Abstract
Objective
To compare the association between perinatal events and the pattern and extent of brain injury on early MRI in newborns with and without therapeutic hypothermia for hypoxic-ischemic encephalopathy (HIE).
Study design
We performed a cohort study of 35 treated and 25 non-treated neonates who underwent MRI. The injury patterns were defined a priori as: normal (N), watershed (WS) or basal ganglia/thalamus (BG/T) predominant, as well as a dichotomous outcome of moderate-to-severe versus mild-no injury.
Results
Neonates with hypothermia had less extensive WS and BG/T injuries, and a greater proportion had normal imaging. Therapeutic hypothermia was associated with a decreased risk of both BG/T injury (RR 0.29, 95% CI 0.10-0.81, p = 0.01) and moderate-severe injury. Neonates with sentinel events showed a decrease in BG/T predominant injury and increase in normal imaging. All neonates with decreased fetal movements had injury, predominantly WS, regardless of therapeutic hypothermia.
Conclusion
These results validate reports of reduced brain injury following therapeutic hypothermia, and suggest that perinatal factors are important indicators of response to treatment.
Search terms: Neonatal, MRI, Hypoxia-ischemia, Hypothermia therapy
Hypoxic-ischemic encephalopathy (HIE) occurs in 1-600/1000 live births, and often results in significant morbidity and mortality [1]. Randomized control trials of therapeutic hypothermia for HIE have demonstrated a reduction in death or severe disability, with a number needed to treat of 5-9 neonates; thus, many centers now utilize therapeutic hypothermia for newborns with HIE [2-4]. The severity and pattern of brain injury, as assessed by magnetic resonance imaging (MRI), has been widely studied as a predictor of outcome in infants with HIE [5-11], and is increasingly utilized and recognized as a biomarker for long-term outcome, especially when combined with advanced techniques such as Diffusion Weighted Imaging (DWI) and Proton Magnetic Resonance Spectroscopy (MRS) [12, 13].
Several studies show less severe cortical and deep gray nuclear injury on MRI in neonates who had therapeutic hypothermia [11, 14, 15]. Imaging from a sub-cohort of the TOBY trial showed reduced injury on conventional MRI (T1 and T2 weighted imaging) at a median age of 8 days (range of 2- 30 days) in treated subjects. Therapeutic hypothermia was associated with a reduction of lesions in the basal ganglia or thalamus and, compared with non-cooled infants, treated subjects were more likely to have normal scans. Findings on MRI were predictive of later neuromotor abnormalities [11]. The relationships between antenatal factors and imaging findings following hypothermia were not assessed, although in the pre-hypothermia era perinatal sentinel events were associated with injury to the deep gray nuclei on MRI [16].
It remains to be determined whether perinatal events are associated with MRI findings in neonates treated with therapeutic hypothermia. Therefore, it was our objective to examine the effect of therapeutic hypothermia on patterns of injury as assessed by early MRI (acquired within the first week of life) in a single-center cohort of neonates with HIE and to determine the differential impact of therapeutic hypothermia on MRI findings in neonates with certain perinatal events (decreased fetal movements and sentinel events). We hypothesized that newborns treated with therapeutic hypothermia would have less severe injury, as assessed by early MRI (including conventional and DWI), when compared with a historical cohort of neonates who did not receive therapeutic hypothermia, and that neonates with decreased fetal movements, suggesting prolonged exposure to hypoxia-ischemia, would have a less significant reduction in injury after therapeutic hypothermia compared with those with an acute asphyxial (sentinel) event.
METHODS
Neonates admitted to the Intensive Care Nursery at UCSF were enrolled in an ongoing study to determine whether MRI findings can serve as a predictor of outcome following HIE from 2004 to present.
Our therapeutic hypothermia program started in November 2007. Institutional eligibility criteria for therapeutic hypothermia include the following: 1) Birth at greater than or equal to 36 weeks post-menstrual age, 2) The presence of one or more of the following: an APGAR score of less than five at ten minutes of life, a history of prolonged resuscitation at birth, the presence of severe acidosis defined as a cord pH or any arterial or venous pH of less than 7.0 within 60 minutes of birth, or a base deficit of greater than -12 from cord blood or any arterial blood gas within 60 minutes of life, and 3) The presence of moderate-severe encephalopathy identified by the attending neonatologist or pediatric neurologist. Encephalopathy was defined as abnormal mental status ranging from a hyperalert state to comatose, with associated abnormalities of tone, abnormal neonatal or deep tendon reflexes, the presence of clinical seizures, or an abnormal background pattern or seizures as identified by amplitude integrated electroencephalography.
Per protocol, cooling was initiated within 6 hours of life. For neonates born outside our center, passive cooling was performed during transport from referring institutions. Whole body cooling was achieved with a blanket cooling device (Cincinnati Subzero Blanketrol III), regulated by the infant’s core temperature measured with a rectal probe. Neonates were maintained at 33.5C for 72 hours and monitored with aEEG and continuous video-EEG for the duration of the cooling, and for 24 hours after rewarming. During cooling, all neonates were sedated with morphine administered as a continuous infusion to minimize shivering.
Of the 72 neonates referred to UCSF for evaluation of encephalopathy and possible therapeutic hypothermia, 5 were excluded for late referral (after the first 6 hours of life), and 5 infants were excluded because they did not have moderate encephalopathy, resulting in 62 infants started on therapeutic hypothermia; of these, 46 completed the full 72 hours and 35 were enrolled in our MRI study. Reasons for early termination of hypothermia and non-enrollment are shown in the Figure. Rewarming prior to completion of therapy, and redirection of care to comfort measures was at the discretion of the attending neonatologist and pediatric neurologist.
Figure.
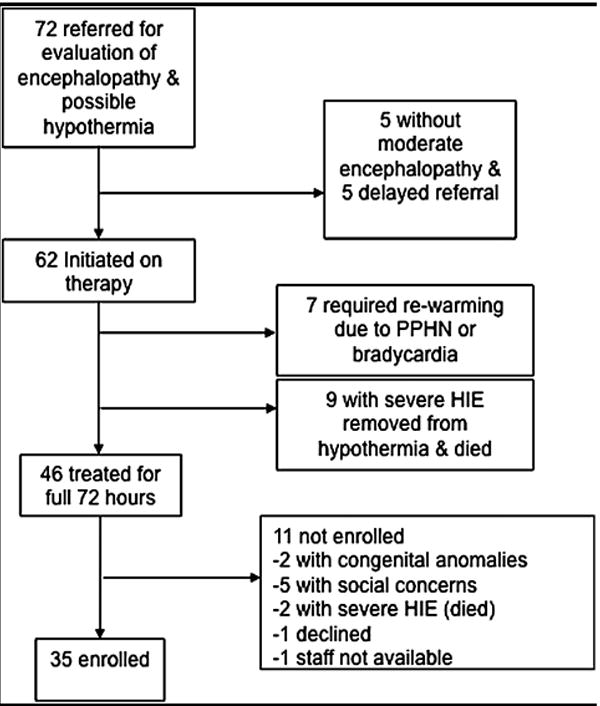
Flow diagram depicting study subject selection
Non-treated neonates were selected from our MRI study cohort based on eligibility for hypothermia using our institution-specific criteria. Between June 2004 and initiation of our therapeutic hypothermia program (November 2007), 20 of 38 infants enrolled in our MRI study also met our hypothermia criteria, and were selected as controls. An additional five infants admitted after 2007 that were ineligible for therapeutic hypothermia based on time of referral (> 6 hours of life) were enrolled and categorized as non-treated infants. The increase in number of neonates eligible for our MRI study after 2007 is related to increased referrals to our center for therapeutic hypothermia. The Committee on Human Research at UCSF approved the research protocol. Parents of eligible neonates were approached and written informed consent was obtained.
Clinical data were collected by trained neonatal research nurses, and included information regarding prenatal, perinatal, and postnatal variables. Variables included sex, gestational age, birthweight, mode of delivery, APGAR score at 1, 5, 10 minutes of life, pH, and base deficit from the umbilical artery or first newborn blood gas within 1 hour of life, a resuscitation score reflecting the amount of resuscitation required at birth: 1 = no intervention, 2 = blow-by oxygen, 3 = endotracheal suctioning, 4 = bag-mask positive pressure ventilation, 5 = endotracheal intubation with positive pressure ventilation, 6 = endotracheal intubation with ventilation and medication, and the need for chest compressions. Perinatal data including maternal report of decreased fetal movements, fetal distress during labor (variable decelerations, late decelerations, fetal bradycardia, fetal tachycardia, lack of fetal heart rate variability), and perinatal sentinel events (placental abruption, uterine rupture, umbilical cord accident, neonatal anemia/hypovolemia) were also collected. An encephalopathy score, assessing mental status, ability to feed, need for respiratory support, tone, reflexes, and the presence of seizures was assigned on day of life 1-3 [17]. A detailed neurologic exam was performed within 24 hour of imaging and a neuromotor score (0-6) was assigned based on abnormalities of tone, reflexes and power and cranial nerve dysfunction [18].
Magnetic Resonance Imaging
Neonates were transported to the MRI scanner in MR compatible incubator, accompanied by a neonatology fellow/attending and a team of trained research nurses. Imaging was performed with a dedicated neonatal head coil, [19]. Scans were obtained at a median of 5 days of life in the hypothermia patients and 4 days of life in the non-treated patients. The difference in the timing of scans was due to several factors including treatment with hypothermia, as scans were not performed during cooling. A series of standard MR sequences were performed for clinical assessment of the neonatal patient on a 1.5T GE scanner (GE Healthcare, Milwaukee, USA) that include 1) T1 weighted sagittal and axial spin-echo images with TR/TE of 500/11, 4mm thickness, 1 excitation, 192×256 encoding matrix; 2) T2 weighted axial dual echo, spin-echo with TR of 3sec, TE of 60 and 120ms, 192×256 encoding matrix, 4mm thickness. DWI was performed using a spin echo EPI diffusion sequence with TE/TR 99/7000ms, FOV 180mm, 128×128, 3mm slice thickness (no skip), b value of 700 s/m2, 6 directions, and 3 averages; some infants had data obtained in 30 directions. The total examination time was approximately 1 hour.
A neuroradiologist blinded to the clinical course reviewed the MRI images. We used a previously validated MR scoring system for acute and subacute signal abnormalities, and the extent of injury in the basal ganglia/thalamus (BG/T) region (scored from 0 to 4) and watershed (WS) region (scored from 0 to 5), (Table I) [5]. Each sequence (T1, T2 and DWI) was assigned a BG/T and WS score. The scores represent the extent of injury observed, and were used to categorize images into two additional outcome measures. 1) Predominant pattern of injury - normal, WS predominant, or BG/T predominant injury. The WS pattern was assigned when the WS region scores were higher than the BG/T scores. The BG/T pattern was assigned when the BG/T scores were higher or as high as the WS region scores. Neonates with total brain injury (maximum BG/T and WS scores) were assigned to the BG/T pattern. 2) A dichotomous outcome of normal-mild injury (normal imaging or WS score of ≤ 2 or BG/T score of ≤ 1) vs. moderate-severe brain injury (WS score of ≥ 3 or BG/T score of ≥ 2) was modeled after a similar classification scheme found to be predictive of outcome in the TOBY trial [11].
Table 1.
MRI Brain Injury Scoring System
| Score | Findings |
|---|---|
| Basal ganglia/Thalamus | |
| 0 | Normal or isolated focal cortical infarct |
| 1 | Abnormal signal in the thalamus |
| 2 | Abnormal signal in the thalamus and lentiform nucleus (LN) |
| 3 | Abnormal signal in the thalamus, LN, and perirolandic cortex |
| 4 | More extensive involvement |
| Watershed | |
| 0 | Normal |
| 1 | Single focal infarction |
| 2 | Abnormal signal in anterior or posterior watershed white matter (WM) |
| 3 | Abnormal signal in anterior or posterior watershed cortex and WM |
| 4 | Abnormal signal in both anterior and posterior watershed zones |
| 5 | More extensive cortical involvement |
Data Analysis
Statistical analysis was performed using Stata software version 9.2 (Stata Corporation, College Station, Texas). Clinical variables were compared using either Chi-square or Fisher exact test for categorical variable, Student T-test for continuous variables, or Wilcoxon rank-sum for non-parametric continuous variables. Wilcoxon rank-sum was used to assess the difference in the extent of injury (comparing the maximal score assigned to the WS and BG/T regions) and the predominant pattern of injury between the treated and non-treated infants. The relative risks of normal imaging and basal ganglia predominant injury in treated infants were calculated. Logistic regression was used to assess the association between therapeutic hypothermia and moderate-severe brain injury. Clinical factors that differed between the two groups (at p < 0.05) and were associated with the outcome (time of MRI scan and initial level of encephalopathy) were included in the regression model. A P value of ≤ 0.05 was considered to be significant.
RESULTS
Non-treated neonates had a higher encephalopathy score on the first day of life compared with those who were treated, 5.5 vs. 4, p=0.0001, and were imaged, on average, one day earlier, day of life 4 (range 2-7) vs. day of life 5 (range 4-9) (Table II). The neuromotor assessment at the time of MRI was similar between the two groups.
Table 2.
Clinical Characteristics
| Control, n=25 | Cooled, n=35 | p-value | |
|---|---|---|---|
| Male Sex | 16 (64%) | 19 (54%) | 0.5 |
| Gestational Age (weeks) | 39.4 (±1.5) | 39.4 (±1.3) | 0.8 |
| Birthweight (gm) | 3428 (±552) | 3346 (±685) | 0.6 |
| Cesarean Section | 17 (68%) | 20 (57%) | 0.4 |
| APGAR 1 min | 1 (0, 3) | 2 (0, 3) | 0.8 |
| APGAR 5 min | 4 (2, 5) | 3 (1, 6) | 0.7 |
| APGAR 10 min | 5.5 (3, 7) | 5 (2, 7) | 0.3 |
| Cord pH | 7.0 (±0.23) | 6.96 (±0.21) | 0.4 |
| Base Deficit | 14 (±7) | 16 (±9) | 0.4 |
| Resuscitation Score | 5 (4, 6) | 5 (4, 6) | 0.9 |
| Cardiac Massage | 7 (28%) | 14 (40%) | 0.3 |
| Inborn | 5 (20%) | 4 (11%) | 0.5 |
| Decreased Fetal Movements | 7 (28%) | 5 (14%) | 0.2 |
| Fetal Distress | 20 (91%) | 25 (78%) | 0.3 |
| Perinatal Sentinel Event | 2 (8%) | 8 (23%) | 0.17 |
| Encephalopathy Score Day 1 | 5.5 (4, 6) | 4 (3, 6) | 0.01 |
| Neonatal Death | 3 (12%) | 2 (6%) | 0.6 |
| Time to Therapeutic Temperature (hrs) | 5.3 (±1.8) | ||
| Day of Life at MRI | 4 (2, 6) | 5 (4, 6) | 0.001 |
| Neuromotor Score at MRI | 2 (1, 4) | 2 (1, 4) | 0.7 |
Data presented as number (%), mean (± std. deviation) or median (p25, p75). P-value for Fisher exact, Student t-test, or rank-sum.
MRI Findings
Overall there was less extensive brain injury in the treated neonates (Table III). In cooled neonates, the extent of injury to deep gray nuclei and WS regions was significantly less, both with a p-value of 0.02.
Table 3.
MRI Findings
| Control, n=25 | Hypothermia, n=35 | p-value | |
|---|---|---|---|
| Extent of Injury to WS Region | |||
| 0 - Normal | 6 (24) | 17 (48) | 0.02* |
| 1 - Single focal infarction | 1 (4) | 5 (14) | |
| 2 - Abnormal signal in anterior or posterior watershed white matter | 3 (12) | 3 (9) | |
| 3 - 2+ WS cortex | 4 (16) | 3 (9) | |
| 4 - Abnormal signal in anterior & posterior WS zones | 5 (20) | 3 (9) | |
| 5 - 4+ more extensive cortical involvement | 6 (24) | 4 (11) | |
| Extent of Injury to BG/T Region | |||
| 0 - Normal or isolated focal cortical infarct | 14 (56) | 28 (80) | 0.02* |
| 1 - Abnormal signal in thalamus | 0 | 2 (6) | |
| 2 - 1+ lentiform nucleus | 2 (8) | 2 (6) | |
| 3 - 2+ perirolandic cortex | 2 (8) | 1 (2) | |
| 4 - 3+ more extensive involvement | 7 (28) | 2 (6) | |
| Predominant Pattern of Injury | |||
| Normal | 4 (16) | 16 (46) | 0.003* |
| Watershed | 11 (44) | 15 (43) | |
| Basal Ganglia/Thalamus | 10 (40) | 4 (11) | |
| Total Brain injury | 6 (24) | 2 (6) | 0.06** |
| Moderate-Severe Brain Injury | 19 (76%) | 12 (34%) | 0.002** |
Data presented as number (%). WS – watershed, BG/T – basal ganglia/thalamus, WM – white matter.
Wilcoxan rank-sum,
Fisher exact test
Treated neonates were more likely to have normal imaging, with a relative risk of 2.86 (95%CI 1.08-7.52, p = 0.02) (Table III). This difference is likely due to the reduction in the frequency of BG/T predominant injury, with a relative risk of BG/T predominant injury of 0.29, 95% CI (0.10-0.81), p = 0.01. There were fewer treated neonates with total brain injury, but this was not significant, p = 0.06.
Similar results were seen using the dichotomous outcome of moderate-severe brain injury. Fewer treated neonates had moderate-severe brain injury with an odds ratio 0.16, 95% CI 0.05-0.52, p=0.002 (Table III). Multivariable logistic regression including terms for severity of encephalopathy on the day of birth, and timing of MRI resulted in an adjusted odds ratio of 0.04, 95% CI 0.004-0.3, p=0.002.
No significant differences were identified in the incidences of perinatal sentinel events or decreased fetal movements between the non-treated and treated neonates, although the numbers were small in both groups (Table II). Among subjects with perinatal sentinel events (n=10), 5 had normal imaging, 2 had WS predominant injury, and 3 had BG/T predominant injury. Only 2 non-treated neonates had an identifiable sentinel event, both with predominant BG/T injury. In those with sentinel events that received therapeutic hypothermia, 5 (62.5%) had normal imaging, 2 (25%) had WS predominant imaging, and 1(12.5%) had BG/T predominant injury. Therapeutic hypothermia was associated with decreased risk of BG/T predominant injury, RR 0.13, 95% CI 0.02 – 0.78, p=0.02. Injury was considered moderate-severe in 2 (100%) non-treated and 3 (37.5%) of 8 treated neonates, although this difference was not significant, p=0.4.
All neonates with a history of decreased fetal movements (n=12) had brain injury, 10 (83%) with WS predominant injury and 2 (17%) with BG/T predominant injury. WS predominant injury was the most common pattern of injury in both treated (80%) and non-treated neonates (86%) with decreased fetal movement (p =1). The injury was considered moderate-severe in fewer of the cooled neonates with decreased fetal movements (3/5 – 60%, vs. 5/7 – 71%), though the difference was not significant, p=1.
DISCUSSION
Using conventional MRI with DWI early after cooling, we found that neonates treated with therapeutic hypothermia have less extensive injury and a have a higher rate of normal MRI when compared with a comparable historical cohort, and that there is a differential effect of therapeutic hypothermia in babies who present with perinatal sentinel events as compared with those with a history of decreased fetal movements. Additionally, therapeutic hypothermia was protective for moderate-severe brain injury, even after accounting for timing of imaging and initial severity of encephalopathy.
The observation that the effect of therapeutic hypothermia appears different in the setting of a perinatal sentinel event versus decreased fetal movements is a critical new finding. Neonates with acute perinatal sentinel events who received therapeutic hypothermia showed a clear reduction in BG/T predominant injury, with more infants having normal imaging. There was a trend of decreased moderate-severe injury, although this difference was not significant, likely related to the small numbers of infants with sentinel events. The effect of therapeutic hypothermia was less clear in those whose mothers reported decreased fetal movements. All neonates with decreased fetal movements had some degree of injury, despite hypothermia, with persistence of watershed predominant injury. In this group, a trend of decreased incidence of moderate-severe injury was found, but this was not significant. These findings suggest that hypothermia may be less beneficial when there has been hypoxia-ischemia remote from the time of birth.
These results support the hypothesis that hypothermia lessens secondary energy failure after an acute ischemic event, and that it may not be as effective in situations where chronic hypoxia or ischemia contributes to the insult. Previous human and animal studies have shown selective regional benefit of hypothermia [14, 15, 20]. Some postulate that the cortex and the deep gray nuclei are highly metabolically active and, therefore, hypothermia may preferentially favor these regions [1]. Other potential reasons for selective benefit include differences in the pathogenesis of injury to the deep gray nuclei vs. watershed/parasagittal injury. Injury to the deep gray nuclei typically occurs after an acute profound asphyxial event such as an umbilical cord emergency, severe fetal bradycardia, or uterine rupture, all catastrophic (and usually recognized) events [16, 21]. Recognition of these events leads to urgent delivery, prompt resuscitation and evaluation, and initiation of hypothermia therapy; this rapid response may lead to more effective neuroprotection and, therefore, less severe/extensive injury to the deep gray nuclei. In contrast, injury to the watershed (parasagittal) regions is thought be associated with more prolonged but less severe hypoxia/reduction in blood flow (prolonged partial asphyxia) or repeated episodes of partial (relatively low grade) ischemia, which may be more difficult to recognized; thus, delivery of the neonate and initiation of therapeutic hypothermia may be delayed [22]. In our cohort, therapeutic hypothermia seemed to decrease the extent of injury in watershed regions, with fewer infants having injury extending to the watershed cortex and fewer having involvement in both anterior and posterior regions; perhaps this indicates an acute component of injury (responsive to therapeutic hypothermia) superimposed on the (non-responsive) chronic injury from previous ischemic episodes.
Our results showing reduced overall burden of injury in the setting of therapeutic hypothermia support the recent findings from the TOBY trial, where imaging was obtained later, at a median of 8 days, with a range of 2-30 days. An important finding of the TOBY study was that injury on MRI was predictive of outcome at 18 age months of age. Our data, coupled with the strength of the RCT trial, suggest that utilizing MRI in the first week of life may provide important prognostic information for use as a biomarker in future trials and to enable conversations regarding goals of care and prognosis. DWI can facilitated earlier identification of injury, as T1 and T2 weighted findings may take longer to develop [23-25]. Further study utilizing advanced imaging techniques with early serial imaging may help to determine the best timing for imaging in this population [13, 26].
There are several limitations to our study. As this is a cohort study of newborns enrolled in an ongoing longitudinal study of MRI predictors of outcome in HIE, there is potential for the selection of less severe cases, as one of the primary goals of the study is to determine predictors of long-term neurodevelopmental outcome. However, newborns with severe HIE are not commonly enrolled in our study as they are likely to undergo redirection of care and die. This practice has not changed over the course of our study. Any potential difference would be non-differential with regard to cooled and non-cooled subjects, as our groups are comparable based on the severity of acidosis, the clinical presentation (APGAR score), and amount of resuscitation required at birth. Our groups also differed in the severity of initial encephalopathy and the age at scan, but hypothermia was still protective after adjusting for these differences, and both groups were imaged within the timeframe that would be expected to reveal injury [25]. In clinical practice, the timing of the scan will depend on access, stability of the baby after cooling, and questions needed to be answered from the MRI.
An important limitation is the difference in the proportions of neonates with decreased fetal movement and sentinel events in the two groups. Increasing the sample size may further clarify the effect of therapeutic hypothermia in the setting of various perinatal events.
Acknowledgments
The authors thank the neonatal research nurses of the Pediatric Clinical Research Center and Laurel Haeusslein at UCSF for their work on this study.
Supported by NIH/NCRR UCSF-CTSI (grant UL1 RR024131) and NIH (grant P50 NS035902-12). S.B. was supported by NIH (training grant 5T32HD007162), H.G. is supported by NINDS (1K23NS66137), and J.V. was supported by the APS/SPR Student Research Program. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/ and Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Abbreviations
- HIE
hypoxic-ischemic encephalopathy
- MRI
magnetic resonance imaging
- DWI
diffusion weighted imaging
- WS
watershed
- BG/T
basal ganglia/thalamus
- WBC
whole body cooling
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volpe J. Neurology of the Newborn. 5. Philadelphia: WB Saunders Comapny; 2008. [Google Scholar]
- 2.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007:CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. Bmj. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 5.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Boichot C, Walker PM, Durand C, Grimaldi M, Chapuis S, Gouyon JB, et al. Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology. 2006;239:839–48. doi: 10.1148/radiol.2393050027. [DOI] [PubMed] [Google Scholar]
- 7.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Hunt RW, Neil JJ, Coleman LT, Kean MJ, Inder TE. Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics. 2004;114:999–1003. doi: 10.1542/peds.2003-0935-L. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford MA, Pennock JM, Counsell SJ, Mercuri E, Cowan FM, Dubowitz LM, et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics. 1998;102:323–8. doi: 10.1542/peds.102.2.323. [DOI] [PubMed] [Google Scholar]
- 10.Twomey E, Twomey A, Ryan S, Murphy J, Donoghue VB. MR imaging of term infants with hypoxic-ischaemic encephalopathy as a predictor of neurodevelopmental outcome and late MRI appearances. Pediatr Radiol. 2009 doi: 10.1007/s00247-010-1692-9. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2009 doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzopardi D, David Edwards A. Magnetic resonance biomarkers of neuroprotective effects in infants with hypoxic ischemic encephalopathy. Semin Fetal Neonatal Med. 2010 doi: 10.1016/j.siny.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Thayyil S, Chandrasekaran M, Taylor A, Bainbridge A, Cady EB, Chong WK, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–95. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 14.Inder TE, Hunt RW, Morley CJ, Coleman L, Stewart M, Doyle LW, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145:835–7. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–6. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 16.Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121:906–14. doi: 10.1542/peds.2007-0770. [DOI] [PubMed] [Google Scholar]
- 17.Miller SP, Latal B, Clark H, Barnwell A, Glidden D, Barkovich AJ, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190:93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 18.Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol. 1999;21:788–93. doi: 10.1016/s0887-8994(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 19.Dumoulin CL, Rohling KW, Piel JE, Rossi CJ, Giaquinto RO, Watkins RD, et al. Magnetic resonance imaging compatible neonate incubator. Magn Reson Engineering. 2002;15:117–88. [Google Scholar]
- 20.Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003;53:65–72. doi: 10.1002/ana.10402. [DOI] [PubMed] [Google Scholar]
- 21.Myers RE. Two patterns of perinatal brain damage and their conditions of occurrence. Am J Obstet Gynecol. 1972;112:246–76. doi: 10.1016/0002-9378(72)90124-x. [DOI] [PubMed] [Google Scholar]
- 22.Myers RE. Fetal asphyxia due to umbilical cord compression. Metabolic and brain pathologic consequences. Biol Neonate. 1975;26:21–43. doi: 10.1159/000240714. [DOI] [PubMed] [Google Scholar]
- 23.Rutherford M, Malamateniou C, McGuinness A, Allsop J, Biarge MM, Counsell S. Magnetic resonance imaging in hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010 doi: 10.1016/j.earlhumdev.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Barkovich AJ, Westmark K, Partridge C, Sola A, Ferriero DM. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J Neuroradiol. 1995;16:427–38. [PMC free article] [PubMed] [Google Scholar]
- 25.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27:533–47. [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant. Clin Perinatol. 2009;36:859–80. vii. doi: 10.1016/j.clp.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


