Abstract
The prevalence of obesity in the pediatric population has increased in the last two decades and represents a serious health concern with potential impact on transplantation outcomes. We studied the effect of weight, by age-adjusted body mass index (BMI) percentiles on 1,281 pediatric patients (ages 2-19) with severe aplastic anemia transplanted between 1990 and 2005. The study population was divided into five weight groups: underweight, risk of underweight, normal BMI range, risk of overweight and overweight, according to age-adjusted BMI percentiles. Cox proportional hazards regression models for survival and acute graft-versus-host disease (aGVHD), performed using weight groups as the main effect and the normal BMI range (26-75th percentile) as the baseline comparison, found higher mortality among overweight children (>95th percentile adjusted for age). Weight at transplantation did not increase the adjusted risk of grades III-IV aGVHD. One- and two-year overall survival rates were 60% and 59% for overweight children, compared to rates greater than 70% in children with lower BMI at both time points (p<0.001). Other significant factors associated with survival included race and region, donor type, conditioning regimens in related donor transplants, performance score and year of transplant. In conclusion, overweight children with aplastic anemia have worse outcomes after HCT. The impact of obesity on survival outcomes in children should be discussed during pre-transplant counseling.
Keywords: bone marrow transplant, obesity, aplastic anemia, children
INTRODUCTION
The child obesity problem has become epidemic in the United States and other industrialized countries. The percent of obese children has doubled or tripled between the late 1970s and 2000, from approximately 5% to 15% in the United States, to 13.2% in the western region and to 19.1% in the southern region of the European Union (1, 2). This percentage remained stable from 2000 to 2006 in the United States, with 15.5% of children at or above the 95% mark for Body Mass Index (BMI) (3).
Obese adults with malignant diseases experience more transplant-related toxicity and worse overall and event-free survivals compared to normal weight patients after autologous hematopoietic cell transplantation (HCT) (4-6). Similar outcomes were also observed after allogenic HCT in obese adults (7). More recently, a large retrospective study demonstrated an increased risk of grade II-IV acute graft-versus-host disease (aGVHD) and infection complications associated with obesity after transplantation (8). Currently, there are few large-scale studies addressing under- and over-nutrition and how they impact transplant outcomes in the pediatric population. Children who are either underweight or obese have worse survival rates after chemotherapy for leukemia when compared to normal weight children (9, 10). After transplantation, the data are scant and one report demonstrated no impact of obesity on outcomes (7).
Thus, the aim of this study is to assess the effect of children’s BMI on bone marrow transplant outcomes. In order to best minimize potential confounders, such as including malignant disease where weight can be a surrogate of disease status at transplant, intensity or recovery from prior treatments and multiple types of conditioning regimens for different diseases, a population of patients with severe aplastic anemia (SAA) was selected.
PATIENTS AND METHODS
Data Sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive HCT to a Statistical Center located at the Medical College of Wisconsin in Milwaukee and at the National Marrow Program (NMDP) Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. The CIBMTR maintains an extensive database of detailed patient-, transplant-, disease-related information for transplants performed worldwide and prospectively collects data longitudinally with yearly follow-ups. Computerized checks for discrepancies, physician review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review by the Institutional Review Boards of NMDP and the Medical College of Wisconsin.
Patients
Our study population included pediatric patients aged 2 to 19 years, who received a first allogeneic HCT for SAA between 1990 and 2005, reported to the CIBMTR. One thousand, three hundred and fifty-four patients met the initial selection criteria. We excluded 37 patients from teams with inadequate follow-up or inconsistent reporting over the study period in order to reduce selection and reporting bias of patients. This left 1,317 patients to be included in the analysis. Corrective action plan (CAP) modeling addresses the potential bias introduced by the exclusion of non-consenting surviving patients, by randomly excluding the same percentage of deceased patients using a weighted randomized scheme. This process is used to adjust for over-sampling of dead patients in the consented cohort.(11) CAP modeling in this dataset excluded an additional 37 unrelated donor patients. Our final study population included 860 HLA-matched siblings (Sibs) and 421 unrelated donor (URD) transplant recipients from 91 reporting centers worldwide.
Obesity has previously been defined using the BMI in adults. This index has been validated and shown to be of utility in children ages 2 to 19 years of age for predicting underweight, normal weight and overweight individuals (12, 13). We divided patients into five groups based on their age-adjusted body mass index (BMI). Age-adjusted BMIs were calculated using 2000 Centers for Disease Control and Prevention (CDC) BMI-for-age growth charts to obtain percentile rankings, which were then used as an indicator to assess the BMI of the study population with reference to the general population(14, 15). Weight categories were defined as follows: underweight < 5th percentile (n= 109); at risk for underweight 5th percentile to < 25th percentile (n= 196); normal weight 25th percentile to 75th percentile (n= 527); at risk for overweight 76th percentile to the 95th percentile (n= 306); overweight, > 95th percentile (n=143). The sample size of this cohort allowed us to include patients in the threshold percentiles by adding groups at risk for underweight and overweight, which could demonstrate a possible dose-effect relationship of BMI and outcomes.
Follow-up was updated for all patients in the data file and each patient enrolled in the study had a minimum of two years of potential follow-up.
Study End Points and Variable
Primary endpoints were 100-day mortality, acute GVHD grades III-IV (aGVHD III-IV), and overall survival (OS). 100-day mortality was defined as death from any cause on or before day 100 post transplant. Patients alive at last observation with fewer than 100 days of follow-up were not considered at risk for this event. aGVHD III-IV was defined as development of grades III or IV aGVHD using the Glucksberg system, which grades GVHD based on the pattern and severity of abnormalities in skin, gastrointestinal and liver (16). This event was summarized by the cumulative incidence estimate, with death and second transplant as competing risks. OS was defined as time to death from any cause; surviving patients were censored at time of last follow-up.
Variables selected for analysis include: age (2-5 vs. 6-10 vs.11-15 vs. 16-19), Lansky performance score (≥90* vs. <90 vs. missing), donor-recipient CMV serology, race and ethnicity, country or region, number of transfusions prior to transplant (<20, 20-50,>50 units), time from diagnosis to transplant (<6 months vs. ≥6 months), donor type and HLA matching [Sibs vs. URD matched, URD partially matched and UR mismatched according to Weisdorf et al (17)], conditioning regimen (separately by donor type), year of transplant (1990-1993 vs. 1994-1997, 1998-2001, 2002-2005), and GVHD prophylaxis [cyclosporine/methotrexate (CSA/MTX) vs. T-cell depletion, CSA ±other and tacrolimus ± other). Selection of conditioning regimens varies depending on type of donor, thus conditioning regimens used in sibling and unrelated donor transplants were analyzed separately.
Statistical Analysis
Variables related to patient, disease, and transplant characteristics were compared using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. A p-value of <0.05 was considered significant for differences among weight groups. Univariate probabilities for overall survival and 100-day mortality were calculated using the Kaplan-Meier estimator. Univariate probability of aGVHD III-IV was calculated using the cumulative incidence function, with death as the competing risk (18).
In the multivariate analysis, a forward stepwise Cox proportional hazards model was used to select covariates for inclusion in the model(19). Since the primary objective was to compare the BMI percentile groups (main effect), we forced BMI into the main model while selecting other covariates that may affect that particular outcome. Models for overall survival and aGVHD III-IV were fitted for URD and HLA identical sibling donors separately, then combined because of their similar effects of baseline weight on outcomes. Conditioning regimen was analyzed separately by donor type. Patient region and race were entered into the models as interaction terms for patients who received transplantation in the U.S. and South America with available race/ethnicity information. All variables were examined to ensure that they complied with the proportional hazards assumption, and interactions with the main effect were tested in the model. Comparison across five distinct groups as the main the effect required adjustments for multiple comparisons. Bonferroni corrections were applied to allow adjustment for multiple comparisons between each weight group and the normal weight group. A P value <0.0167 was therefore considered statistically significant, whereas the P values for inclusion in the final models of all other potentially confounding covariates were set at <0.05. Analyses were performed using the SAS 9.2 statistical package.
RESULTS
Patient Characteristics
A total of 1,281 patients were included in the analysis. The number of patients in each weight category was: underweight 109 (8.5%), at risk for underweight 196 (15.3%), normal weight 527 (41.1%), at risk for overweight 306 (23.8%) and overweight 143 (11.1%). Comparisons of patient characteristics by weight groups are listed in Table 1 and by donor type in Table 2. Obese patients more frequently received an URD graft (18% URD and 8% Sibs) and received their transplant in the U.S. (60%). Underweight and at risk of underweight patients were older than patients in the other weight groups (p<0.01), and 66% of patients in these weight groups where from Europe, the Middle East and South American regions. The distribution of Lansky performance scores at transplant, prior transfusions, and time from diagnosis to transplant was the same for all weight groups. URD transplants were performed more commonly after 1998. URD recipients more commonly received transplants after six months from diagnosis and received total body irradiation (TBI) containing regimens than Sibs recipients.
Table 1.
Patients ages 2 to 19 years who received allogeneic hematopoietic cell transplantation for severe aplastic anemia, by weight groups, between 1990 and 2005.
| Characteristics | Under weight | At risk for under weight | Healthy weight | At risk for over weight | Over weight | P-value |
|---|---|---|---|---|---|---|
| Number of patients | 109 | 196 | 527 | 306 | 143 | |
| Sib: URD ratio | 2.6 :1 | 3:1 | 2.2:1 | 2:1 | 0.88:1 | |
| Age at transplant | 12 (2 - 19) | 12 (2 - 19) | 12 (2 - 19) | 11 (2 - 19) | 10 (2 - 18) | <.001 |
| 2-5 | 27 (25) | 21 (11) | 59 (11) | 33 (11) | 24 (17) | |
| 6-10 | 19 (17) | 56 (29) | 143 (27) | 104 (34) | 53 (37) | |
| 11-15 | 35 (32) | 57 (29) | 181 (34) | 95 (31) | 48 (34) | |
| 16-19 | 28 (26) | 62 (32) | 144 (27) | 74 (24) | 18 (13) | |
| Male gender | 74 (68) | 109 (56) | 307 (58) | 157 (51) | 76 (53) | 0.03 |
| Lansky performance score ≥90 | 70 (64) | 132 (67) | 355 (67) | 193 (63) | 92 (64) | 0.58 |
| Race/Ethnicity | <.001 | |||||
| White | 55 (50) | 108 (55) | 331 (63) | 185 (60) | 82 (57) | |
| Black | 7 (6) | 18 (9) | 40 (8) | 26 (8) | 10 (7) | |
| Hispanic | 3 (3) | 5 (3) | 27 (5) | 21 (7) | 21 (15) | |
| Asian | 13 (12) | 33 (17) | 65 (12) | 39 (13) | 12 (8) | |
| Pacific Islanders/Native American | 3 (2) | 0 | 6 (1) | 0 | 4 (3) | |
| Middle Eastern/North Africa | 17 (16) | 21 (11) | 34 (6) | 18 (6) | 6 (4) | |
| Mixed/Unknown | 10 (10) | 11 (6) | 19 (3) | 15 (5) | 8 (5) | |
| Patient Region | <.001 | |||||
| USA | 28 (26) | 58 (30) | 175 (33) | 98 (32) | 86 (60) | |
| Canada | 5 (5) | 12 (6) | 31 (6) | 15 (5) | 9 (6) | |
| Europe | 14 (13) | 41 (21) | 111 (21) | 64 (21) | 13 (9) | |
| Asia | 14 (13) | 22 (11) | 56 (11) | 38 (12) | 10 (7) | |
| Australia/New Zealand | 3 (3) | 3 (2) | 21 (4) | 14 (5) | 7 (5) | |
| Mideast/Africa | 23 (21) | 23 (12) | 39 (7) | 17 (6) | 5 (3) | |
| Central/South America | 22 (20) | 37 (19) | 94 (18) | 60 (20) | 12 (8) | |
| Year of transplant | 0.41 | |||||
| 1990-1993 | 26 (24) | 50 (26) | 144 (27) | 75 (25) | 26 (18) | |
| 1994-1997 | 36 (33) | 53 (27) | 172 (33) | 92 (30) | 49 (34) | |
| 1998-2001 | 24 (22) | 45 (23) | 114 (22) | 71 (23) | 42 (29) | |
| 2002-2005 | 23 (21) | 48 (24) | 97 (18) | 68 (22) | 26 (18) | |
| Disease-related | ||||||
| Previous transfusions | 73 (67) | 133 (68) | 362 (69) | 198 (65) | 66 (46) | <0.001 |
| Number of donor exposures | 0.004 | |||||
| <20 | 29 (27) | 60 (31) | 172 (33) | 90 (29) | 33 (23) | |
| 21-50 | 22 (20) | 35 (18) | 94 (18) | 57 (19) | 11 (8) | |
| >50 | 18 (17) | 33 (17) | 72 (14) | 42 (14) | 17 (12) | |
| Unknown | 4 (4) | 4 (2) | 19 (4) | 7 (2) | 3 (2) | |
| Time from Diagnosis to Transplant-median | 4 | 3 | 4 | 5 | 8 | |
| < 6 months | 65 (60) | 137 (70) | 311 (59) | 163 (53) | 61 (43) | <.001 |
| ≥ 6 months | 43 (39) | 59 (30) | 215 (41) | 142 (46) | 82 (57) | |
| IST prior to transplant | 49 (45) | 103 (53) | 307 (58) | 199 (65) | 106 (74) | <.001 |
| Transplant-related | ||||||
| Sibling donors | 79 (72) | 147 (75) | 363 (69) | 204 (67) | 67 (47) | <.001 |
| Unrelated donors | 30 (28) | 49 (25) | 164 (31) | 102 (33) | 76 (53) | |
| Well matched | 10 (9) | 19 (10) | 47 (9) | 34 (11) | 18 (13) | |
| Partially matched | 12 (11) | 15 (8) | 49 (9) | 31 (10) | 29 (20) | |
| Mismatched | 8 (7) | 15 (8) | 68 (13) | 37 (12) | 29 (20) | |
| Donor/Recipient CMV match | 0.41 | |||||
| Any positive | 71 (65) | 140 (72) | 341(65) | 211(70) | 92 (64) | |
| Negative | 30 (28) | 42 (21) | 154 (29) | 79 (26) | 46 (32) | |
| Unknown | 8 (7) | 14 (7) | 32 (6) | 16 (5) | 5 (3) | |
| Conditioning regimen | 0.01 | |||||
| Cy ± other | 28 (26) | 47 (24) | 142 (27) | 70 (23) | 19 (13) | |
| Cy + TBI ± other | 18 (17) | 28 (14) | 71 (13) | 39 (13) | 39 (27) | |
| Cy + BU ± other | 18 (17) | 25 (13) | 51 (10) | 38 (12) | 14 (10) | |
| Cy + ATG ± other | 44 (40) | 92 (47) | 257 (49) | 156 (51) | 70 (49) | |
| TBI dosage group ≤1300 cGy | 19 (17) | 36 (18) | 101 (19) | 60 (20) | 49 (34) | <.001 |
| ATG use -Yes | 53 (49) | 98 (50) | 276 (52) | 169 (55) | 76 (53) | 0.72 |
| GVHD prophylaxis | .36 | |||||
| T-cell depletion | 15 (14) | 19 (10) | 65 (12) | 27 (9) | 20 (14) | |
| CSA + MTX ± other | 75 (69) | 140 (71) | 389 (74) | 216 (70) | 95 (67) | |
| CSA only | 16 (15) | 27 (14) | 54 (11) | 46 (15) | 14 (10) | |
| Tacrolimus ± other | 3 (2) | 6 (3) | 11 (2) | 9 (3) | 9(6) | |
| Other | - | 4(2) | 8 (1) | 8(3) | 5(3) | |
| Median follow-up of survivors, mths | 71 | 65 | 74 | 62 | 59 |
Abbreviations: ATG = antithymocyte-globulin; BMI = body mass index; Bu = Busulfan; CMV = cytomegalovirus; CSA = cyclosporine; Cy = cyclophosphamide; GVHD = graft vs. host disease; IST = immune suppression therapy; MTX = methotrexate, TBI = total body irradiation; mths=months
Table 2.
Characteristics of patients ages 2-19 who received an allogeneic hematopoietic cell transplantation for severe aplastic anemia by donor type, HLA-match sibling (Sibs) or an unrelated donor (URD) between 1990 and 2005.
| Characteristics of patients | Sibs | URD | P-value |
|---|---|---|---|
| Number of patients | 860 | 421 | |
| Lansky performance score≥90 | 532 (62) | 310 (74) | <0.001 |
| BMI groups | <0.001 | ||
| Underweight | 79 (9) | 30 (7) | |
| At risk for underweight | 147 (17) | 49 (12) | |
| Healthy weight | 363 (42) | 164 (39) | |
| At risk for overweight | 204 (24) | 102 (24) | |
| Overweight | 67 (8) | 76 (18) | |
| Time from dx to tx median, months | 2 (<1 -86) | 16 (<1 -150) | <0.001 |
| < 6 months | 669 (78) | 68 (16) | |
| ≥6 months | 191 (22) | 350 (83) | |
| Conditioning regimen | <0.001 | ||
| Cy ± other | 283 (33) | 23 (5) | |
| Cy + TBI ± other | 30 (3) | 165 (39) | |
| Cy +BU ± other | 128 (15) | 18 (4) | |
| Cy+ ATG ± other | 410 (48) | 209 (50) | |
| GVHD prophylaxis | <0.001 | ||
| T-cell depletion | 16 (2) | 130 (31) | |
| CSA + MTX ± other | 699 (81) | 212 (50) | |
| CSA only | 123 (14) | 34 (8) | |
| Tacrolimus ± other | 8 (1) | 30(7) | |
| Other | 14 (2) | 15(4) | |
| Year of transplant | <0.001 | ||
| 1990-1993 | 254 (30) | 67 (16) | |
| 1994-1997 | 289 (34) | 113 (27) | |
| 1998-2001 | 177 (21) | 119 (28) | |
| 2002-2005 | 140 (16) | 122 (29) | |
| Patient region | <0.001 | ||
| United States | 142 (17) | 303 (72) | |
| Canada | 52 (6) | 20 (5) | |
| Europe | 180 (21) | 63 (15) | |
| Asia | 122 (14) | 18 (4) | |
| Australia/New Zealand | 37 (4) | 11 (3) | |
| Mideast/Africa | 105 (12) | 2 (<1) | |
| Central/South America | 222 (26) | 3 (<1) | |
| Median Follow-up of survivors (months) | 74 (3-206) | 58 (3-194) |
Abbreviations: ATG = antithymocyte-globulin; BMI = body mass index; Bu = Busulfan; CMV = cytomegalovirus; CSA = cyclosporine; Cy = cyclophosphamide; GVHD = graft vs. host disease; IST = immune suppression therapy; MTX = methotrexate, TBI = total body irradiation; dx=diagnosis; tx=transplant.
Busulfan-containing regimens were mostly used in HLA-matched donor transplants from centers outside the U.S. Anti-thymocyte globulin (ATG) was commonly used for both Sibs and URD transplants, and cyclosporine and methotrexate were the most common GVHD prophylaxis treatments, being used in 81% and 50% of Sibs and URD transplants, respectively.
Acute GVHD
The probability of aGVHD III-IV at day 100 was 8% [95% Confidence Interval (CI), 4- 14%], 8% (95% CI, 5-13%), 11% (95% CI, 8-14%), 15% (95% CI, 11-19%) and 24% (95% CI, 18-32%) for underweight, at risk of underweight, normal weight, at risk of overweight and overweight patients respectively (p<0.01). Multivariate analysis of aGVHD III-IV adjusted for significant covariates: race/ethnicity/region, donor type, HLA matching, conditioning regimen (Sibs only) and GVHD prophylaxis, resulting in a relative risk of 1.55 (95% CI, 1.00-2.41, p=0.05) for the overweight group compared to the normal weight group. The overall p-value for BMI in the aGVHD III-IV model was 0.15. The varying impacts of significant covariates on development of aGVHD III-IV are summarized in Table 3. It is noteworthy that reductions in the risk of aGVHD III-IV were seen in children receiving transplants in Asia [relative risk (RR) 0.18, 95% CI, 0.06-0.61, p<0.01] compared to Caucasian children in the U.S.; and recipients of T-cell depleted grafts (RR 0.32, 95% CI, 0.18-0.54, <0.01) compared to cyclosporine/methotrexate. There was an increase in the risk for aGVHD III-IV in recipients of URD regardless of HLA matching (Table 3) compared to recipients of Sibs. Among Sibs transplants, recipients of busulfan-containing regimens (RR 4.75, 95% CI, 1.88-12.02, p<0.01) had a higher risk compared to the cyclophosphamide and ATG combination (Cy/ATG).
Table 3.
Multivariate analyses of grades III-IV acute GVHD and mortality of children with severe aplastic anemia who received allogeneic hematopoietic cell transplantation from 1990 to 2005.
| Variable | N | aGVHD | P-value | Overall mortality | P-value |
|---|---|---|---|---|---|
| Age-adjusted BMI | 0.147 | 0.012 | |||
| Underweight | 108 | 0.7 (0.34-1.44) | 0.332 | 1.01(0.65-1.57) | 0.972 |
| At risk of underweight | 196 | 0.84 (0.48-1.48) | 0.551 | 1.38 (0.99-1.91) | 0.057 |
| Normal | 525 | 1 | 1 | ||
| At risk of overweight | 304 | 1.09 (0.73-1.63) | 0.673 | 1.22 (0.93-1.61) | 0.142 |
| Overweight | 142 | 1.55 (1-2.41) | 0.05 | 1.71 (0.24-2.35) | 0.001 |
| Race/Region | 0.02 | 0.004 | |||
| U.S., Caucasian | 303 | 1 | 1 | ||
| U.S., A.A. | 50 | 1.61 (0.92-2.82) | 0.09 | 1.84 (1.17-2.91) | 0.01 |
| U.S., Hispanic | 47 | 1.09 (0.59-2.02) | 0.78 | 1.13 (0.07-1.83) | 0.61 |
| U.S., API | 24 | 0.63(0.22-1.77) | 0.38 | 0.46 (0.17-1.26) | 0.13 |
| U.S., Other | 15 | 0.28 (0.04-2.04) | 0.22 | 1.02 (0.41-2.53) | 0.97 |
| Canada | 71 | 0.28 (0.1-0.8) | 0.02 | 0.58 (0.32-1.07) | 0.08 |
| Europe | 241 | 0.67 (0.41-1.1) | 0.11 | 0.92 (0.65-1.31) | 0.66 |
| Asia | 140 | 0.18 (0.06-0.61) | 0.005 | 1.34 (0.85-22.11) | 0.21 |
| Australia/New Zealand | 48 | 0.91 (0.35-2.33) | 0.84 | 0.48 (0.22-1.04) | 0.06 |
| Middle East | 107 | 1.09 (0.45-2.67) | 0.84 | 1.6 (0.95-2.71) | 0.08 |
| South America, White | 123 | 0.36 (0.12-1.04) | 0.06 | 0.78 (0.44-1.38) | 0.39 |
| South America, Black | 43 | 0.53 (0.13-2.14) | 0.37 | 1.64 (0.87-3.09) | 0.13 |
| South America, Hispanic | 27 | 0.43(0.66-3.25) | 0.41 | 0.98 (0.38-2.53) | 0.97 |
| South America, Unknown | 31 | 0.34 (0.07-1.65) | 0.18 | 1.06 (0.52-2.18) | 0.87 |
| Donor Type, HLA Matching | <0.001 | <0.001 | |||
| Matched Sibling Donor | 855 | 1 | 1 | ||
| URD, Matched | 125 | 7.05(3.52-14.11) | <0.001 | 4.8 (2.91-7.94) | <0.001 |
| URD, Partially Matched | 136 | 10.12 (5.34-19.2) | <0.001 | 8.36 (5.55-12.59) | <0.001 |
| URD, Mismatched | 154 | 11.74 (6.17-22.33) | <0.001 | 7.99 (5.32-12) | <0.001 |
| Conditioning Regimen (Sibling only) | 0.01 | <0.001 | |||
| Cy+ATG | 407 | 1 | 1 | ||
| TBI+/-Other | 53 | 0.66 (0.14-3.14) | 0.60 | 1.36 (0.68-2.75) | 0.38 |
| Bu+/-Other | 133 | 4.75(1.88-12.02) | 0.001 | 3.13 (1.85-5.29) | <0.001 |
| Cy+/-Other (No ATG) | 262 | 1.51 (0.72-3.2) | 0.28 | 1.92 (1.24-2.96) | 0.003 |
| GVHD Prophylaxis | <0.001 | ||||
| CSA+MTX+/-Other | 912 | 1 | - | ||
| CSA+/-Other | 165 | 2.57 (1.71-3.84) | <0.001 | - | |
| T-cell depletion | 144 | 0.32 (0.18-0.54) | <0.001 | - | |
| Tacrolimus ± other | 37 | 1.13 (0.59-2.15) | 0.72 | - | |
| Other | 12 | 6.27 (1.33-29.69) | 0.02 | - | |
| Lansky Performance Score | - | - | <0.001 | ||
| >=90 | 838 | - | - | 1 | |
| <90 | 417 | - | - | 1.71 (1.36-2.14) | <0.001 |
| Missing | 20 | - | - | 2.48 (1.14-5.38) | 0.02 |
| Year of transplant | - | - | <0.001 | ||
| 1990-1993 | 321 | - | - | 1 | |
| 1994-1997 | 400 | - | - | 0.94 (0.7-1.26) | 0.70 |
| 1998-2001 | 294 | - | - | 0.77 (0.55-1.06) | 0.11 |
| 2002-2005 | 260 | - | - | 0.44 (0.29-0.66) | <0.001 |
Abbreviations: AA= African Americans; API = Asians and Pacific Islanders; ATG = antithymocyte-globulin; BMI = body mass index; Bu = Busulfan; CMV = cytomegalovirus; CSA = cyclosporine; Cy = cyclophosphamide; GVHD = graft vs. host disease; IST = immune suppression therapy; MTX = methotrexate, TBI = total body irradiation.
Survival Outcomes
Mortality at 100 days after transplant was 13% (95% CI, 7-20), 15% (95% CI, 10-20%), 12% (95% CI, 9-14%), 17% (95% CI, 13-21%) and 29% (95% CI, 22-37%) for underweight, at risk of underweight, normal weight, at risk of overweight and overweight patients, respectively (p<0.01). Adjusted survival curves according to weight groups are summarized in Figure 1. Multivariate analysis of mortality, which was adjusted for for race/ethnicity/region, donor type, HLA matching, conditioning regimen (Sibs only), Lansky performance score, and years after transplant resulted in a relative risk of death of 1.71 (95% CI, 1.24-2.35, p<0.01) in overweight patients, compared to patients in the healthy weight category. Figure 2, summarizes the magnitude of the effect of weight on mortality across all groups.
Figure 1.
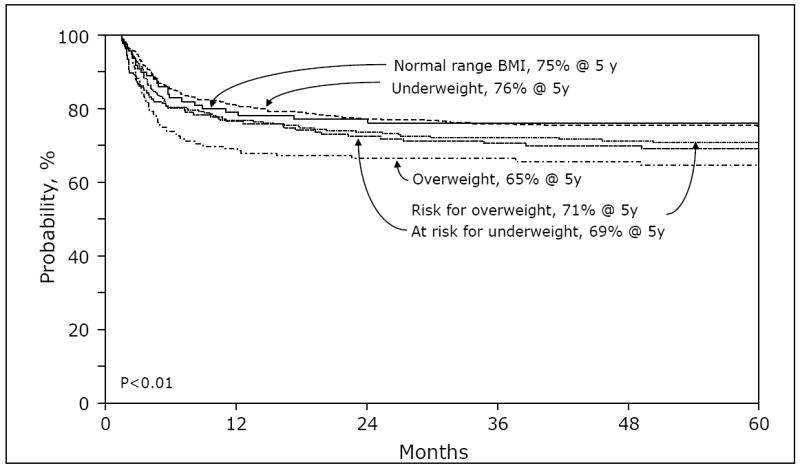
Adjusted probability of overall survival of children with severe aplastic anemia who received allogeneic hematopoietic cell transplantation from 1990 to 2005, by weight groups.
Figure 2.
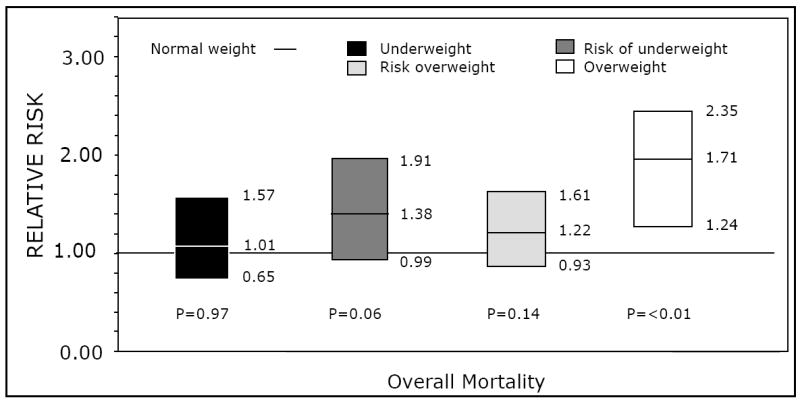
Multivariate analysis for overall mortality in children with severe aplastic anemia who received bone marrow transplantation from 1990 to 2005, according to age-adjusted body mass index percentiles.
The different impacts of significant covariates on mortality are summarized in Table 3. It is noteworthy that reductions in mortality were observed in patients who received transplantation during the period from 2002 to 2005 (RR 0.44, 95% CI, 0.29-0.66, p<0.01), compared to patients transplanted from 1990 to 1993. Increases in mortality were observed in African-American recipients in the U.S. (RR 1.84, 95% CI, 1.17-2.91, p<0.01) compared to Caucasians in the same country. Recipients of URD, regardless of HLA matching (Table 3), and patients with Lansky performance score of < 90 (RR 1.71, 95% CI, 1.36-2.14, p<0.01) at time of transplant. Among Sibs transplants, recipients of busulfan-containing regimens experienced higher mortality (RR 3.13, 95% CI 1.85-5.29, p<0.01) compared to those receiving Cy/ATG. Mortality was higher with Bu-containing conditioning regimens compared to cyclophosphamide/ATG, among recipients of Sibs grafts (RR, 3.13; 95% CI. 1.85-5.29, P<0.01)
Causes of Death
A total of 363 patients died during the course of the study: 204 URD (48%) and 159 Sibs (18%) transplant recipients. The attributed causes of death reported by transplant centers were infection (24%), graft failure (18%), organ failure (17%), GVHD (14%), idiopathic pneumonia syndrome (12%), hemorrhage (9%), post-transplant cancer (2%) and unknown causes (4%). The most common cause of death among URD recipients was infection (n=52, 25%), and among Sibs transplant recipients was graft failure (n=37, 23%).
DISCUSSION
This study found that overweight children (age-adjusted BMI >95th percentile) with severe aplastic anemia experienced worse survival outcomes after allogeneic hematopoietic cell transplantation compared to patients who were not overweight. This demonstrates that obesity in this homogenous population has a significant negative impact on survival and possibly on complications post HCT. Overweight children with severe aplastic anemia have no apparent increase in rates of aGVHD III-IV in that region.
The effect of body weight on transplant outcomes is the topic of several studies. The results, however, vary depending on the transplant indication, type of transplant and patient population. This question was addressed initially in recipients of autologous HCT for malignant diseases. Obese adults, defined according to BMI, experienced higher mortality rates after autologous HCT for non-Hodgkin’s lymphoma and acute myeloid leukemia (AML) (4, 6). Subsequent studies using the CIBMTR database addressed this question in 4,681 adult recipients of autologous HCT for lymphoma. Obese patients experienced similar survival outcomes compared to overweight patients and to patients in the normal weight range. Transplant-related mortality was 2.5 times greater for patients in the underweight group compared to all the other weights (5). Similar results, i.e. comparable survival outcomes of obese and non-obese patients and shortened survival in underweight patients, were demonstrated in patients with AML receiving HCT (20). Despite these observations in large cohort of patients, obesity was identified as an independent prognostic factor for non-relapse mortality after HCT and is a component of the HCT-Comorbidity Index (21).
The results from obesity studies in the adult population might not directly apply to children. The definition of obesity in adults utilizes a single BMI measurement at time of transplant. In children, age adjustments are necessary for defining weight groups and it is uncertain whether BMI in adults or age-adjusted BMI in children have the same prognostic impact. Chemotherapy dose adjustments based on weight to minimize toxicity are likely to be different between children and adults(10). Children with leukemia at the opposite ends of the weight scale, overweight and underweight, suffer from higher mortality during standard chemotherapy induction treatments (9, 10). Studies on the impact of weight on post transplant outcomes in the pediatric population are scant. One single-institution study analyzed 54 children with BMI ≥ 95th among a cohort of 325 recipients of allogeneic HCT for treatment of several diseases. Five-year overall survival probabilities were 47% in the overweight group compared to 70% in the non-overweight group (p=0.02)(22).
The effect of obesity on aGVHD is less clear. Univariate analysis in our study demonstrated a higher rate of aGVHD III-IV in the overweight group, which was likely related to the higher proportion of recipients of URD recipients in this group. After adjusting the analysis for donor type and other variables, this effect of weight and aGVHD III-IV disappeared.
A study from the Japanese group also demonstrated a strong relationship between weight at time of transplant and GVHD (8). This study analyzed patients of all ages with malignant diseases. Although the univariate analysis in the Japanese study demonstrated a strong association between weight and both acute and chronic GVHD, this association disappeared in multivariate analysis. In our analysis, other factors were strongly associated with development of aGVHD III-IV in patients with severe aplastic anemia. As expected, URD recipients and HLA disparity were associated with higher rates of aGVHD III-IV. Recipients from Asia experienced less frequent aGVHD III-IV, which could also be attributed to lower rates of aGVHD in URD recipients in Asia, due to less HLA gene variability in the region.
Race and region also were associated with survival outcomes. Interestingly, there was higher rate of mortality among African American compared to Caucasian children in the U.S. No data on socio-economic status was collected for these patients, which could impact outcomes along with access to care. Improvements in survival outcomes in the later periods are attributed to improvements in donor and patient selection, and have also been described in other studies (23). The effect of conditioning regimen was analyzed separately in Sibs and URD HCT. The use of busulfan in Sibs recipients was associated with worse survival. There was no effect of conditioning regimens and survival outcomes in URD HCT.
This is the largest study ever done to assess the effect of weight on allogeneic transplant outcomes in children with severe aplastic anemia. However, this is a retrospective study, utilizing registry data that spans a 15-year period. The decision to proceed to transplantation was made by individual transplant physicians as part of center-specific protocols or as standard of care. Despite the number of cases, some variables such as dose-adjustment practices and detailed prior treatment, including transfusion, were not present in all patients and could not be formally examined. Another limitation of this study was the definition of obesity. The weight charts in which the age-adjusted BMIs were calculated are normalized to the U.S. population, but the study utilized children outside the U.S. This might explain the higher number of patients from Asia, South American and Europe in the underweight groups, which could clarify why this group did not experience poorer survival rates, as other studies on underweight children have shown. Small numbers of underweight children in the U.S. did not allow for a meaningful subset analysis to answer this question.
In conclusion, our study indicates that obese children with severe aplastic anemia have higher mortality rates following allogeneic HCT compared to normal weight children. There was no impact of weight on development of aGVHD III-IV in this population. Obesity is not an easily modifiable risk factor, and optimizing nutrition and weight pretransplant is difficult to achieve due to the urgency of the procedure. The impact of obesity on transplant outcomes for this disease should be discussed during pre-transplant counseling. Prospective studies with a better definition of obesity, using anthropometrics in children, are important to further understanding the association between obesity and post-transplant outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson-Leach R, Lobstein T. Estimated burden of paediatric obesity and co-morbidities in Europe. Part 1. The increase in the prevalence of child obesity in Europe is itself increasing. International Journal of Pediatric Obesity. 2006;1:26–32. doi: 10.1080/17477160600586614. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics US. Hyattsville, MD: National Center for Health Statistics; 2003. [Google Scholar]
- 3.Ogden CL, Carroll MD, Flegal KM. High Body Mass Index for Age Among US Children and Adolescents, 2003-2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 4.Meloni G, Proia A, Capria S, et al. Obesity and autologous stem cell transplantation in acute myeloid leukemia. Bone Marrow Transplant. 2001;28:365–367. doi: 10.1038/sj.bmt.1703145. [DOI] [PubMed] [Google Scholar]
- 5.Navarro WH, Loberiza JFR, Bajorunaite R, et al. Effect of Body Mass Index on Mortality of Patients with Lymphoma Undergoing Autologous Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2006;12:541–551. doi: 10.1016/j.bbmt.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Tarella C, Caracciolo D, Gavarotti P, et al. Overweight as an adverse prognostic factor for non-Hodgkin’s lymphoma patients receiving high-dose chemotherapy and autograft. Bone Marrow Transplant. 2000;26:1185–1191. doi: 10.1038/sj.bmt.1702692. [DOI] [PubMed] [Google Scholar]
- 7.Fleming DR, Rayens MK, Garrison J. Impact of obesity on allogeneic stem cell transplant patients: A matched case-controlled study. The American Journal of Medicine. 1997;102:265–268. doi: 10.1016/S0002-9343(96)00450-0. [DOI] [PubMed] [Google Scholar]
- 8.Fuji S, Kim S-W, Yoshimura K-i, et al. Possible Association between Obesity and Posttransplantation Complications Including Infectious Diseases and Acute Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation. 2009;15:73–82. doi: 10.1016/j.bbmt.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and Outcome in Pediatric Acute Lymphoblastic Leukemia. J Clin Oncol. 2007;25:2063–2069. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 10.Lange BJ, Gerbing RB, Feusner J, et al. Mortality in Overweight and Underweight Children With Acute Myeloid Leukemia. JAMA. 2005;293:203–211. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]
- 11.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75:978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 13.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132:204–210. doi: 10.1016/s0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- 14.http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 15.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-Matching for Retrospective Analysis of Unrelated Donor Transplantation: Revised Definitions to Predict Survival. Biology of Blood and Marrow Transplantation. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 19.Cox D. Regression Models and Life Tables. J R Stat Soc B. 1972;4:187–200. [Google Scholar]
- 20.Navarro WH, Agovi MA, Logan BR, et al. Obesity Does Not Preclude Safe And Effective Myeloablative Hematopoietic Cell Transplantation (Hct) For Acute Myeloid Leukemia (Aml) In Adults. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2010.04.009. Epub date 2010/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)- specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulley S, Gassas A, Dupuis LL, et al. Inferior outcomes for overweight children undergoing allogeneic stem cell transplantation. Br J Haematol. 2008;140:214–217. doi: 10.1111/j.1365-2141.2007.06900.x. [DOI] [PubMed] [Google Scholar]
- 23.Kahl C, Leisenring W, Deeg HJ, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long-term follow-up. Br J Haematol. 2005;130:747–751. doi: 10.1111/j.1365-2141.2005.05667.x. [DOI] [PubMed] [Google Scholar]


