Abstract
In this study we employed a novel technique to examine the neural basis of written spelling by having subjects touch-type single words on an fMRI compatible QWERTY keyboard. Additionally, in the same group of participants we determined if task-related signal changes associated with typed spelling were also co-localized with or separate from those for reading. Of particular interest were the left inferior frontal gyrus, left inferior parietal lobe as well as an area in the left occipitotemporal cortex termed the Visual Word Form Area (VWFA), each of which have been associated with both spelling and reading. Our results revealed that typed spelling was associated with a left hemisphere network of regions which included the inferior frontal gyrus, intraparietal sulcus, inferior temporal/fusiform gyrus, as well as a region in the superior/middle frontal gyrus, near Exner's area. A conjunction analysis of activation associated with spelling and reading revealed a significant overlap in the left inferior frontal gyrus and occipitotemporal cortex. Interestingly, within the occipitotemporal cortex just lateral and superior to the VWFA we identified an area that was selectively associated with spelling, as revealed by a direct comparison of the two tasks. These results demonstrate that typed spelling activates a predominantly left hemisphere network, a subset of which is functionally relevant to both spelling and reading. Further analysis revealed that the left occipitotemporal cortex contains regions with both conjoint and dissociable patterns of activation for spelling and reading.
Keywords: reading, spelling, typing, FMRI, Exner's Area, VWFA
Introduction
The act of writing involves the conversion of ideas to the written word. This method of expression permeates nearly every aspect of modern society and is flexible in the sense that it can be carried out via innumerable output modalities ranging from organizing simple objects into letters and words, to cursive handwriting, or to typing on a keyboard. What are the neural substrates that underlie this pervasive and dynamic cognitive process? Psycholinguistic models have long attempted to discern the various cognitive components of written spelling and thus provide a foundation upon which to explore its neural basis. Written spelling is generally considered it to begin with central processing involving semantics which is followed by the retrieval of lexical and/or sub-lexical representations that are transiently stored in a working memory system called the graphemic buffer. These more central processes are followed by peripheral components involving the generation of modality specific motor plans. For handwriting these motor commands are associated the production of specific allographs (i.e. the various forms that a letter can take such as upper/lower case or cursive/print font) which requires the generation of allographic motor plans that are specific to the letter shape to be formed. Keyboard typing on the other hand involves the generation of motor command that are not directly related to the letter shape but instead involves the pairing of fingers to keys on a standard keyboard. Interestingly, the more central cognitive components of spelling appear to be the similar to those of reading except in the reverse functional order. For instance, whereas spelling-to-dictation first invokes semantic and/or sub-lexical processing of an auditory word form which is then converted to an orthographic word form and then written, reading-out-loud entails an orthographic word form to be recognized, processed semantically and/or sub-lexically, converted to an auditory word from, and then spoken. Although the function of these components may be different for each task, they may rely on common phonological, orthographic, and semantic representations and thus shared neural substrates. These descriptions of the cognitive architecture underlying written spelling provide a theoretical foundation upon which to explore the brain basis of written spelling via neuroimaging techniques.
Although numerous neuropsychological studies have explored the cognitive components and to a limited extent the neurobiological basis of writing, only a few neuroimaging studies have attempted to examine the functional anatomy of written spelling in non-impaired individuals. In recent years some fMRI studies have attempted to answer this question by employing a handwriting task as well as tasks which require participants to either indicate whether a specific letter is present in an auditorily presented word or whether two auditorily presented words are spelled similarly. No study has used keyboard typing to examine the brain basis of written spelling, which is surprising considering its relevance in today's society as a common form of writing. In the proceeding introduction we will explore typing vis-à-vis the known neurobiological basis of writing and discuss how previous work guides predictions for the brain basis of typed spelling.
It has long been known that keyboard typing is an efficient form of written expression that involves the pairing of single key presses to individual letters on a standard keyboard space. By comparison, handwriting requires the formation of individual letters as the word is written. Hence, typing does not require the formation of the specific letter shapes such as upper/lower case or cursive/print motor commands, i.e. allographic processing, whereas handwriting does. In terms of exploring the brain basis of handwritten spelling it therefore becomes critical to account for the act of forming letters. Specifically, studies which have demonstrated that drawing (or merely viewing) single letters, independent of spelling, can activate areas associated with written spelling such as the left premotor and occipitotemporal cortex. Although, previous neuroimaging studies of handwriting have employed appropriate tasks to control for allographic processing, the use of typed spelling excludes the need to control for this processes altogether and therefore is uniquely suited to examine the neural substrates of written spelling in an even more controlled manner. Therefore, exploring the brain basis of typed spelling will not only provide insight into this highly relevant (and to date unexplored) form of spelling, but provides a unique opportunity to examine a written spelling modality that does not require allographic processing.
At the same time, typing and handwriting share many critical cognitive processes. At the more peripheral output processing stage both typing and handwriting require the conversion of graphemes to manual motor commands. The neural representation of this particular process is thought to reside in a region of the left premotor cortex just anterior to the hand primary motor area termed Exner's area. This region has long been associated with the generation of graphemic-motor commands required for normal handwriting. Interestingly, a recent study reported that a lesion in the proximity of this region led to a typing impairment (dystypia) in addition to a transient impairment in handwriting. This suggests that a portion of this area could be associated with a common process involved in both handwriting and typing. For instance it could be associated with graphemic buffer processing, which is shared across handwriting and typing; or it could be associated with the conversion of grapheme-to-motor sequences following to allographic processing. Additionally, the left superior parietal lobe is associated with peripheral component processing as demonstrated by lesions in this area leading to impaired generation of accurate movement sequences during written expression, known as apractic agraphia, as well as impairment in the generation of correct typed motor sequences. The association of this left frontal-parietal network with written spelling is also supported by neuroimaging studies of handwriting, as well as one study involving the production of motor sequences on a QWERTY keyboard. In general, these studies suggest that typed spelling is associated with a left hemisphere frontal-parietal network.
At the more central processing stages of written language it has been suggested that both spelling and reading share processes that involve the retrieval and storage of lexical and sub-lexical representations. Support for this idea comes from the behavioral literature which suggests that reading and spelling abilities are correlated in typically-developing children and that developmental reading impairments tend to be associated with poor spelling. Further, spelling accuracy for words predicts the priming effects on those same words during a lexical decision task, suggesting that reading and spelling may actually share lexical representations (Burt 2002).
Not surprisingly, some of the same regions associated with lexical processing in written spelling have also been associated with reading. In particular, lesions to the left occipitotemporal cortex have been associated with impaired lexical access during written spelling as well as reading. One region in particular in the left occipitotemporal cortex termed the Visual Word Form Area (VWFA) has consistently been identified in neuroimaging studies of reading. Interestingly, activation in or around the VWFA has also been identified in neuroimaging studies that have involved various spelling tasks such as handwritten spelling, a task that required subjects to determine if an auditorily presented word contained a given letter, and a task that required subjects to compare the spelling of two auditorily presented words. Along with the left occipitotemporal cortex, lesions in the posterior portion of the left inferior parietal lobe, including the angular gyrus, have long been associated with deficits in lexical access during both writing and reading. In the area of developmental reading disability, cerebral blood flow level in the left angular gyrus was shown to correlate with reading skill in dyslexic adults. However, neuroimaging studies in normal readers have not found the expected association of the left angular gyrus with reading or handwritten spelling. Taken together, the occipitotemporal cortex has been associated with lexical processing for both reading and spelling whereas this relationship for the posterior parietal cortex exists with a lesser degree of certainty.
Sub-lexical processes that underlie spelling and reading have also been associated with common brain regions. In particular, left perisylvian cortex lesions that include either the supramarginal gyrus (SMG) or inferior frontal gyrus (IFG) have been associated with impaired pseudoword spelling as well as pseudoword reading. It is uncertain, however, the extent to which these lesion-induced impairments are specific to written language as opposed to more general phonological deficits. Neuroimaging studies that have involved a more specific exploration of these sub-lexical processes also support the idea that perisylvian regions including the left SMG and IFG play a significant role phoneme-grapheme mapping in spelling and reading. Overall, both lesion and neuroimaging work suggests that sub-lexical processing would be associated with perisylvian regions including the left SMG and IFG.
The main goal of this study was to use fMRI to examine the brain basis of spelling via keyboard typing. Additionally, we explored the degree to which spelling shares neural resources with reading, as well as the degree to which spelling and reading demonstrate dissociable patterns of activation. This approach allows for a better characterization of the neural substrates that underlie the more central processes associated with spelling as well as reading. In order to accomplish this we employed a novel written spelling task that involved typing auditory words on an MRI compatible QWERTY keyboard. We also conducted a separate reading experiment in order to make direct comparisons between brain activity associated with these two tasks.
Methods
Participants
Seventeen (7 male and 10 female) right-handed, healthy adults (Mean age = 23.2; Range = 18-27 years) participated in the experiment. Right-handedness was determined using the Edinburgh Handedness Inventory. All participants were monolingual English speakers with no history of neurological or learning disorders. Standard neuropsychological tests for reading and spelling were administered in order to ensure that none of the participants had below normal reading or spelling abilities (i.e. each subject had a standard reading score of > 85 on either test). Participants were also required to have normal or corrected-to-normal vision, and be able to type on an American QWERTY keyboard at a word per minute (WPM) rate of at least 50 without looking at their hands. This minimum WPM rate was assessed using a standard typing test program. All participants were recruited from the graduate and undergraduate population at Georgetown University. Experimental procedures were approved by Georgetown University's Institutional Review Board and written informed consent was obtained from all subjects prior to the experiment.
Stimuli
Two word lists of 40 items each were obtained from the CELEX Lexical Database, one for the spelling experiment and the other for the reading experiment. For each list an equal number of 3, 4, 5, and 6 letter nouns were used. None of the words were homophones (e.g. bear/bare). Words from each list were matched on numerous linguistic parameters including word frequency, orthographic neighborhood count, number of syllables, age of acquisition rating and imagability. Word frequency and orthographic neighborhood count were obtained from the MCWord Orthographic Database; age of acquisition and imagability ratings were obtained from the MRC database.
Auditory words for the spelling task were obtained from Linguistic Data Consortium (http://www.ldc.upenn.edu/cgi-bin/aesl/aesl); the words had a mean duration of 550 ms with a duration range between 292 and 745 ms Each audio file was processed in MATLAB (The Mathworks, MA) in order to standardize the root mean square amplitude and addend varied periods of silence to the beginning of each audio file to ensure each audio file was 750 ms in duration. Visual word images for the reading test were developed using MATLAB (The Mathworks, MA) to eliminate background variations and to standardize image size to approximately 2×4° visual angle. Stimuli were presented using Presentation software (Neurobehavioral Systems, Inc. Albany, CA).
fMRI Tasks
Subjects participated in separate spelling and reading fMRI experimental runs conducted in a single session. A summary description of each experiment is presented in Figure 1.
Figure 1.
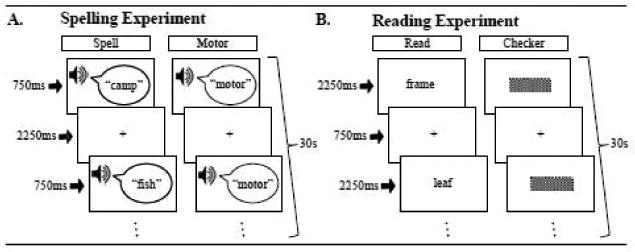
Designs for the spelling and reading experiments. Both experiments used an fMRI block design that involved three different types of conditions presented pseudorandomly in 4 blocks of 30 second duration each. Both experiments had a Fixation condition which involved fixating on a cross in the center of the screen (Fix). A. The spelling experiment involved either hearing a word and then typing it (Spell) or a control condition which involved hearing the word “motor” and then performing a pre-practiced keypress motor sequence (Motor). B. The reading experiment involved reading visual words (Read) or a control condition involving attending to visual checkerboards (Checker).
Spelling Experiment
We presented pseudorandomly ordered 30sec blocks of stimuli which consisted of a spelling task, a motor control task, or a fixation cross resting condition. No block was followed directly by a block of the same type. For the spelling and motor task condition blocks, 10 auditory stimuli were presented for 750 ms with 2250 ms silent intervals between each. A fixation was present throughout the task period. During the spelling condition subjects were instructed to type the different auditorily presented words on an MRI compatible keyboard (Mag Design and Engineering: http://www.magconcept.com). The percentage of keypresses on a QWERTY keyboard required by the right and left hand was calculated for each word (e.g. the word ‘camp’ requires 50% usage from both the right and left hand) and equated to ensure that there was no bias in hand use across the entire list. Specifically, the left hand was required for the spelling experiment word list on average for 50.9% (SD=18.5%) of the keypresses and the right hand was required on average 49.1% (SD=18.5%) of the keypresses. During the motor control task subjects were presented with 10 consecutive auditory presentations of the word “motor” and would type a pre-learned motor sequence (learned just prior to the scan session) that involved 4 alternating finger movements from each hand, e.g. “JFKDLS;A” as typed on the QWERTY keyboard. Subjects were verbally instructed to respond to the auditory stimuli as quickly as possible while minimizing errors and unnecessary movements; subjects were also informed not to correct any mistakes that were made. A fixation cross was constantly present during the fixation blocks. Subjects were instructed to maintain their eyes on the cross during both the fixation and task blocks.
Reading Experiment
This experiment was adapted from a paradigm developed by Cohen et al.. We presented pseudorandomly ordered 30sec blocks of visual words, checkerboards, or a fixation cross resting condition. Blocks were ordered so that no block was followed directly by a block of the same condition. For the word and checkerboard conditions, 10 visual stimuli were presented for 2250 ms, with a 750 ms fixation interval between each. For the word condition, subjects covertly read each word. In order to ensure that participants read each word, they were informed that there would be a short recognition memory task immediately after the scan. For the checkerboard control condition subjects were instructed to attend to all stimuli. Finally, as for the spelling condition above, the fixation cross was constantly present during the fixation blocks. For both experiments, there were a total of 4 blocks per condition.
Immediately after the scan, a recognition memory test was administered to the subject while still in the scanner. An excel spreadsheet with a list of 50 words was presented to the subjects; half of the words were “old” (i.e. presented during the reading experiment”) and half of the words were “new” (i.e. not presented during the reading or spelling experiment). Subjects responded by typing the “y” or “n” key in the cell adjacent to each word.
MRI practice session
Each participant was required to perform a keyboard typing task while lying in a mock MRI scanner in order to ensure that they could perform the task prior to participation in the actual experiment. For this the participant wore headphones and a typical QWERTY keyboard with an extended cord was placed on their waist. An angled mirror placed above their head allowed them to view a computer screen positioned at the rear of the mock scanner. Participants were instructed to keep their hands on the home keys when at rest; home keys were identifiable to the touch by a small tab on the keys corresponding to the index fingers (“j” and “f”). Participants first performed the same typing test performed in an earlier session on a standard computer in order to ensure that typing ability was retained while in a supine position. Subjects then practiced the spelling experiment for the fMRI scan (different stimuli were used in the practice and fMRI sessions).
MRI Acquisition
After screening for metal objects, the participants were securely positioned in a 3.0 Tesla Siemens Trio scanner. Great care was taken to ensure subjects were in a comfortable typing position prior to the start of the scanning session. The MRI compatible keyboard (Mag Design and Engineering, http://www.magconcept.com) was attached to a small plastic tray and positioned on the participants waist; foam padding was placed under the subject's arms to add comfort if requested. Visual stimuli were projected onto a rear screen with a LCD projector and viewed through an angled mirror. Auditory stimuli were heard binaurally through electrostatic MRI-compatible noise-cancellation headphones (STAX, http://www.stax.co.jp). Head movement was minimized by small foam cushions placed on the sides of the participant's head. Two continuous-acquisition functional EPI sequences, one each for the reading and spelling experiments, were acquired using a 12-channel head coil with the following parameters: flip angle = 90°, TR = 2000 ms, TE = 30 ms, FOV = 205 mm, 64 by 64 matrix, 37 axial slices (thickness = 3, no gap; in-plane resolution = 3.2 × 3.2 mm2. Each run had a total of 192 volumes (60 volumes each for the experimental and control conditions and 72 volumes for the fixation rest condition) and scan time of 6min, 24sec. We also acquired 3D T1-weighted MPRAGE images with the following parameters: TR/TE 1600/4.38 ms, FOV=256, 160 axial slices; effective resolution of 1 mm3 with a scan time of 4 min 28 sec.
Behavioral Data Analysis
Behavioral data obtained from the spelling experiment were processed in MATLAB (The Mathworks, MA). Accurate trials were defined as those for which subjects produced the correct key-press sequence for a given word. Since we were primarily interested in spelling errors as opposed to typing errors, - which could involve a slip in a keypress such that the adjacent key is pressed or if the subject's hands were temporarily shifted laterally to the right or left while lying in the scanner - we did not consider keypresses that were either one to the right or left of the correct key to be incorrect (e.g. for the letter ‘s’, responses such as ‘a’ or ‘d’ were considered correct). Reaction time was measured as the time from the end of the stimulus presentation (i.e. 750 ms after the start of the trial) to the start of the first keypress. Response duration was measured as the time from the end of the stimulus presentation to the final keypress for a give word. Inter-key-interval (IKI) was calculated to yield the average response time difference between consecutive keypresses for each trial involving a motor sequence response.
Behavioral data from the post scan word recognition memory test was recorded in analyzed in Microsoft Excel. The proportion of hits and false alarms were used to calculate the sensitivity index or d-prime (d′) for each subject. The d′ is an unbiased estimate of the sensitivity of the new/old comparison and is calculated by comparing the normalized hit rate to that of the normalized false alarm rate. This measure was calculated to confirm that each participant performed at above chance level (i.e. d′ > 0), which would suggest that they did not ignore the visual word stimuli during the reading experiment.
MRI Data Analysis
All preprocessing and statistical analysis of the fMRI data was performed using the software package SPM5. The anatomical MPRAGE was normalized to a standard Neurological Institute (MNI) reference anatomical template brain. After discarding the first 4 functional scans of each EPI acquisition, we corrected for head motion by realignment of each scan to the first image, co-registering the functional scans with the MPRAGE anatomical scan, and then normalizing them via the same warping parameters used to normalize the MPRAGE scan. The images were then resliced to 2×2×2 mm3 and smoothed with an isotropic 6.4 mm Gaussian kernel.
In order to ensure that the fMRI data was not confounded by excessive head motion, we applied criteria for inclusions of functional data based on the motion parameters obtained during the realignment of each functional scan. Entire runs were included only if their overall motion in the vector sum of the x, y, and z direction movement was less than one voxel (3.2 mm). Individual scans were excluded if the vector sum motion in the x, y, and z dimensions exceeded 2 mm compared to the prior scan. No runs or scans had to be discarded.
Generation of task-specific within-groups maps
A whole-brain statistical analysis was performed separately for the reading and spelling experiments in each subject. We first performed a temporal filtering with a high pass filter (128sec) as well as applied an autoregressive (AR 1) model to account for serial correlations. We then modeled the hemodynamic activity for each experimental condition in the reading experiment (reading, checkerboard, and fixation) and the spelling experiment (spelling, motor, and fixation) with the standard hemodynamic response function (HRF). We further obtained a global average signal across every time point and after verifying that there were no correlations of the global signal with the experimental conditions, applied global scaling by adding the global signal into the regression model. In order to account for confounds due stimulus timed movement we also included the 6 motion parameters (roll, pitch, yaw, x, y, z) from this experiment into the regression model for each experiment. Additionally we included reaction time and inter-key-interval behavioral data for the spelling experiment as regressors of no interest. These regressors were calculated by obtaining the average behavioral response over the course of individual spelling and motor blocks (regardless of accuracy) and assigning this average to every timepoint in the corresponding spelling or motor blocks. Adding these regressors controls for at least some of the variability in activation due to variability reaction time and inter-key-interval responses both within and across the spelling and motor conditions.
The contrast images from both the reading and spelling experiments from each subject were then input into a second-level random effects analysis to allow for population level inferences. An uncorrected threshold of p<0.0001 was initially applied for each contrast of interest and only clusters with a minimum of 30 contiguous voxels and that had a cluster-level corrected p<0.05 were reported. Cluster extent threshold was obtained from the CorrClusTh.m program (Thomas Nichols; http://www.sph.umich.edu/∼nichols/JohnsGems5.html) which reports the statistically appropriate size threshold for identifying cluster-level corrected clusters at an alpha level of p<0.05 based on the number of voxels in the data, smoothness of the data, and uncorrected threshold used (i.e. p<0.001). Anatomical localization of the statistical maps was determined by superimposing t-maps on a normalized structural image averaged across all subjects and referencing them to the SPM Anatomy Toolbox Atlas as well as the Anatomical Automatic Labeling atlas developed for MNI space. Visual inspection and activation maps were carried out by way of the SPM viewing program xjview (http://www.alivelearn.net/xjview8/). Clusters peaks are reported in both MNI and Talairach (TAL) coordinates.
Areas of activation associated with spelling were identified by examining the Spell>Motor contrast. In order to exclude clusters in which typed spelling activation was negative in relation to the baseline fixation condition, we applied a mask generated via a Spell>Fix contrast at a low threshold of p<0.05 uncorrected to the activation maps generated in the Spell>Motor contrast. Areas of activation associated with reading were identified by performing a Read>Checker contrast. In order to excluded clusters in which reading activation was negative in relation to the baseline fixation condition, we applied a mask of the Reading>Fix contrast at a low threshold of p<0.05 uncorrected.
Spatial co-localization between spelling and reading
We then identified regions that were significantly active for both the spelling and reading conditions by performing a conjunction analysis using the statistical maps generated in the prior analyses. Specifically, we attempted to identify clusters that were significantly active for both the Spell>Motor and Reading>Checker contrasts. The same masks applied in each of the Spelling and Reading experiments separately were combined into a single map and further applied to the conjunction map in order to ensure that the spelling and reading activation were both greater than their respective fixation baselines. This conjunction of spelling and reading can be interpreted as a logical AND operation. We additionally performed a Region of Interest (ROI) analysis on the data from this conjunction analysis in order to further test our hypotheses of co-localization in the left occipitotemporal and parietal cortices. Four different ROIs we identified by using the WFU pickatlas; these included the bilateral occipitotemporal cortex (combining only the inferior temporal and fusiform gyri), superior parietal lobe, angular gyrus, and supramarginal gyrus.
No cluster-level corrected clusters were identified at an initial uncorrected threshold of p<0.0001 for either the conjunction whole-brain or ROI analyses, therefore in order to further explore these data a more lenient uncorrected threshold of p<0.001was employed for each analysis. The appropriate cluster extent thresholds were identified via the aforementioned CorrClusTh.m program (Thomas Nichols; http://www.sph.umich.edu/∼nichols/JohnsGems5.html) and only clusters that surpassed a cluster-level corrected p<0.05 were reported for each analyses.
Differences between spelling and reading
Although there are numerous differences between the tasks associated with spelling and reading (i.e. one involves viewing visual words and the other involves execution of a complex motor response), a direct comparison of the spelling and reading activation maps afforded us an opportunity to further explore and interpret the findings from the aforementioned statistical comparisons. We performed a direct contrast between spelling and reading by first generating direct contrasts between the Spell>Motor and Read>Checker conditions during the 1st level single subject analysis (e.g. (Spell>Motor) > (Read>Checker)), and then entered these contrasts into a 2nd-level one-way t-test.
Results
Spelling: Behavioral Results
For the spelling experiment the mean spelling accuracy (after adjusting for instances when the subject mistakenly shifted their finger to the left or right) was 86% with a range from 77 to 97%. Even without the adjustment, accuracy levels were still relatively high with a mean of 81% and a range of 65 to 97%. The mean reaction time for correct trials on spelling task was on 358 ms (SD = 92 ms) with an average IKI of 164 ms (SD = 6 ms) and total response duration of 916 ms (SD = 165 ms). These results indicate that participants could perform the spelling task with relatively high accuracy levels within approximately a second after stimulus presentation.
Participants had no difficulty performing the motor task; mean accuracy level was 98% (SD = 2%). The mean reaction time for correct trials on motor task was on -37 ms (SD = 29 ms); it should be noted that the reaction time was calculated from the end of the auditory presentation which was 750 ms from the start of each trial. This indicates that for the motor task subjects on average responded 37 ms prior to the end of the auditory presentation of the word “motor”. Additionally, the average IKI was 164 ms (SD = 6 ms) and total response duration was 1190 ms (SD = 89 ms) for the motor task. That is, participants performed the motor task with high accuracy and within just over a second after the stimulus presentation. Because there was no need for a decision about the auditory stimulus (i.e. “motor) they tended to respond prior to the end of the stimulus presentation.
Because this non-linguistic motor task was employed to control for the auditory input and motor output that are irrelevant to spelling, a direct comparison of the behavioral performances was conducted to ensure minimal differences between the motor and the spelling task. Critically, participants did not demonstrate a significant difference in the IKI when comparing the motor with the spelling condition, as determined via a paired t-test; t (16) = 1.71, p = 0.12. Participants did however demonstrate significantly faster reaction times for the motor as compared to the spelling task (paired t-test: t (16) = 10.59, p = 1.22e-8). A direct comparison of the response duration was not performed because this comparison is also inherent to the IKI measure and the number of keypresses is not comparable across the tasks (i.e. average 4 keypresses for the spelling task and 8 for the motor control task). These results suggest that although the spelling and motor tasks were equated on IKI performance, the motor task involved reaction times that were significantly faster than in the spelling task. In order to control for this behavioral difference in the generation of statistical maps for the spell>motor tasks, these measures were added as regressors of no interest in the multiple-regression model.
Reading: Behavioral Results
We obtained responses from the reading experiment post scan recognition memory test for 16 out of the 17 subjects (data from one subject was coded incorrectly and had to be discarded). The average percent accuracy was 62% with a range of 32% to 100%. Although accuracy provides a valuable measure of recognition memory, the d′ provides a more sensitive measure of whether subjects could discriminate between words that were and were not presented, because it incorporates both the hit and false alarm rate. The average d′ was calculated to be 2.3 with a range of 0.8 to 5.6. Behavioral performance from each subject is presented in Table 1. These post-scan results indicate that each subject performed at an above chance level (d′ > 0), suggesting that these subjects attending to the written words while in the scanner and therefore no fMRI data was excluded.
Table 1. Behavioral performance on Reading experiment post-scan recognition memory test.
| Subject | Hit | False Alarm | d-prime |
|---|---|---|---|
| 1 | 88% | 12% | 2.35 |
| 2 | NA | NA | NA |
| 3 | 32% | 0% | 4.73 |
| 4 | 60% | 0% | 5.45 |
| 5 | 44% | 12% | 1.02 |
| 6 | 52% | 8% | 1.46 |
| 7 | 36% | 8% | 1.05 |
| 8 | 76% | 4% | 2.46 |
| 9 | 64% | 4% | 2.11 |
| 10 | 44% | 12% | 1.02 |
| 11 | 84% | 4% | 2.75 |
| 12 | 68% | 8% | 1.87 |
| 13 | 72% | 24% | 1.29 |
| 14 | 60% | 4% | 2.00 |
| 15 | 52% | 8% | 1.46 |
| 16 | 64% | 32% | 0.83 |
| 17 | 100% | 72% | 4.62 |
| Mean | 62% | 13% | 2.28 |
| Min | 32% | 0% | 0.83 |
| Max | 100% | 72% | 5.45 |
Spelling: task-specific within-group maps
Regions that demonstrated greater activation for the spelling task as compared to the motor task are reported in Figure 2.A and Table 2. An uncorrected threshold of p<0.0001 was used for this contrast and only clusters surpassing a cluster-level correction of p<0.05 are reported. The results from this map reveal a predominance of left hemisphere clusters associated with typed spelling. We identified a number of left hemisphere frontal cortex regions including inferior frontal gyrus (BA 44/45), insular cortex (BA 13), superior/middle frontal gyrus (BA 6), supplementary motor area (BA 6), as well as anterior cingulate gyrus (BA 24). The only region identified in the right hemisphere was a located in the frontal lobe in insular cortex (BA 13). In parietal cortex we found left supramarginal gyrus (BA 40) and posterior intraparietal sulcus extending to portions of the superior parietal lobe and middle occipital gyrus. In the temporal and occipitotemporal cortex task-related signal change for spelling was observed in left hemisphere superior temporal gyrus (BA 22) which extended in to the middle temporal gyrus (BA21). Additionally we identified left fusiform gyrus (BA 37) activation which extended laterally into the inferior temporal gyrus and posteriorly into the left inferior occipital gyrus (BA 19). Finally, a region in the right cerebellum, specifically the more medial portion of lobe VI was found.
Figure 2.
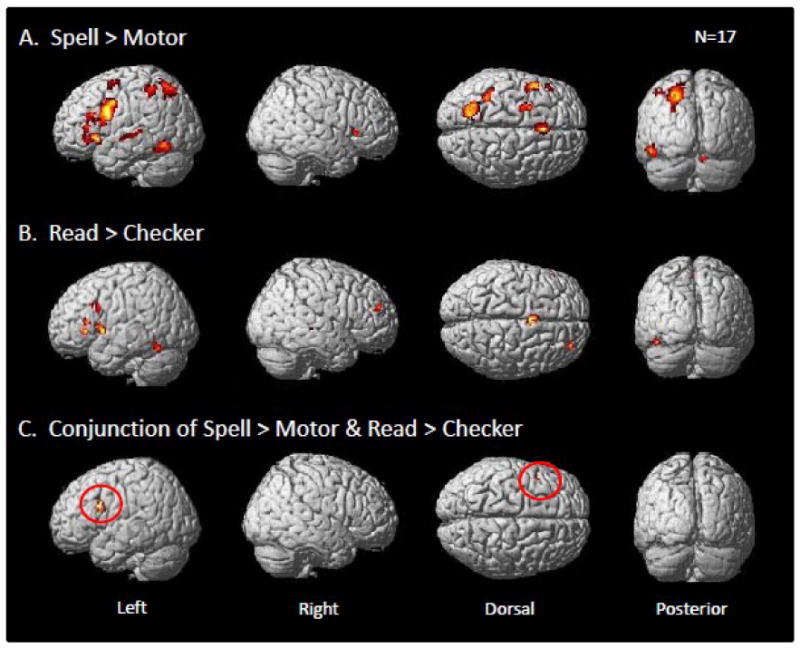
Whole brain contrast maps for the spelling and reading experiments. Each was projected on a standard rendered SPM template brain. Only clusters surpassing a corrected cluster-threshold of p<0.05 are shown. (A) Map of clusters for the Spell>Motor contrast with an inclusive mask of the Spell>Fix contrast (p<0.05, uncorrected). (B) Map of clusters for the Read>Checker contrast with an inclusive mask of the Read>Fix contrast (p<0.05, uncorrected). (C) A conjunction of effects for both the Spell>Motor and Read>Checker contrasts with an inclusive mask of the Spell>Fix and Read>Fix contrasts (p<0.05 uncorrected) for each. The red circles identify a single cluster in the inferior frontal gyrus (BA 44) that demonstrated a significant overlap across spelling and reading in this whole brain analysis.
Table 2. List of anatomical regions, volumes, maximal z-values, and peak coordinates for the Spell>Motor, Read>Checker, and Conjunction of Spell>Motor & Read>Checker contrasts. N=17 (p<0.05 corrected).
| Anatomical Region (Estimated Brodmann area) | volume (voxels) | Zmax | MNI Coordinates | TAL Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| Spell>Motor [inclusive mask of Spell>Fix (p<0.05, uncorr.)] | ||||||||
| Frontal L Inferior Frontal Gyrus (BA 44) | 1412 | 6.9 | -46 | 6 | 30 | -44 | 1 | 31 |
| L Insula (BA 13) | -32 | 22 | -2 | -31 | 19 | 4 | ||
| R Insula (BA 13) | 88 | 4.9 | 42 | 22 | 2 | 38 | 18 | 8 |
| L Supplementary Motor Area (BA 6) | 331 | 5.6 | 0 | 20 | 46 | -2 | 13 | 47 |
| L Anterior Cingulate (BA 24) | 75 | 5.6 | 0 | 8 | 26 | -1 | 3 | 28 |
| L Superior Frontal Gyrus (BA 6) | 197 | 4.7 | -22 | -6 | 52 | -22 | -12 | 50 |
| L Middle Frontal Gyrus (BA 6) | 4.4 | -26 | 4 | 56 | -26 | -3 | 54 | |
| Parietal L Supramarginal Gyrus (BA 40) | 178 | 4.9 | -36 | -46 | 50 | -35 | -49 | 44 |
| L Superior Parietal Lobule (BA 7) | 584 | 5.2 | -28 | -60 | 46 | -28 | -62 | 39 |
| Temporal L Superior Temporal Sulcus (BA22/21) | 81 | 4.9 | -62 | -20 | 0 | -58 | -20 | 1 |
| Occipitotemporal L Fusiform Gyrus (BA 37) | 183 | 5.2 | -44 | -50 | -16 | -42 | -47 | -15 |
| L Inferior Occipital Gyrus (BA 19) | 5.2 | -46 | -64 | -14 | -44 | -60 | -15 | |
| Cerebellum R Cerebellum | 56 | 5.7 | 10 | -74 | -22 | 8 | -69 | -22 |
| Read>Checker [inclusive mask of Read>Checker (p<0.05, uncorr.)] | ||||||||
| L Inferior Frontal Gyrus (BA 44) | 76 | 5.2 | -46 | 10 | 6 | -44 | 7 | 10 |
| L Inferior Frontal Gyrus (BA 44) | 0 | 4.2 | -42 | 12 | 28 | -40 | 7 | 30 |
| Frontal L Inferior Frontal Gyrus (BA 45) | 37 | 5.3 | -54 | 28 | 0 | -51 | 25 | 6 |
| L Inferior Frontal Gyrus (BA 45) | 39 | 4.5 | -44 | 26 | 10 | -42 | 22 | 15 |
| L Middle Frontal Gyrus (BA 9) | 57 | 4.2 | -36 | 20 | 32 | -35 | 14 | 34 |
| L Supplementary Motor Area (BA 6) | 139 | 5.7 | -4 | 4 | 54 | -5 | -3 | 53 |
| R Middle Frontal Gyrus (BA 10) | 33 | 4.5 | 30 | 48 | 20 | 27 | 41 | 27 |
| Temporal R Middle Temporal Gyrus (BA 21) | 33 | 4.4 | 46 | -36 | 0 | 41 | -36 | 2 |
| Occipitotemporal L Fusiform Gyrus (BA 37) | 86 | 5.3 | -40 | -56 | -14 | -38 | -53 | -14 |
| Conjunction Spell>Motor & Read>Checker [inclusive masks of Spell>Fix and Read>Fix (p<0.05, uncorr.)] | ||||||||
| Frontal L Inferior Frontal Gyrus (BA 44) | 97 | 3.7 | -42 | 12 | 30 | -40 | 7 | 31 |
Reading: task-specific within-group maps
Regions that demonstrated greater activation for the reading task as compared to the checker viewing task are reported in Figure 2.A and Table 2. An uncorrected threshold of p<0.0001 was used for this contrast and only clusters surpassing a cluster-level correction of p<0.05 are reported. As indicated in Figure 2.B and Table 2, this task was associated with activity in four regions in the left inferior frontal gyrus, with local maxima falling onto BA 44 and BA 45. Additionally we identified regions in left middle frontal gyrus (BA 9), supplementary motor area (BA 6) and fusiform gyrus (BA 37). In the right hemisphere activity was observed in the middle frontal (BA 9) and middle temporal gyri (BA 21).
Spatial co-localization between spelling and reading
As indicated in Figure 2.C and Table 2, the only region that demonstrated a conjunction of effects across both spelling and reading was found in the left inferior frontal gyrus (BA 44) (MNI peak = -42 12 30; TAL peak = -40 7 31). Although these findings confirmed part of our initial hypothesis that spelling and reading would be co-localized in the left inferior frontal gyrus, it did not confirm our hypothesis that they would be co-localized in the left parietal and occipitotemporal cortex. In order to further explore these hypotheses we performed an additional ROI analysis of the inferior temporal/fusiform gyri, superior parietal lobe, supramarginal gyrus and angular gyrus, bilaterally. These regions were identified with the WFU PickAtlas toolbox. A relatively liberal uncorrected threshold of p<0.001 was applied and only a cluster-level correction of p<0.05 is reported. While this more focused ROI analysis also did not reveal any conjunction of effects for spelling and reading in the parietal cortex ROIs, there was a significant conjunction of effects in the left inferior temporal/fusiform gyrus ROI (MNI peak = -46 -56 -14; TAL peak = -44 -52 -14). These results are shown in Figure 3.
Figure 3.
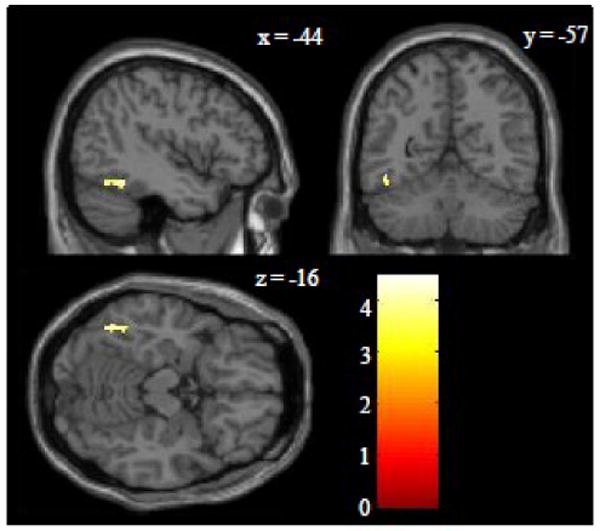
Region of interest conjunction analysis in the occipitotemporal cortex. This analysis involved a conjunction of the Spell>Motor and Read>Checker contrasts with an inclusive mask of the Spell>Fix and Read>Fix contrasts (p<0.05 uncorrected for each). The region of interest included the bilateral inferior temporal and fusiform gyri. A Z-score color scale is shown in the lower right corner. The only cluster that surpassed a corrected threshold of p<0.05 was in the left mid-fusiform (BA 37) with peak Z-score of 4.0, peak MNI coordinates of -46 -56 -14, peak TAL coordinates of -44 -52 -14, and volume of 52 voxels.
Differences between spelling and reading
Regions that demonstrated greater activation for the spelling task (Spell>Motor) as compared to the reading task (Read>Checker) are reported in Figure 4.A/B and Table 3. An uncorrected threshold of p<0.0001 was used for each contrast and only clusters surpassing a cluster-level correction of p<0.05 were explored. Interestingly, the results from this map revealed a similar map to that identified in the Spell>Motor map (Figure 1.A and Table 2). Specifically, the results reveal a number of frontal regions, including the left inferior frontal gyrus (BA 44), superior frontal gyrus (BA 6), and middle frontal gyrus (BA 6). They also revealed an area in left superior parietal cortex, which extended from the anterior to the posterior portion of the intraparietal sulcus, and numerous smaller right superior parietal cortex regions around BA 1, 2 and 7. We also observed left superior temporal gyrus (BA 22) extending into the superior temporal sulcus. Finally, spelling compared to reading resulted in more activity in the left inferior temporal gyrus (BA 37) (MNI peak = -48 -64 -6; TAL peak = -46 -61 -8). Note that this region is just lateral and superior (Euclidean distance of 11 mm3) to the peak of the conjunction analysis between spelling and reading shown in Figure 3 (MNI peak = -46 -56 -14; TAL peak = -44 -52 -14). To more clearly illustrate the spatial distributions of our findings in the left ventral visual stream, Figure 5 provides a visual representation of this region along with the area identified for spelling in the within-group analysis and the conjunction analysis for spelling and reading.
Figure 4.
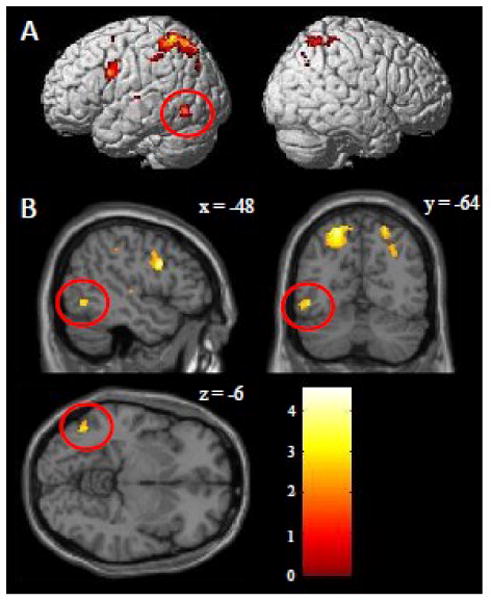
Whole brain contrast maps directly comparing the activations underlying spelling versus reading. A contrast of the (Spell>Motor) > (Read>Checker) was performed (the opposite contrast is not show, but described in the text). Only clusters surpassing a corrected cluster-threshold of p<0.05 are shown. (A) Whole brain cluster map projected on a standard rendered SPM template brain. (B) Slice cluster map focused on the left occipitotemporal cortex. The red circles identify a cluster in the left inferior temporal gyrus (BA 37) that is a both lateral and superior to the VWFA with peak Z-score of 5.1, peak MNI coordinates of -48 -64 -6, peak TAL coordinates of -46 -61 -8 and volume of 72 voxels. A Z-score color scale is shown in the lower right corner.
Table 3. List of anatomical regions, volumes, maximal z-values, and peak coordinates for the (Spell>Motor) > (Read>Checker) contrasts as well as the (Read>Checker) > (Spell>Motor) contrasts. N=17 (p<0.05 corrected).
| Anatomical Region | cluster volume | Zmax | MNI Coordinates | TAL Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| (Spell>Motor) > (Read> Checker) | ||||||||
| Frontal L Inferior Frontal Gyrus (BA 44) | 223 | 6.3 | -48 | 6 | 28 | -46 | 2 | 29 |
| L Superior Frontal Gyrus (BA 6) | 81 | 4.5 | -22 | -4 | 50 | -22 | -10 | 48 |
| L Middle Frontal Gyrus (BA 6) | 0 | 4.1 | -24 | 4 | 56 | -24 | -3 | 54 |
| Parietal R Superior Parietal Lobule (BA 7) | 72 | 4.3 | 30 | -64 | 38 | 26 | -65 | 33 |
| R Post-central gyrus (BA 2) | 34 | 4.1 | 36 | -46 | 56 | 32 | -50 | 51 |
| R Post-central gyrus (BA 1) | 0 | 4.1 | 42 | -38 | 62 | 37 | -43 | 57 |
| L Superior Parietal Lobule | 1391 | 6.2 | -20 | -64 | 56 | -20 | -66 | 48 |
| R Superior Parietal Lobule (BA 7) | 210 | 5.4 | 22 | -62 | 56 | 19 | -65 | 49 |
| Temporal L Superior Temporal Gyrus (BA 22) | 32 | 4.3 | -52 | -18 | 6 | -49 | -19 | 7 |
| Occipitotemporal L Inferior Temporal Gyrus (BA 37) | 72 | 5.1 | -48 | -64 | -6 | -46 | -61 | -8 |
| (Read>Checker) > (Spell>Motor) | ||||||||
| Frontal L Middle Cingulate Gyrus | 132 | 5.0 | -2 | -8 | 44 | -3 | -13 | 43 |
| Occipital L Cuneus (BA 18) | 67 | 5.3 | -12 | -94 | 22 | -13 | -91 | 15 |
| R Cuneus (BA 18) | 116 | 5.1 | 12 | -86 | 32 | 10 | -85 | 25 |
Figure 5.
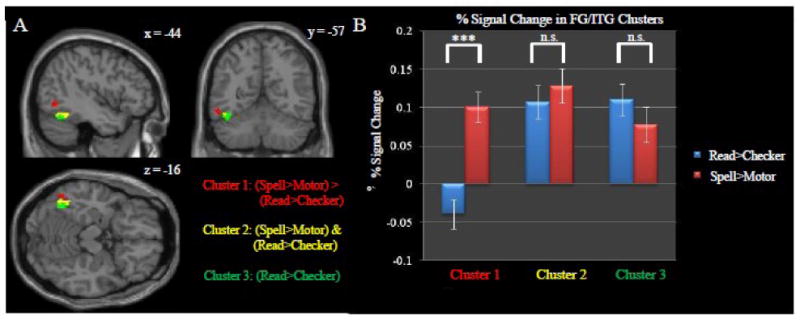
Summary of the spelling and reading results obtained for the left occipitotemporal cortex. Included in this summary are the clusters associated with the (Spell>Motor) > (Read>Checker), (Spell>Motor) & (Read>Checker), and the Read>Checker contrasts. In order to facilitate a direct comparison, each map was generated within the ITG/FG ROI with an uncorrected threshold of p<0.001 (i.e. same statistical constraints used in Figure 3). Only clusters surpassing a corrected cluster-threshold of p<0.05 are shown. (A) Red corresponds to a cluster from the (Spell>Motor) > (Read>Checker) contrast. Yellow corresponds to the same cluster presented in Figure 3 showing the conjunction of Spell>Motor & Read>Checker activation. Green corresponds to the cluster of Read>Checker masked by Read>Fix (p<0.05, uncorrected). (B) Percent signal change values from each of the three clusters. Although there were no significant differences between activation associated with spelling and reading in the left mid-fusiform gyrus (i.e. the VWFA), there was a significant difference in the left inferior temporal gyrus just lateral/superior to this area (p<0.0001, corresponding to ***).
Regions that demonstrated greater activation for the reading task (Read>Checker) as compared to the spelling task (Spell>Motor) are reported in Table 3. The only regions that demonstrated greater activation during reading relative to spelling were in the left middle cingulate gyrus and bilateral cuneus (BA 18).
Discussion
To our knowledge, this is the first fMRI study to use keyboard typing to study the brain basis of spelling. In support of our predictions, typed spelling was associated with numerous left hemisphere regions previously implicated in spelling in both the imaging and lesion literature including the left inferior frontal gyrus, superior/middle frontal gyrus, inferior/superior parietal lobe and occipitotemporal cortex. It was further determined that a subset of these left hemisphere regions involved in spelling were also associated with reading, including the left inferior frontal gyrus and, using an ROI analysis, the left occipitotemporal cortex. Direct comparisons between activity associated with spelling versus reading revealed numerous regions that demonstrated greater activation for the spelling compared to the reading task, but few regions for the inverse comparison. Specifically, an area with a significant preference for spelling was identified in the inferior temporal gyrus, located just lateral to the left mid-fusiform gyrus which in turn was characterized by a shared response to both spelling and reading (as revealed by the conjunction analysis). Together these results provide an interesting picture of shared neuronal representation for spelling and reading in the medial portion of the left occipitotemporal cortex (typically associated with the VWFA), with a functional specialization for spelling located lateral and superior from there. All of these results are examined in more detail in the following discussion.
Inferior Frontal Gyrus and Insular Cortex
In the inferior frontal gyrus we identified a large amount of activation associated with typed spelling including the left BA 44/45 and bilateral insular cortex The portion of the left IFG including the pars opercularis (BA 44) is of particular interest because it has often been associated with phonological processing of written language. Interestingly, we not only observed activation in the left IFG (BA 44) for spelling, but also found that a portion of this region was conjointly active with reading. These results suggest that the activation identified in the left IFG (BA 44) may be associated with phonological and/or orthographic demands involved in both spelling and reading. Results from a more recent fMRI determined that both the left mid-fusiform (BA 37) and IFG (BA 44) were associated with spelling knowledge for low greater than high frequency words as well as reading words. This suggests that the left IFG (BA 44) may play a specific role in orthographic specific processing. This conclusion is further supported by a lesion study which examined a large cohort of patients 24 hours after the onset of a left hemisphere stroke; specifically they determined that damage to the left BA 44/45 was associated with impaired access to orthographic representations.
We also identified a portion of the left BA 44 which demonstrated greater activation in the Spell>Motor contrast as compared to the Read>Checker contrast. This difference between spelling and reading in BA 44 could be associated with the unique phonological demands required for spelling such as the requisite holding of a short term phonological representation in memory prior to spelling. This interpretation is supported by the observation of bilateral anterior insula activation that was not also observed in the reading task which in previous studies has been associated with various auditory processing demands.
Although both typed spelling and reading activated the left BA 45 there was a lack of co-localization in this region; this suggests that this region may be involved in task demands specific to each task. Some work suggests that the more ventral left IFG including BA 45 activation identified in the typed spelling task could be associated with more general semantic processing. This interpretation is not directly supported by the results of our study though; if the left BA 45 was purely involved in semantic processing we would expect it to be conjointly active during both the spelling and the reading tasks. Another interpretation is that portions of the left IFG activation are not necessarily associated with processing phonological, lexical or semantic representations per se, but instead is involved in the coordination of task dependent activity in posterior areas associated with representations used for either spelling or reading. This interpretation would support the claim that the clusters of activation identified in the left IFG could be predominantly task dependent which would explain the minimal overlap of activation in this region across both spelling and reading tasks as compared to the extent of activation due to each task separately. Further work will be required to parse apart the nature of activation in this general area regarding how it is involved in processing representations as well as purely task dependent activation.
In general, these results indicate that typed spelling relies on inferior frontal regions previously associated with the phonological processing, and at least a portion of the left IFG (BA 44) is also active for reading.
Superior/Middle Frontal Gyrus
We also identified activation associated with typed spelling in the left superior/middle frontal gyrus. Importantly, this cluster appears to be near a region termed Exner's area which has long been associated with handwritten spelling, particularly allographic processing required in the generation of motor commands specific to the various shapes and sizes of handwritten letters (e.g. upper or lower case). For this reason, it was intriguing to identify activation in this same general location during a typed spelling task which does not require any allographic processing. As a way to compare our typed spelling results to that of handwritten spelling we examined a few recent studies that involved handwriting of whole words. The first used both intra-operative recording sessions and fMRI to identify a region in the left superior/middle frontal gyrus that corresponded to the functional requirements of Exner's area. In order to quantify the proximity of the cluster identified in our study (MNI peak = -22 -6 52) to that of the Exner's area cluster identified in two separate groups of right and left handed subjects examined in the Roux et al. study (Right hand group MNI peak = -26 -8 45 and Left hand group MNI peak = -26 -4 50), we calculated the Euclidean distance between the peaks of these clusters and the one identified in our study. The results of this calculation identified a distance of approximately 8 and 5 mm between the peak of our cluster and those in the right and left hand groups respectively. We further compared our findings to those from an fMRI study involving handwriting of Japanese phonograms which attributed a cluster in the left superior frontal gyrus (MNI peak = -24 -9 51) to be associated with Exner's area, and which had only a 4 mm Euclidean distance from our peak. Finally, we examined the Exner's area cluster identified in an fMRI study involving generative handwriting (peak MNI = -30 -4 58), and found it has a Euclidean distance of 10 mm from the peak reported in our study. In general, these findings indicate that the focus of activation associated with typed spelling in this study is approximately within 1 cm distance from previous reports of activation around Exner's area. Based on these results it can be inferred that the region identified in this study may be associated with partially common or at least adjacent neuronal populations to that of Exner's area.
Admittedly, one possibility is that the activation focus in the left middle/superior frontal gyri is due to behavioral differences across the spelling and motor control task. Although the IKI was equated for across both tasks, there were significant reaction time differences such that participants responded much faster for the motor task as compared to the spelling task. In order to account for the possible interpretation that this area is merely associated with either modulating reaction time or IKI rate we added both of these behavioral measures as regressors of no interest into the first-level analysis. This analysis accounted for at least some of the variability in the signal due to the behavioral profiles associated with the spelling and motor tasks; therefore we do not feel that activation in this area is due purely to behavioral differences. Another possibility is that activation in this region is associated with differences in the motor output between the spelling and motor tasks. For instance the spelling task involved on average four consecutive keypresses, whereas the motor task involved eight. Although the motor requirements are not equated, it should be noted that the motoric output demands associated with the motor task are greater than that for the spelling task and therefore any activation associated purely with number of sequential keypresses should not be present in spelling>motor contrast map. In sum, although we cannot completely rule out the possibility that performance differences are driving the activation reported in this premotor region, the use of regressors in the analysis and the greater motor requirements necessary to perform the motor task, make this unlikely.
Based on the constraints of our task and the findings that this cluster of activation is within one centimeter of previous reports of Exner's area, we favor an interpretation that this region is relevant to typed spelling processes that might be common to both keyboard typing and handwriting. This finding is partially supported by a previous study which reported that a lesion to the left middle frontal gyrus led to a transient handwriting deficit alongside a more persistent and selective deficit in keyboard typing. Two possible functional roles are that it could be associated graphemic buffer processing which is involved in the temporary storage of graphemic representations prior to the formation of written motor commands and therefore rely on the same populations of neurons independent of whether the word is handwritten or typed. Likewise, this area could be associated with the conversion of graphemic representations to manual motor sequences for handwriting and typing in a modality dependent manner (i.e. post-allographic processing). It would be possible to explore these interpretations with further experiments that examine whether there are dissociable sub-populations of neurons in this area that are associated with typing and handwriting respectively. Although further work is required to directly compare the handwritten and typed spelling, these results suggest that a portion of the left superior/middle frontal gyrus may be important region for written spelling regardless of whether it is handwritten or typed.
Parietal Cortex
The activation map associated with typed spelling supports long standing notions that the left parietal cortex is important for written production. In particular, typed spelling activation was identified in both the posterior and anterior portions of the left parietal cortex including portions of both the supramarginal gyrus (SMG) and superior parietal lobe (SPL). In the more anterior parietal lobe we observed typed spelling activation in the left SMG which fits with previous studies indicating that lesions to the left SMG have been associated with an impairment in the sub-lexical processing demands of spelling as measured by selective deficits in pseudoword spelling. This is supported by neuroimaging studies which find that the left SMG is associated with phoneme-grapheme mapping used to spell Japanese phonograms. It was interesting to find that there was no conjoint activation in the left SMG for spelling and reading given a recent lesion study which reported that the left SMG is critical for real/pseudoword spelling and reading. One interpretation is that typed spelling may rely on the left SMG due to its inherent reliance on the conversion of auditory words to their written form, and that a similar finding may be found in a reading that involved a comparatively reciprocal task of reading a word out loud as opposed to covert reading.
In the more posterior parietal lobe we identified SPL (BA 7) activation that extended along the intraparietal sulcus and into the middle occipital gyrus. Previous studies have reported that lesions to the left SPL led to deficits in the generation of correct sequence of movements required for handwriting, and that the left SPL has also been observed in fMRI studies of handwriting. Although no lesion studies have specifically implicated the left SPL in typed spelling, one previous fMRI study which examined the production of typing motor sequences indicated a reliance on the SPL. This suggests that the left SPL activation identified in our study could be associated with the generation of bimanual motor sequences involved in typing. An additional interpretation of activation in this area is based on reports which claim that lesions to the left angular gyrus (AG) impair irregular word spelling which suggests that it is critical to output orthographic lexical processing. Although we did not observe AG activation, one possibility is that a portion of the left IPS is functionally relevant to lexical processing which underlies both spelling and reading, and that the bulk of lesion literature which supports the claim that the left AG is associated with lexical processing may not have the resolution to dissociate left posterior IPS from AG. Further study is required to determine the specific functionality of the left IPS/SPL and surrounding cortex in written spelling.
It is important to note that contra to our prediction, we did not observe overlapping activation for spelling and reading in the left parietal cortex, regardless of whether we employed anatomical ROIs focal the superior parietal lobe, supramarginal gyrus, or angular gyrus. Although, initially unexpected, it should be noted that the reading task employed did not activate the parietal cortex and that this is consistent with findings from previous work which employed a similar reading paradigm i.e.. We interpret this null finding as being due to differences in task-dependent activation, such that if we employed a reading task that involved more demanding phonological and/or semantic processing (as opposed to just covert reading) such as utilized in other studies that examined the neural substrates of spelling/reading e.g., we would observe more overlap of activation across spelling and reading in the parietal cortex, particularly the inferior parietal lobe. Further studies need to be carried out which modulate the reading task phonological/semantic demands and then assess the degree to which this modulates activation overlap with spelling in order to confirm this.
Left Occipitotemporal Cortex
Many studies have reported that lesions to the left occipitotemporal cortex region are associated with spelling impairments. Specifically, lesions to this region have been associated with phonologically plausible errors in irregular word spelling, which suggests that it plays a critical role in output orthographic lexical processing. These findings are also supported by neuroimaging studies which require word spelling knowledge. In confirmation of this previous work, we identified a typed spelling cluster in the left fusiform (BA 37) (MNI peak = -44 -50 -16) which extended laterally into the inferior temporal gyrus and posteriorly into the left inferior occipital gyrus (BA 19). Although, the left occipitotemporal cortex has previously been associated with spelling, classically this region has been associated with reading. In particular the left mid-fusiform gyrus region known as the Visual Word Form Area due to its selective response properties to reading visual words. The relevance of the VWFA to reading has been established by studies documenting that left occipitotemporal cortex lesions can lead to pure alexia as well as neuroimaging studies which have found activation in this region during reading tasks. Not surprisingly we observed activation associated with reading in the left fusiform gyrus (MNI peak = -40 -56 -14), which is close to previously reported coordinates of the VWFA based on the review of numerous reading experiments (MNI average peak = -43 -55 -17).
Previous studies of spelling, as well as the identification of a typed spelling activation in the left occipitotemporal cortex suggested that there would be significant co-localization of spelling and reading activation in this region. Although, we did not initially identify conjoint activation in this region via a whole-brain analysis (Figure 2.C) we did identify a cluster of conjoint activation across both reading and spelling after an ROI analysis (Figure 3). In order to further explore the functional relevance of the left occipitotemporal cortex in spelling and reading we also performed a contrast between the spelling and reading tasks. Interestingly, there was significantly greater activation associated with the spelling task as compared to the reading task in the left inferior temporal gyrus (indicated by the red circles in Figure 4). This cluster (MNI peak = -48 -64 -6) was just lateral/superior by 8mm to the cluster associated with the conjunction of both spelling and reading (MNI peak = -46 -56 -14). In turn, both of these clusters were just lateral to the region associated with only reading as can be seen Figure 5.A. Figure 5 also shows the average percent signal change across subjects in these regions and demonstrates that within the region typically termed the VWFA there is no significant difference between activation levels associated with spelling and reading, but in a region just lateral to the VWFA in the inferior temporal gyrus there is significantly greater activation associated with spelling as compared to reading.
These results complement previous studies which suggest that the left occipitotemporal cortex, including the VWFA, could be a heterogeneous structure that has functionally dissociable regions which may be involved in more than just visual word processing but also auditory and even somatosensory word processing. In particular, these results fit with a study involving both unimodal and multimodal auditory and visual word processing which determined that medial aspects of the left occipitotemporal cortex are associated with unimodal visual word processing and that more lateral aspects are associated with multimodal visual/auditory word processing. Based on this work, one interpretation is that spelling may call upon more lateral multimodal regions of the visual word form system in order to convert an auditory word from to its orthographic form as compared to reading which does not require the same degree of multimodal processing. Additionally, other work suggests that a portion of the lateral inferior temporal lobe may be associated with single letter processing. One such study found activation in a lateral inferior temporal gyrus after attending to individual visual letter stimuli as compared to attending to colors or non-linguistic symbolic stimuli. Another such study identified lateral left inferior temporal gyrus activation that was associated with a with a single letter working memory task in an adult population and further reported that activation originating from this same region in a pediatric population was correlated with scores on a standardized spelling test. Based on these previous studies this more lateral inferior temporal region may be associated with multimodal processing and/or single letter processing that in some way is relevant to spelling. Although the exact function of this region is unknown, these data support the claim that spelling may call upon a more spelling-specific region in a portion of the inferior temporal gyrus that is just lateral and superior to that of the VWFA.
A recent study by Rapp and Lipka also identified overlapping of activation across spelling and reading in the left occipitotemporal cortex using a different spelling task. Specifically, this group employed a reading task that is similar to the one used in this study, but a spelling task that required subjects to press a button if an auditory word contains a specific letter and did not involve the written expression of the word (Rapp and Lipka 2010). Our results complement theirs in that we identified overlapping activation in both the left IFG and fusiform gyrus; in addition our results fit with theirs in that we also observed that the activation associated with spelling tended to be more lateral to that of the activation associated with reading. Unlike in Rapp and Lipka 2010 though, we identified dissociable activation patterns across typed spelling and reading in the left inferior temporal gyrus. This discrepancy could be either because Rapp and Lipka did not perform a direct comparison between spelling and reading, or that typed spelling may rely more heavily on this inferior temporal gyrus region compared to the spelling task employed in their study (i.e. press a button if an auditory word contained a specific letter) due to potentially greater demands on the spelling system when a word is actively spelled during the experiment. Further work is needed to clarify the degree to which there is task dependent spelling activation in the left occipitotemporal cortex.
Overall, these results support the view that a portion of the left BA 37 is associated with orthographic representations that are common to reading and spelling, although further work is required to characterize the heterogeneity of this region as it relates to shared and dissociable processing shared across spelling and reading.
Medial Prefrontal Cortex
Typed spelling was also associated with activation in medial frontal regions including the left anterior cingulate cortex (ACC) and left supplementary motor area (SMA). The anterior cingulate activation was not surprising considering its long standing association with task dependent processing, in particular tasks that involve the monitoring of potential errors. Typed spelling inherently involves the monitoring of errors as the word is typed, which during our experiment may have been particularly demanding considering the lack of visual confirmation that a word was typed correctly. The left SMA activation could be associated with sequence processing associated the production of learned word-specific keypress sequences specific to typing as suggested by previous work, which finds that temporary lesions to this region lead to impairments in the production of a complex sequence of movements. One other possible interpretation though is that typed spelling involves the generation of the phonological sequence associated with producing a word. Interestingly, there was also left SMA activation that was associated with reading, but it was not co-localized with typed spelling. These results are consistent with previous studies of reading and suggests that typed spelling and reading may call upon differential portions of the left SMA in order to carry out their respective tasks. Overall, typed spelling appears to be associated with medial frontal regions which may be associated with the executive task demands required to type a word as well as the sequence processing associated with not only the keypresses, but also which may be phonological in nature.
Conclusions
These findings provide some of the first insights into the neuronal substrates that underlying spelling via typing. In general, typed spelling proved to be a useful technique to explore the brain basis of written spelling and how this spelling network is conjointly active with reading. First, we found that typed spelling activates a predominantly left hemisphere network including the inferior frontal gyrus, middle/superior frontal gyrus, supramarginal gyrus, superior parietal lobe, and fusiform gyrus consistent with the results from previous studies of spelling that, to date, have primarily handwriting. Second, this network includes a region in the left superior/middle frontal gyrus near Exner's area that has been previously primarily associated with the grapheme-motor programs in handwriting. Third, using a conjunction analysis of spelling and reading we identified co-localized activation in the left inferior frontal gyrus (BA 44) and after an additional ROI analysis, the left inferior temporal cortex (BA 37). Finally, we found that in lateral inferior temporal gyrus the activation associated with typed spelling was greater than that for reading, while in the more medial portion typically associated with the VWFA there was no significant difference between spelling and reading. This indicates that although both spelling and reading rely on common lexical representations in the left occipitotemporal cortex, there may be task dependent representations in lateral portion of this region as well. Future studies will need to determine the extent to which typed spelling shares neurobiological resources with other written spelling modalities such as handwriting and also whether employing reading experiments which varying demands on phonological and lexical processing would recruit different degrees of conjoint and dissociable activation associated with spelling and reading.
Research Highlights.
Typed spelling activated a left hemisphere network of language related areas.
Typed spelling activation was observed near Exner's area.
Typed spelling and reading activation was co-localized to left IFG and ITG.
Activity for spelling was found lateral to reading in left occipitotemporal cortex.
Acknowledgments
This work was supported by NICHD (R01HD056107), NIDCD (F31DC009545) and the NSF (SBE 0541953 Science of Learning Center). We would like to thank Maximilian Riesenhuber, David Roeltgen, Darlene Howard, and John VanMeter for their advice on experimental design and our participants for volunteering their time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, et al. The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage. 1998;8(3):302–6. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Alario FX, et al. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076(1):129–43. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Alexander MP, et al. Lesion localization in apractic agraphia. Arch Neurol. 1992;49(3):246–51. doi: 10.1001/archneur.1992.00530270060019. [DOI] [PubMed] [Google Scholar]
- Baayen R, et al. Technical report, Linguistic Data Consortium. Philadelphia, PA: University of Pennsylvania; 1993. The CELEX Lexical Database. [Google Scholar]
- Bamiou DE, et al. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev. 2003;42(2):143–54. doi: 10.1016/s0165-0173(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Beauvois MF, Derouesne J. Lexical or orthographic agraphia. Brain. 1981;104(Pt 1):21–49. doi: 10.1093/brain/104.1.21. [DOI] [PubMed] [Google Scholar]
- Beeson P, et al. The neural substrates of writing: A functional magnetic resonance imaging study. Aphasiology. 2003;17(Numbers 6-7/June-July 2003):647–665. [Google Scholar]
- Berninger VW, et al. Writing and reading: connections between language by hand and language by eye. J Learn Disabil. 2002;35(1):39–56. doi: 10.1177/002221940203500104. [DOI] [PubMed] [Google Scholar]
- Bitan T, et al. Shifts of effective connectivity within a language network during rhyming and spelling. J Neurosci. 2005;25(22):5397–403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, et al. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002;16(1):7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, et al. Modality independence of word comprehension. Hum Brain Mapp. 2002;16(4):251–61. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, et al. Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Dev Sci. 2007;10(4):441–51. doi: 10.1111/j.1467-7687.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, et al. Developmental increases in effective connectivity to brain regions involved in phonological processing during tasks with orthographic demands. Brain Res. 2008;1189:78–89. doi: 10.1016/j.brainres.2007.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Canter GJ. Neuropsychological analysis of a typewriting disturbance following cerebral damage. Brain Lang. 1987;30(1):147–64. doi: 10.1016/0093-934x(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Buchel C, et al. A multimodal language region in the ventral visual pathway. Nature. 1998;394(6690):274–7. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Buckner RL, et al. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123(Pt 3):620–40. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Miceli G. The structure of graphemic representations. Cognition. 1990;37(3):243–97. doi: 10.1016/0010-0277(90)90047-n. [DOI] [PubMed] [Google Scholar]
- Carter CS, et al. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10(1):49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Chialant D, Caramazza A. Perceptual and lexical factors in a case of letter-by-letter reading. Cogn Neuropsychol. 1998;15:167–201. doi: 10.1080/026432998381258. [DOI] [PubMed] [Google Scholar]
- Cloutman L, et al. A neural network critical for spelling. Ann Neurol. 2009;66(2):249–53. doi: 10.1002/ana.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22(1):466–76. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, et al. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage. 2004;23(4):1256–70. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Cohen L, et al. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(Pt 5):1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, et al. Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex. 2003;13(12):1313–33. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Collins DL, et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17(3):463–8. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology. 1981b;33A:497–505. [Google Scholar]
- Dejerine J. Sur un cas de cecite verbale avec agraphie, suive d'autopsie. C R Societé du Biologie. 1891;(43):197–201. [Google Scholar]
- Dejerine J. Contribution a l'étude anatomopathologique et clinique des différentes variétés de cécité verbale. Mem Soc Biol. 1892:61–90. [Google Scholar]
- Della-Maggiore V, et al. An empirical comparison of SPM preprocessing parameters to the analysis of fMRI data. Neuroimage. 2002;17(1):19–28. doi: 10.1006/nimg.2002.1113. [DOI] [PubMed] [Google Scholar]
- Ehri L, Wilce L. Does learning to spell help beginners learn to read words? Reading Research Quarterly. 1987;22(1):47–65. [Google Scholar]
- Eickhoff SB, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Exner S. Untersuchungen über die Lokalisation der Functionen in der Grosshirnrinde des Menschen. Wien: W Braumuller 1881 [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5(2):79–83. [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci U S A. 1998;95(3):914–21. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, et al. Specific reading and phonological processing deficits are associated with damage to the left frontal operculum. Cortex. 2006;42(4):624–43. doi: 10.1016/s0010-9452(08)70399-x. [DOI] [PubMed] [Google Scholar]
- Flowers DL, et al. Attention to single letters activates left extrastriate cortex. Neuroimage. 2004;21(3):829–39. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, et al. Conjunction revisited. Neuroimage. 2005;25(3):661–7. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gaillard R, et al. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50(2):191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63(8):1403–8. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gerloff C, et al. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120(Pt 9):1587–602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Price CJ. The constraints functional neuroimaging places on classical models of auditory word processing. J Cogn Neurosci. 2001;13(6):754–65. doi: 10.1162/08989290152541421. [DOI] [PubMed] [Google Scholar]
- Glezer LS, et al. Evidence for highly selective neuronal tuning to whole words in the “visual word form area”. Neuron. 2009;62(2):199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, et al. Functional magnetic resonance imaging of motor, sensory, and posterior parietal cortical areas during performance of sequential typing movements. Exp Brain Res. 1998;121(2):153–66. doi: 10.1007/s002210050447. [DOI] [PubMed] [Google Scholar]
- Henderson EH. Developmental concepts of word. In: Henderson EH, Beers JW, editors. Developmental and cognitive aspects of learning to spell: a reflection of word knowledge. Newark, Del.: International Reading Association; 1980. [Google Scholar]
- Henderson VW. Chapter 37 Alexia and agraphia. Handb Clin Neurol. 2009;95:583–601. doi: 10.1016/S0072-9752(08)02137-4. [DOI] [PubMed] [Google Scholar]
- Henry ML, et al. The role of left perisylvian cortical regions in spelling. Brain Lang. 2007;100(1):44–52. doi: 10.1016/j.bandl.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A, Kane A, Tuffiash E, Beauchamp N, Barker P, Jacobs M, Wityk R. Neural substrates of the cognitive processes underlying spelling: Evidence from MR diffusion and perfusion imaging. Aphasiology. 2002;16(4/5/6):425–438. [Google Scholar]
- Hillis A, Rapp B. Cognitive and neural substrates of written language comprehension and production. In: Gazzaniga M, editor. The new cognitive neurosciences. 2004. [Google Scholar]
- Hillis A, Rapp R. Cognitive and neural substrates of written language: comprehension and production. In: Gazzaniga MS, editor. The cognitive neurosciences III. Cambridge, Mass: The MIT press; 2005. pp. 775–787. [Google Scholar]
- Hillis AE, et al. The roles of the “visual word form area” in reading. Neuroimage. 2005;24(2):548–59. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- James KH, Gauthier I. Letter processing automatically recruits a sensory-motor brain network. Neuropsychologia. 2006;44(14):2937–49. doi: 10.1016/j.neuropsychologia.2006.06.026. [DOI] [PubMed] [Google Scholar]
- James KH, et al. Letter processing in the visual system: different activation patterns for single letters and strings. Cogn Affect Behav Neurosci. 2005;5(4):452–66. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Jobard G, et al. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Katanoda K, et al. A functional MRI study on the neural substrates for writing. Hum Brain Mapp. 2001;13(1):34–42. doi: 10.1002/hbm.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahata N, et al. Alexia with agraphia due to the left posterior inferior temporal lobe lesion--neuropsychological analysis and its pathogenetic mechanisms. Brain Lang. 1988;33(2):296–310. doi: 10.1016/0093-934x(88)90070-3. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, et al. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21(3):946–53. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Libertus ME, et al. Developmental changes in category-specific brain responses to numbers and letters in a working memory task. Neuroimage. 2009;44(4):1404–14. doi: 10.1016/j.neuroimage.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcamp M, et al. Visual presentation of single letters activates a premotor area involved in writing. Neuroimage. 2003;19(4):1492–500. doi: 10.1016/s1053-8119(03)00088-0. [DOI] [PubMed] [Google Scholar]
- Longcamp M, et al. Premotor activations in response to visually presented single letters depend on the hand used to write: a study on left-handers. Neuropsychologia. 2005;43(12):1801–9. doi: 10.1016/j.neuropsychologia.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lubrano V, et al. Writing-specific sites in frontal areas: a cortical stimulation study. J Neurosurg. 2004;101(5):787–98. doi: 10.3171/jns.2004.101.5.0787. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matsuo K, et al. Discrimination of Exner's area and the frontal eye field in humans--functional magnetic resonance imaging during language and saccade tasks. Neurosci Lett. 2003;340(1):13–6. doi: 10.1016/s0304-3940(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Max Type LITE. Typing Tutor. 2006 http://www.askmesoft.com/maxtype_lite.htm.
- McCandliss BD, et al. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An On-Line Orthographic Database of the English Language. 2005 http://www.neuro.mcw.edu/mcword/
- Menichelli A, et al. Allographic agraphia: a case study. Cortex. 2008;44(7):861–8. doi: 10.1016/j.cortex.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Desmond JE. Left superior parietal cortex involvement in writing: integrating fMRI with lesion evidence. Brain Res Cogn Brain Res. 2001;12(2):337–40. doi: 10.1016/s0926-6410(01)00063-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10(2):181–92. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Morris D, Perney J. Developmental Spelling as a Predictor of First-Grade Reading Achievement. The Elemantary School Journal. 1984;84(4):440–457. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–133. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otsukia M, et al. Dystypia: Isolated Typing Impairment without Aphasia, Apraxia or Visuospatial Impairment. European Neurology. 2002;47(3):136–140. doi: 10.1159/000047971. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes A. Random effects analysis. In: K F, Kiebel S, Nichols T, Penny W, editors. Human Brain Function. San Diego: Academic Press; 2006. [Google Scholar]
- Philipose LE, et al. Neural regions essential for reading and spelling of words and pseudowords. Ann Neurol. 2007;62(5):481–92. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, et al. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19(3):473–81. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Pugh KR, et al. Cerebral organization of component processes in reading. Brain. 1996;119(Pt 4):1221–38. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rapcsak S, Beeson P. Neuroanatomical correlates of spelling and writing. In: Hillis A, editor. The Handbook of Adult Language Disorders. New York: Psychology Press; 2002. pp. 71–100. [Google Scholar]
- Rapcsak SZ, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62(12):2221–9. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, et al. Phonological dyslexia and dysgraphia: cognitive mechanisms and neural substrates. Cortex. 2009;45(5):575–91. doi: 10.1016/j.cortex.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B. Uncovering the cognitive architecture of spelling. In: Hillis A, editor. Handbook on Adult Language Disorders: integrating Cognitive Neuropsychology, Neurology and Rehabilitation. Philadelphia: Psychology Press; 2002. [Google Scholar]
- Rapp B, Caramazza A. From graphemes to abstract letter shapes: levels of representation in written spelling. J Exp Psychol Hum Percept Perform. 1997;23(4):1130–52. doi: 10.1037//0096-1523.23.4.1130. [DOI] [PubMed] [Google Scholar]
- Rapp B, Lipka K. The Literate Brain: The Relationship between Spelling and Reading. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritaccio AL, et al. The role of dominant premotor cortex and grapheme to phoneme transformation in reading epilepsy. A neuroanatomic, neurophysiologic, and neuropsychological study. Arch Neurol. 1992;49(9):933–9. doi: 10.1001/archneur.1992.00530330055016. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP, Heilman KM. Lexical agraphia. Further support for the two-system hypothesis of linguistic agraphia. Brain. 1984;107(Pt 3):811–27. doi: 10.1093/brain/107.3.811. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP, Heilman KM. Review of Agraphia and a Proposal for an Anatomically-Based Neuropsychological Model of Writing. Applied Psycholinguistics. 1985;6(n3):205–29. [Google Scholar]
- Roux FE, et al. The graphemic/motor frontal area Exner's area revisited. Ann Neurol. 2009;66(4):537–45. doi: 10.1002/ana.21804. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, et al. A functional lesion in developmental dyslexia: left angular gyral blood flow predicts severity. Brain Lang. 1999;70(2):187–204. doi: 10.1006/brln.1999.2158. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, et al. Parietal dysgraphia: Characterization of abnormal writing stroke sequences, character formation and character recall. Behav Neurol. 2007;18(2):99–114. doi: 10.1155/2007/906417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Perceptual, cognitive, and motoric aspects of transcription typing. Psychol Bull. 1986;99(3):303–19. [PubMed] [Google Scholar]
- Schulte-Korne G. Annotation: Genetics of reading and spelling disorder. J Child Psychol Psychiatry. 2001;42(8):985–97. doi: 10.1111/1469-7610.00797. [DOI] [PubMed] [Google Scholar]
- Shallice T. Phonological agraphia and the lexical route in writing. Brain. 1981;104(3):413–29. doi: 10.1093/brain/104.3.413. [DOI] [PubMed] [Google Scholar]
- Stevenson J, et al. A twin study of genetic influences on reading and spelling ability and disability. J Child Psychol Psychiatry. 1987;28(2):229–47. doi: 10.1111/j.1469-7610.1987.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Sugihara G, et al. Interindividual uniformity and variety of the “Writing center”: a functional MRI study. Neuroimage. 2006;32(4):1837–49. doi: 10.1016/j.neuroimage.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic-atlas of the human brain 3-Dimensional proportional system: approach to cerebral imaging. New-York: Thieme Medical Publishers Inc.; 1988. [Google Scholar]
- Tsapkini K, Rapp B. The orthography-specific functions of the left fusiform gyrus: Evidence of modality and category specificity. Cortex. 2009 doi: 10.1016/j.cortex.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, et al. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Warnke A. Reading and spelling disorders: Clinical features and causes. European Child & Adolescent Psychiatry. 1999;8(3):III/2–III/12. doi: 10.1007/pl00010689. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, et al. Woodcock-Johnson III Test of Achievement. Itasca, IL: Riverside Publishing Company; 2001. [Google Scholar]
- Xu B, et al. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb Cortex. 2001;11(3):267–77. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]


