Abstract
Understanding the functional role of the left lateral parietal cortex in episodic retrieval requires characterization of both spatial and temporal features of activity during memory tasks. In a recent study using magnetoencephalography (MEG), we described an early parietal response in a cued-recall task. This response began within 100 milliseconds of the retrieval cue and lasted less than 400 milliseconds. Spatially, the effect reached significance in all three anatomically defined left lateral parietal subregions included in the study. Here we present a multimodal analysis of both hemodynamic and electrophysiologic responses in the same cued-recall paradigm. Functional MRI (fMRI) was used to more precisely reveal the portion of the parietal cortex with the greatest response. The MEG data set was then reanalyzed to show the early MEG time course of the region identified by fMRI. We found that the hemodynamic response is greatest within the intraparietal sulcus. Further, the MEG pattern in this region shows a strong response during the first 300 milliseconds following the cue to retrieve. Finally, when individual-dipole MEG activity is analyzed for the left cortical surface over the early 300-millisecond time window, significant recall-related activity is limited to a relatively small portion of the left hemisphere that overlaps the region identified by fMRI in the intraparietal sulcus.
Keywords: parietal, memory, retrieval, recall, fMRI, MEG
1. Introduction
Recent efforts to assign a functional role for the prominent activations in left lateral parietal cortex during episodic retrieval tasks have produced competing hypotheses. One hypothesis holds that retrieved information is stored in an “episodic buffer” supported by the left parietal cortex (Baddeley, 2000; Vilberg and Rugg, 2008a; Wagner et al., 2005). Another hypothesis states that left parietal cortex participates in directing attention internally to memory search (Cabeza, 2008; Ciaramelli et al., 2008). Others have proposed that parietal cortex does not directly participate in retrieval and instead reflects the subjective experience of recollection (Ally et al., 2008).
The relatively high spatial resolution of functional magnetic resonance imaging (fMRI) has provided evidence for a further functional dissociation between left hemisphere dorsal parietal and ventral parietal cortex. In particular, ventral parietal activity has been associated with the episodic buffer. Some have questioned dorsal parietal involvement in retrieval, suggesting it may only reflect “processes downstream of retrieval” (Vilberg and Rugg, 2008a, 2008b). Under the attention to memory hypothesis, however, ventral parietal activity arises from attentional capture by retrieved information in an automatic, bottom-up process, and dorsal parietal activity supports goal-driven, top-down direction of attention to retrieval (Cabeza, 2008; Ciaramelli et al., 2008).
We recently proposed that these functional hypotheses could be distinguished by the timing of the parietal response (Seibert et al., 2010). Episodic buffer, subjective experience of recollection, and bottom-up attention all require that at least some information has already been retrieved. Top-down attention to memory search, on the other hand, must begin prior to retrieval, and is consistent with an early parietal response. Using magnetoencephalography (MEG) in a cued-recall task, we observed a response in left posterior parietal cortex that began within 100 milliseconds (ms) of the cue and resolved in less than 400 ms. This early and transient activity increase is most consistent with an attentional role. However, the pattern of activity in the three anatomically-defined subregions probed in the study was fairly similar and did not show a dissociation of dorsal and ventral parietal cortices.
Both location and timing are required to characterize parietal activity in retrieval paradigms and improve understanding of its function. Dissociable spatial patterns within the parietal cortex have been observed with fMRI, but the hemodynamic response offers very limited information on timing. Conversely, our MEG results have revealed an early parietal response, but no clear dissociation was observed between the superior and inferior anatomical subregions probed in the study. While fMRI and MEG may measure different aspects of brain activity, both modalities provide important functional insights. The advantage of a multimodal approach is the opportunity to leverage both the spatial resolution of fMRI and the temporal resolution of MEG to investigate retrieval activity in the same region of parietal cortex.
In this manuscript, we present results from a combined analysis of a previously unpublished fMRI data set and our MEG data. We acquired BOLD functional data from subjects performing the same paradigm used in our previous MEG study (Seibert et al., 2010). We expected the hemodynamic response would reveal one or more significant activations within the left lateral posterior parietal cortex. Those regions could then be used as masks for our MEG data to give insight into the temporal dynamics of neural activity in the functional regions of interest (ROI). We hypothesized that this multimodal analysis would confirm MEG findings of recall-associated activity in dorsal and ventral parietal subregions, while painting a more precise picture of the spatiotemporal dynamics of the left lateral parietal response in episodic retrieval.
2. Material and Methods
2.1 Participants
Sixteen healthy, right-handed adults participated in this study. Twelve subjects (mean age: 23.8 ± 3 years; five male) participated in the fMRI study, and eleven subjects (mean age: 23.7 ± 3 years; six male) participated in the MEG study. Seven subjects participated in both the fMRI and MEG studies; of these, four had fMRI first. These studies were approved by the institutional review board of the University of California, San Diego. Subjects gave informed consent prior to the experiment and received $40 for their participation.
2.2 Stimuli
Stimuli were 256 color drawings of common objects selected from Rossion and Pourtois color Snodgrass images (Rossion and Pourtois, 2004). Drawings were paired randomly into 128 pairs. Pairs were screened to remove those with obvious visual or semantic relationships.
2.3 Task
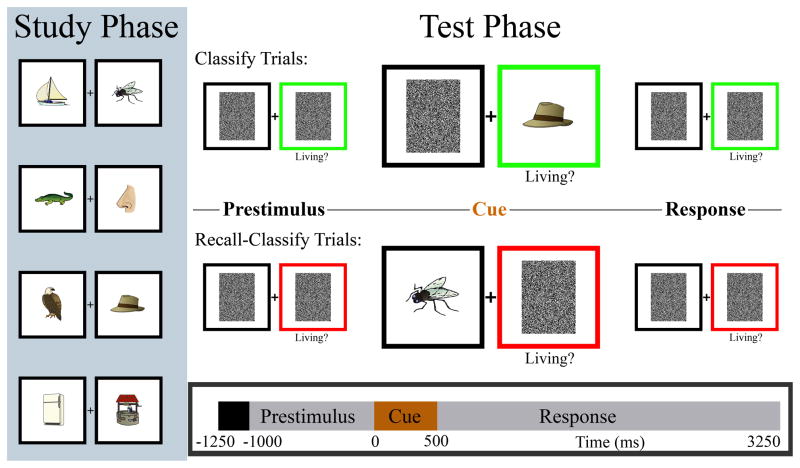
The behavioral task for fMRI sessions was identical to that previously described for MEG sessions (Seibert et al., 2010). Subjects were tested on 128 pairs of drawings of common objects and animals (which they had studied approximately 45 minutes prior to the experiment) while activity was recorded using either fMRI or MEG. In all test phase trials, a single drawing from one of the studied pairs was presented for 500 ms in one of two boxes (Figure 1), followed by an additional 2750-ms response period. During “classify” trials, subjects simply indicated by a finger response whether the presented stimulus was a living or non-living object. During “recall-classify” trials, subjects indicated whether the absent associate of the presented stimulus was a living or non-living object, requiring recall of the paired associate. A colored box, present from 1000 ms prior to stimulus onset, designated the trial type—green for classify and red for recall-classify. A fixation cross presented between two black boxes was shown for the first 250 ms of each trial. Subjects were instructed to respond as quickly and accurately as possible.
Figure 1.
Pair-cued recall task. Subjects viewed each pair for 3 seconds during the study phase (repeated in random order three times). MEG or fMRI recordings were acquired during the test phase (timeline on bottom of figure). In classify trials subjects made a simple living/nonliving judgment on the presented item. In recall-classify trials subjects retrieved the absent associate and then made a living/nonliving judgment on the item in memory. In both conditions the test item was equally likely to appear on the left and right sides. A fixation cross and two black boxes were presented during the initial 250 ms of the trial. The cue period is enlarged only for display in the figure. Reproduced with permission from Seibert et al., 2010.
The test phase comprised 256 trials, presented in eight runs of 32 trials each. Order of presentation of stimulus pairs was pseudorandomized to create ‘trial list A’ and ‘trial list B,’ each containing all pairs. The item presented from each pair, the side of the screen it was presented on, and the condition associated with each pair were all pseudorandomly determined separately for each trial list. All pairs were the same in each list. In the MEG experiment, five subjects were given trial list A and six subjects were given trial list B. In the fMRI experiment, six subjects were given trial list A, and six subjects were given trial list B. Of the seven subjects who participated in both the MEG and fMRI experiments, four were given trial list A first, and three were given trial list B first. The average interval between sessions for these subjects was 7.9 days (range: 5–11 days). During fMRI acquisition, stimuli were presented on a screen visible to the subject via a mirror, and subjects indicated their responses using an MRI compatible button box held in their right hand.
2.4 MRI Acquisition
Imaging was done with a 3T GE scanner at the Center for Functional MRI at the University of California, San Diego. Functional images were acquired using a gradient echo echo-planar, T2*-weighted pulse sequence (repetition time = 1.5 s, one shot per repetition, echo time = 30 ms, flip angle = 90°, bandwidth = 31.25 MHz). Twenty-two slices covering the entire brain were acquired perpendicular to the long axis of the hippocampus with 3.4 × 3.4 × 7 mm voxels. A T1-weighted high-resolution (1 × 1 × 1 mm), three-dimensional magnetization-prepared rapid gradient echo or fast spoiled gradient recalled anatomical dataset was also collected for each subject. An additional T1-weighted structural scan was acquired in the same slice locations as the functional images for use in confirming alignment of functional data to the high-resolution anatomical scan.
2.5 fMRI Analysis
An event-related design was used to examine parietal activity during the recall-classify and classify conditions. These were contrasted with an even-odd digit classification active baseline. Error trials (unsure, incorrect, and no-response) were excluded from the analysis. Trials were jittered with 0, 3, 6, or 9 seconds of baseline to optimize the study design (Dale, 1999).
Data from each run were reconstructed. Slices were temporally aligned and co-registered with a 3D registration algorithm. Voxels outside the brain were removed using a threshold mask of the functional data. A general linear model was constructed using multiple regression analysis, and included six motion regressors from the registration process and regressors for recall-classify and classify condition correct and incorrect responses using the AFNI suite of software (Cox, 1996). For the recall-classify and classify conditions, a hemodynamic response was estimated, within each voxel, for the 15 seconds following trial onset using signal deconvolution.
Derived hemodynamic response time series for each condition were projected to a model of each subject’s cortical surface (Dale et al., 1999; Fischl, Sereno, and Dale, 1999) to facilitate comparison to the MEG analysis, which used surface meshes as the source space for dipole locations. The FreeSurfer software package (version 4.5.0, http://surfer.nmr.mgh.harvard.edu) was used to create a surface mesh for each hemisphere consisting of approximately 160,000 vertices per hemisphere. Anatomical regions of interest were identified through an automated parcellation of the individual surface using the Desikan-Killiany atlas (Desikan et al., 2006; Fischl et al., 2004). Three regions (corresponding to those used in the previous MEG study) were taken from the atlas as ROIs for fMRI analysis: left superior parietal, left inferior parietal, and left supramarginal.
BOLD activity was measured by averaging the expected peak parameter estimates from the hemodynamic response of each condition (corresponding to volumes acquired at 4.75, 6.25, 7.75, and 9.25 seconds following cue onset). Activity differences between conditions were assessed at the ROI and vertex levels. ROI differences were evaluated after averaging BOLD activity from all vertices within the region in each subject’s native space and performing a two-tailed t-test across subjects for recall-classify vs. classify conditions. Vertex-wise analysis (for group-level maps) was performed after registering individual surfaces to a seventh-order icosahedron representation of the FreeSurfer average subject (Fischl et al., 1999). To account for variation in anatomy in the vertex-wise analysis, a conservative iterative smoothing process was applied to each subject’s data before registering to the average surface, as well as to the group-level data after averaging across subjects (iterative smoothing was equivalent to a 6 mm full-width, half-maximum Gaussian kernel).
In addition to the atlas regions, a functional ROI within the left lateral parietal cortex was defined from a t-statistic map of BOLD activity differences between recall-classify and classify conditions. The region was defined as the cluster of supra-threshold vertices adjacent to the peak activity difference within the left lateral posterior parietal cortex, where the threshold was set by controlling the false discovery rate for the entire left hemisphere surface at 0.05 (Genovese et al., 2002). For display of the map, color thresholds were set for t-statistics corresponding to controlling the false discovery rate at 0.05 (minimum) and 0.01 (maximum).
2.6 MEG Analysis
MEG data acquisition, activity estimation using dynamic statistical parametric mapping (Dale et al., 2000), and analysis were all described previously (Seibert et al., 2010). Additionally, the functional ROI defined from the fMRI group map was resampled to a lower-resolution surface mesh used for MEG analysis (approximately 2,500 dipoles per hemisphere). Estimated individual subject MEG data from dipoles within the functional ROI were combined to create an average ROI time series for each condition. As in the previously published analysis, statistical significance of the activity difference between the two conditions was evaluated by paired, two-tailed t-tests across subjects for each of eight 100 ms subperiods (i.e., 100–190 ms, 200–290 ms, etc.), extending from 300 ms prior to cue onset to 500 ms after cue onset. Significance was assessed at the p < 0.01 level, after applying a Bonferroni correction for the eight comparisons.
A t-statistic map of MEG data was calculated for comparison with fMRI results and to visualize the spatial extent of significant MEG activity using a vertex-wise approach. Estimated activity for each condition was averaged across the time periods of significant MEG activity difference in the functional ROI. For each dipole (i.e., each vertex on the low-resolution surface), a t-test across subjects was performed to compare the average recall-classify and classify activity. This group map was then resampled to the high-resolution surface and smoothed (iterative smoothing equivalent to 6 mm full-width, half-maximum Gaussian kernel) for display consistent with the fMRI map. Color thresholds were set for t statistics corresponding to controlling the false discovery rate for the left hemisphere surface at 0.05 (minimum) and 0.01 (maximum).
3. Results
3.1 fMRI Behavioral Results
Mean reaction times (± standard error) from the fMRI experiment were 1743 ± 59 ms for recall-classify, and 1191 ± 46 ms for classify trials, representing a significant difference (p < 0.001, two-tailed t-test), similar to previous studies with this task (Israel et al., 2010; Seibert et al., 2010).
A subject response was recorded within the specified response period in 94% of trials. Of these trials, subjects responded correctly in 97 ± 1% of classify trials and 90 ± 4% of recall-classify trials (mean ± standard error). Only trials with correct responses were included in signal deconvolution and comparisons of activity.
For subjects who participated in both MEG and fMRI sessions there was no significant difference in accuracy in either condition between the first and second sessions (paired t-tests, p = 0.58 and p = 0.97 for recall-classify and classify conditions, respectively).
3.2 Atlas ROI Analysis
MEG results from three left lateral parietal regions of the Desikan-Killiany atlas (Desikan et al., 2006) were published previously. Recall-classify activity was greater than classify activity in each of the three regions for 100 ms subperiods immediately following cue onset, with the superior parietal region showing the greatest effect. Analysis of the BOLD data shows recall-classify activity was greater than classify activity in the left superior parietal (t = 6.1, p < 10−5) and left inferior parietal regions (t = 7.7, p < 10−9), but not left supramarginal (t = −1.4, p = 0.18). Supplementary Figures 1 and 2 show the MEG and fMRI time courses.
3.3 fMRI Vertex Analysis
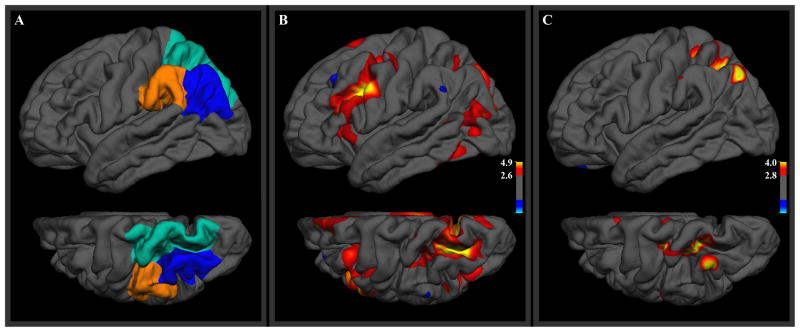
Lateral parietal BOLD activity differences are displayed as t-statistics for each vertex in Figure 2B, alongside the three parietal atlas ROIs (Figure 2A). A prominent region of greater activity for the recall-classify condition was found in the intraparietal sulcus, straddling the entire boundary between the superior and inferior parietal atlas ROIs. A smaller region of greater recall-classify activity was also observed in the most inferior portion of the inferior parietal atlas ROI, though the peak of this cluster was in lateral occipital cortex. t-statistic values for vertices outside the parietal cortex are included in the display for context but such activations outside the a priori ROIs are not discussed further.
Figure 2.
(A) Three anatomical ROIs in the left lateral posterior parietal cortex. (B) Group-level t-statistics for greater BOLD response in the recall-classify condition than in the classify condition. (C) Analogous t-statistics for MEG response, averaged over the period from 0–300 ms after onset of the retrieval cue. The lower image in each frame is a superior view of the left hemisphere, rotated 30 degrees to show the intraparietal sulcus. Thresholds were set for t-statistics corresponding to controlling the false discovery rate over the left hemisphere at 0.05 (minimum) and 0.01 (maximum).
3.4 Functional ROI Analysis
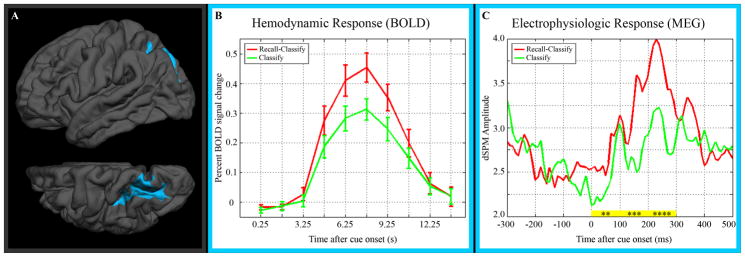
A functional region of interest was defined on the FreeSurfer average cortical surface for the peak cluster of greater recall-classify activity within the left parietal cortex (Figure 3A). The full, derived hemodynamic response for the functional ROI is shown in Figure 3B.
Figure 3.
(A) Functional ROI defined from t-statistic map for greater BOLD activity in the recall-classify than in the classify condition. The lower image is a superior view of the left hemisphere, rotated 30 degrees to show the intraparietal sulcus. (B) Derived hemodynamic response functions (and standard error) for both conditions within the functional ROI. (C) Estimated MEG activity time series. “dSPM” refers to dynamic statistical parametric mapping, the MEG source localization method used. Recall-classify MEG activity was significantly greater than classify activity for the 100 ms time periods indicated in yellow (**: p < 0.001, ***: p < 10−5, ****: p < 10−7).
When this region was applied to the MEG data set, average time series for recall-classify and classify conditions appeared to diverge just prior to cue onset, with a peak difference at ~150 ms (Figure 3C). A temporary convergence is seen at ~100 ms, and the two conditions display similar levels of activity by ~400 ms after stimulus onset. Testing for significant activity difference in 100 ms subperiods revealed significantly greater recall-classify activity over 0–100 ms (t = 4.4, p < 10−3), 100–200 ms (t = 5.3, p < 10−5), and 200–300 ms (t = 6.3, p < 10−7). All MEG p-values are corrected for multiple comparisons using the Bonferroni method for eight comparisons. An exploratory analysis of the early prestimulus period (from 1000 ms prior to cue onset to 300 ms prior to cue onset) did not suggest a MEG activity difference prior to our time period of interest.
3.5 MEG Vertex Analysis
MEG activity time series at each vertex were averaged over 0–300 ms, corresponding to the period of significant MEG activity difference in the functional ROI. Significant vertex-wise MEG activity differences in the lateral parietal cortex over this time window are shown in Figure 2C, with t-statistics displayed for each vertex. The spatial extent of greater recall-classify activity in this analysis is relatively limited to the intraparietal sulcus and neighboring cortex.
4. Discussion
Taking advantage of the complementary strengths of fMRI and MEG, we have described a parietal response to episodic retrieval that is centered on the intraparietal sulcus and has an early, transient time course. Vertex-wise analysis of fMRI data over the left lateral parietal cortex localized the peak region of greater recall-classify activity to the border between the superior and inferior anatomical atlas ROIs, in the intraparietal sulcus. The region identified by localization of the hemodynamic response was then used to probe the temporal dynamics of the electrophysiologic response, which exhibited greater recall-classify activity during the first 300 ms following the cue to retrieve. Vertex-wise analysis of MEG data suggested the retrieval-related electrophysiologic response over this early period was also concentrated in and near the intraparietal sulcus.
The location of the retrieval response described here supports a functional role in episodic retrieval for the intraparietal sulcus, a region that has been highlighted as part of the dorsal parietal cortex in dissociations of parietal activity in retrieval (Cabeza et al., 2008; Ciaramelli et al., 2008; Vilberg and Rugg, 2008a). The chief argument against an episodic retrieval role is based on a consistent observation from fMRI studies using remember/know recognition paradigms that the intraparietal sulcus is associated more with familiarity than with recollection (Ciaramelli et al., 2008; Vilberg and Rugg, 2008a, 2008b). Under one view, these findings are evidence that the intraparietal sulcus does not participate in episodic retrieval, but instead contributes to downstream processes (Vilberg and Rugg, 2008a). Under another view, however, it is argued that the intraparietal sulcus is more active for familiar items because these require greater top-down attention to memory search than recollected items (Cabeza et al., 2008; Ciaramelli et al., 2008). If the subject recognizes a test item in the remember/know paradigm as previously studied (i.e., familiar), but some detail from the study event does not immediately come to mind, the subject should make an effort to retrieve such a memory. In this way, familiar items themselves represent a cue to engage top-down attention to episodic memory search in the remember/know task. Among the advantages of the cued-recall paradigm used in this study are that stimuli in both conditions are equally familiar and that the conditions differ by the presence or absence of an explicit top-down retrieval cue. Thus, the recall-related response during this paradigm, localized to the intraparietal sulcus with both fMRI and MEG, is difficult to reconcile with the suggestion that dorsal parietal involvement in retrieval is limited to familiarity.
The time course of the MEG activity difference also does not support several of the functional hypotheses associated with the left lateral parietal cortex. In particular, the early onset and offset of increased recall-classify activity is not consistent with processing downstream of retrieval. Other functions attributed to left parietal lobe activity similarly imply post-retrieval activity. Some retrieved information is necessary before the parietal cortex could participate in the subjective experience of recollection or in the buffering of that episodic information, but the MEG activity difference in the time courses in Figure 3C is clearly observed during the first 50 ms after onset of the stimulus—before completion of basic visual processing. There remains a possibility that processes associated with successful retrieval might partially contribute to the later portions of the MEG response. If, however, the intraparietal sulcus is either buffering retrieved information or utilizing retrieved information for some process occurring downstream of retrieval, the MEG response might be expected to extend closer to the behavioral response. The mean behavioral response in the retrieval condition comes more than 1400 ms after the period of significant MEG response. The early onset and the transient duration of retrieval-related MEG activity are not readily compatible with hypotheses assigning a post-retrieval role to intraparietal sulcus activity.
Both the timing and location of the present findings are consistent, however, with top-down attention to memory search. The recall-classify condition in our study encourages top-down attention to memory search by requiring retrieval of a specific paired associate and giving the instruction to retrieve prior to presentation of the cue. The intraparietal sulcus may contribute to the direction of internal attention toward episodic memory search or possibly toward a particular target in episodic memory, though the latter is less likely, given the immediate and brief MEG response. Centered in and near the intraparietal sulcus, our findings are largely localized within the dorsal parietal cortex region proposed to be responsible for top-down attention to memory search (Cabeza et al., 2008; Ciaramelli et al., 2008).
A limitation of the present study is the relatively focused time period investigated. The present MEG data set was designed to explore the first 500 ms following cue onset (Seibert et al., 2010) and thus does not preclude the possibility of a later activity difference; however, any such activity difference would likely be distinct from that described here, as there is no significant activity difference in the 300–400 ms or 400–500 ms subperiods.
Another limitation of this study, common to all brain imaging studies comparing two conditions, is that a paired contrast does not provide proof that the process underlying the activity difference is exclusive to only one of the conditions. A graded process may participate in both the recall-classify and classify conditions but still contribute to the activity difference. Ongoing encoding and retrieval are to be expected regardless of experimental task, yet this study controls for incidental memory processes by contrasting the recall condition with a condition that does not require episodic retrieval. Thus, similar underlying processes may contribute to both conditions, but the present findings demonstrate that intraparietal sulcus activity is increased when episodic retrieval is required.
5. Conclusions
Taken together, these findings offer convergent, multimodal evidence for involvement of the intraparietal sulcus in recall and suggest that this region contributes to pre-retrieval processes, such as orienting attention to memory search. The power of integrative analyses in evaluating functional hypotheses is demonstrated by the observation that only one of the several proposed roles for lateral parietal cortex (top-down attention to memory search) is clearly consistent with the parietal response measured in this study. By describing both the location and timing of parietal activity during recall, the present results provide a critical piece of the empirical framework necessary for understanding how the intraparietal sulcus contributes to episodic retrieval.
Research Highlights.
fMRI and MEG show increased left lateral parietal activity with episodic retrieval.
Peak fMRI response localizes to the intraparietal sulcus.
MEG retrieval response in intraparietal sulcus is early and transient (0–300 ms).
Parietal activity in cued recall is consistent with top-down attention to memory.
Supplementary Material
Supplementary Figure 1. (A) Three anatomical ROIs in the left lateral posterior parietal cortex. (B–D) Estimated MEG activity time courses from these regions. “dSPM” refers to dynamic statistical parametric mapping, the MEG source localization method used. 100 ms time periods highlighted with yellow showed significantly greater recall-classify activity than classify activity (*: p < 0.01, **: p < 0.001, ***: p < 10−4, ****: p < 10−7). Adapted with permission from Seibert et al., 2010; ROIs are displayed on the higher-resolution surface used throughout the present study.
Supplementary Figure 2. (A) Three anatomical ROIs in the left lateral posterior parietal cortex. (B–D) Derived hemodynamic response functions from these regions.
Acknowledgments
The authors would like to thank the staff at the Center for Functional MRI and Radiology Imaging Laboratories for their assistance with fMRI and MEG acquisition, respectively. We would also like to thank Sanja Kovacevic, Ksenija Marinkovic, Eric Halgren, Anders Dale, and Christine Smith for suggestions for data analysis.
Abbreviations
- dSPM
dynamic statistical parametric mapping
- BOLD
blood oxygenation level dependent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tyler M. Seibert, Email: tseibert@ucsd.edu.
Sarah I. Gimbel, Email: sisrael@ucsd.edu.
Donald J. Hagler, Jr., Email: dhagler@ucsd.edu.
James B. Brewer, Email: jbrewer@ucsd.edu.
References
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46(7):1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci (Regul Ed) 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia. 2008;46(7):1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46(7):1828–51. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl B, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26(1):55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. NeuroImage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Israel SL, Seibert TM, Black ML, Brewer JB. Going their separate ways: dissociation of hippocampal and dorsolateral prefrontal activation during episodic retrieval and post-retrieval processing. J Cogn Neurosci. 2010;22(3):513–525. doi: 10.1162/jocn.2009.21198. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Seibert TM, Hagler DJ, Brewer JB. Early parietal response in episodic retrieval revealed with MEG. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008a;46(7):1787–99. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Functional significance of retrieval-related activity in lateral parietal cortex: Evidence from fMRI and ERPs. Hum Brain Mapp. 2008b;30(5):1490–1501. doi: 10.1002/hbm.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Three anatomical ROIs in the left lateral posterior parietal cortex. (B–D) Estimated MEG activity time courses from these regions. “dSPM” refers to dynamic statistical parametric mapping, the MEG source localization method used. 100 ms time periods highlighted with yellow showed significantly greater recall-classify activity than classify activity (*: p < 0.01, **: p < 0.001, ***: p < 10−4, ****: p < 10−7). Adapted with permission from Seibert et al., 2010; ROIs are displayed on the higher-resolution surface used throughout the present study.
Supplementary Figure 2. (A) Three anatomical ROIs in the left lateral posterior parietal cortex. (B–D) Derived hemodynamic response functions from these regions.