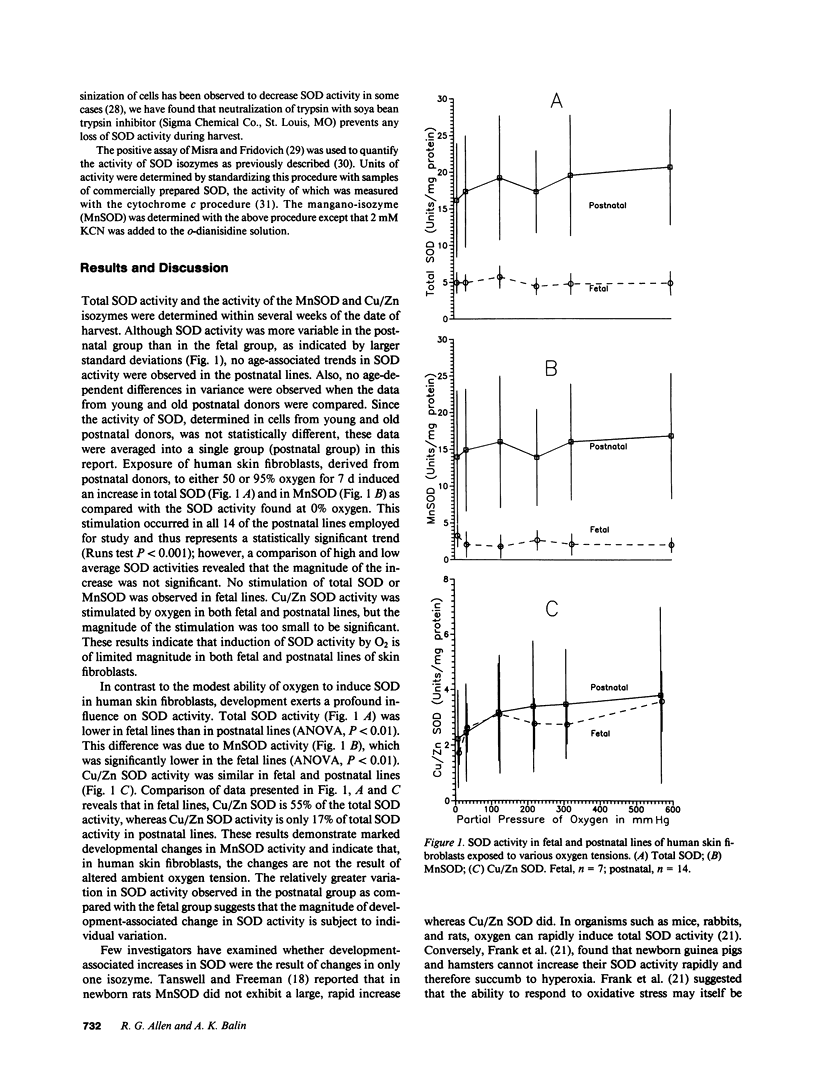
Confluent cultures of human skin fibroblast lines established from fetal and postnatal donors were exposed to a broad range of oxygen tensions (10-600 mmHg) for 1 wk; superoxide dismutase (SOD) activity was subsequently determined. Hyperoxia increased SOD activity slightly in postnatal lines but not in fetal lines. The magnitude of the increase in postnatal lines was not significant. Fetal lines exhibit only about one-fifth the SOD activity observed in postnatal lines. The results indicate that, while development-associated changes in SOD do occur in human cells, these alterations do not result from variations in ambient oxygen tension.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Balin A. K., Reimer R. J., Sohal R. S., Nations C. Superoxide dismutase induces differentiation in microplasmodia of the slime mold Physarum polycephalum. Arch Biochem Biophys. 1988 Feb 15;261(1):205–211. doi: 10.1016/0003-9861(88)90119-1. [DOI] [PubMed] [Google Scholar]
- Allen R. G., Newton R. K., Farmer K. J., Nations C. Effects of the free radical generator paraquat on differentiation, superoxide dismutase, glutathione and inorganic peroxides in microplasmodia of Physarum polycephalum. Cell Tissue Kinet. 1985 Nov;18(6):623–630. doi: 10.1111/j.1365-2184.1985.tb00705.x. [DOI] [PubMed] [Google Scholar]
- Allen R. G., Newton R. K., Sohal R. S., Shipley G. L., Nations C. Alterations in superoxide dismutase, glutathione, and peroxides in the plasmodial slime mold Physarum polycephalum during differentiation. J Cell Physiol. 1985 Dec;125(3):413–419. doi: 10.1002/jcp.1041250308. [DOI] [PubMed] [Google Scholar]
- Autor A. P., Frank L., Roberts R. J. Developmental characteristics of pulmonary superoxide dismutase: relationship to idiopathic respiratory distress syndrome. Pediatr Res. 1976 Mar;10(3):154–158. doi: 10.1203/00006450-197603000-00002. [DOI] [PubMed] [Google Scholar]
- Balin A. K., Fisher A. J., Carter D. M. Oxygen modulates growth of human cells at physiologic partial pressures. J Exp Med. 1984 Jul 1;160(1):152–166. doi: 10.1084/jem.160.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin A. K., Goodman B. P., Rasmussen H., Cristofalo V. J. The effect of oxygen tension on the growth and metabolism of WI-38 cells. J Cell Physiol. 1976 Oct;89(2):235–249. doi: 10.1002/jcp.1040890207. [DOI] [PubMed] [Google Scholar]
- Bucher J. R., Roberts R. J. The development of the newborn rat lung in hyperoxia: a dose-response study of lung growth, maturation, and changes in antioxidant enzyme activities. Pediatr Res. 1981 Jul;15(7):999–1008. doi: 10.1203/00006450-198107000-00005. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Dionisi O., Galeotti T., Terranova T., Azzi A. Superoxide radicals and hydrogen peroxide formation in mitochondria from normal and neoplastic tissues. Biochim Biophys Acta. 1975 Oct 22;403(2):292–300. doi: 10.1016/0005-2744(75)90059-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sousa J. M., Michelson A. M. Variation of superoxide dismutases during the development of the fruit fly Ceratitis capitata L. Biochem Biophys Res Commun. 1976 Nov 22;73(2):217–223. doi: 10.1016/0006-291x(76)90696-3. [DOI] [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Frank L., Groseclose E. E. Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res. 1984 Mar;18(3):240–244. doi: 10.1203/00006450-198403000-00004. [DOI] [PubMed] [Google Scholar]
- Gerdin E., Tydén O., Eriksson U. J. The development of antioxidant enzymatic defense in the perinatal rat lung: activities of superoxide dismutase, glutathione peroxidase, and catalase. Pediatr Res. 1985 Jul;19(7):687–691. doi: 10.1203/00006450-198507000-00010. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Rolph T. P. Metabolism during fetal life: a functional assessment of metabolic development. Physiol Rev. 1985 Apr;65(2):357–430. doi: 10.1152/physrev.1985.65.2.357. [DOI] [PubMed] [Google Scholar]
- Lott T., Gorman S., Clark J. Superoxide dismutase in Didymium iridis: characterization and changes in activity during senescence and sporulation. Mech Ageing Dev. 1981 Oct;17(2):119–130. doi: 10.1016/0047-6374(81)90078-6. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Aiello V. R., Williams T. R. Changes i superoxide dismutase activity and copper during development and ageing in the fruit fly Drosophila Melanogaster. Mech Ageing Dev. 1980 Mar;12(3):279–286. doi: 10.1016/0047-6374(80)90051-2. [DOI] [PubMed] [Google Scholar]
- Mavelli I., Rigo A., Federico R., Ciriolo M. R., Rotilio G. Superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Biochem J. 1982 May 15;204(2):535–540. doi: 10.1042/bj2040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mir-Madjlessi S. H., Taylor J. S., Farmer R. G. Clinical course and evolution of erythema nodosum and pyoderma gangrenosum in chronic ulcerative colitis: a study of 42 patients. Am J Gastroenterol. 1985 Aug;80(8):615–620. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Superoxide dismutase: a photochemical augmentation assay. Arch Biochem Biophys. 1977 May;181(1):308–312. doi: 10.1016/0003-9861(77)90509-4. [DOI] [PubMed] [Google Scholar]
- Nations C., Allen R. G., Balin A. K., Reimer R. J., Sohal R. S. Superoxide dismutase activity and glutathione concentration during the calcium-induced differentiation of Physarum polycephalum microplasmodia. J Cell Physiol. 1987 Oct;133(1):181–186. doi: 10.1002/jcp.1041330124. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Oberley T. D., Buettner G. R. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Med Hypotheses. 1980 Mar;6(3):249–268. doi: 10.1016/0306-9877(80)90123-1. [DOI] [PubMed] [Google Scholar]
- Oberley L. W., Oberley T. D. The role of superoxide dismutase and gene amplification in carcinogenesis. J Theor Biol. 1984 Feb 7;106(3):403–422. doi: 10.1016/0022-5193(84)90038-9. [DOI] [PubMed] [Google Scholar]
- Russanov E. M., Kirkova M. D., Setchenska M. S., Arnstein H. R. Enzymes of oxygen metabolism during erythrocyte differentiation. Biosci Rep. 1981 Dec;1(12):927–931. doi: 10.1007/BF01114962. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Allen R. G., Nations C. Oxygen free radicals play a role in cellular differentiation: an hypothesis. J Free Radic Biol Med. 1986;2(3):175–181. doi: 10.1016/s0748-5514(86)80067-8. [DOI] [PubMed] [Google Scholar]
- Tanswell A. K., Freeman B. A. Pulmonary antioxidant enzyme maturation in the fetal and neonatal rat. II. The influence of maternal iron supplements upon fetal lung catalase activity. Pediatr Res. 1984 Sep;18(9):871–874. doi: 10.1203/00006450-198409000-00013. [DOI] [PubMed] [Google Scholar]
- Van Balgooy J. N., Roberts E. Superoxide dismutase in normal and malignant tissues in different species. Comp Biochem Physiol B. 1979;62(3):263–268. doi: 10.1016/0305-0491(79)90211-6. [DOI] [PubMed] [Google Scholar]
- Van Hien P., Kovács K., Matkovics B. Properties of enzymes. I. Study of superoxide dismutase activity change in human placenta of different ages. Enzyme. 1974;18(6):341–347. [PubMed] [Google Scholar]
- Warshaw J. B., Wilson C. W., 3rd, Saito K., Prough R. A. The responses of glutathione and antioxidant enzymes to hyperoxia in developing lung. Pediatr Res. 1985 Aug;19(8):819–823. doi: 10.1203/00006450-198508000-00008. [DOI] [PubMed] [Google Scholar]
- Yam J., Frank L., Roberts R. J. Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. Pediatr Res. 1978 Feb;12(2):115–119. doi: 10.1203/00006450-197802000-00010. [DOI] [PubMed] [Google Scholar]
- Yamanaka N., Deamer D. Superoxide dismutase activity in WI-38 cell cultures: effects of age, trypsinization and SV-40 transformation. Physiol Chem Phys. 1974;6(2):95–106. [PubMed] [Google Scholar]



