Abstract
Reported here are analyses of the interactions between a select group of solution-phase glycoproteins and a unique boronic acid capture surface. The boronic acid derivative, 4-[(2-aminoethyl)carbamoyl]phenylboronic acid, AECPBA, was synthesized and then immobilized on carboxymethyl dextran surfaces using simple coupling methods. From surface plasmon resonance (SPR) spectroscopy responses, it is found that model glycoproteins interact strongly with the AECPBA surface and subsequently can be readily released from the AECPBA surface using borate buffer. A striking difference between the glycoproteins fetuin and asialofetuin (desialylated fetuin), in terms of glycoprotein binding to the AECPBA surface, indicates that the interaction of glycoproteins with the immobilized AECPBA is dictated by the terminal saccharide of the heteroglycan chain. Surprisingly, secondary interactions of glycosylated and non-glycosylated proteins with the carboxymethyl dextran hydrogel matrix are observed. Importantly, it is demonstrated that use of tris(hydroxymethyl)aminomethane buffer allows for decreased secondary interactions of non-glycosylated proteins on the AECPBA/dextran surface, as noted with the model protein ExtrAvidin.
INTRODUCTION
Glycosylation renders particular functions that are reflected in most of the physico-chemical and biological properties of proteins. Some of the key roles in which glycosylated proteins participate include cellular recognition, protein folding, and protein trafficking.1 On the other hand, aberrant glycosylations—as manifested by changes in glycosylation levels and alterations in glycan structures—have been associated with the development and progression of cancer and other diseases.2–3 As a result, glycosylated proteins have been the subject of many research efforts targeting the elucidation of structure-function relationships.
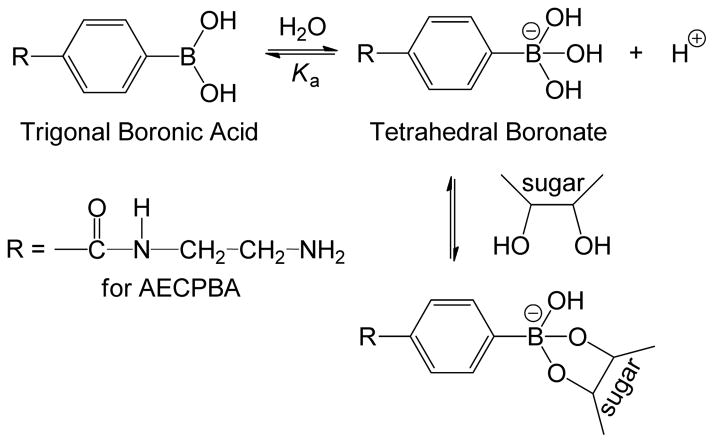
The characteristic of phenylboronic acids to form reversible complexes with diol-containing materials, such as sugars, has led to numerous developments for eventual application in areas such as sensor technology,3–8 drug delivery,9 and affinity chromatography.10–11 Current knowledge on the mechanism of the diol-boronic acid interaction is based on the equilibrium formation of a heterocylic diester from 1,2- or 1,3-diol groups and a tetrahedral boronate ion (Scheme 1);12 this equilibrium is a function of the ionization constant Ka of the boronic acid moiety. Thus, the coordination of diol species is commonly performed at a pH that results in conversion of the trigonal planar boronic acid species into the tetrahedral boronate ion. Although it is generally accepted that the boronate ion is the active binding species, Ishihara and co-workers13 are of the opinion that the neutral planar boronic acid has comparable or even higher reactivity toward diols than the boronate ion, regardless of solution pH.
Scheme 1.
Depiction of phenylboronic acid–sugar equilibrium for 4-[(2-aminoethyl)carbamoyl]phenylboronic acid, AECPBA.
The ability to select, from a diverse protein population, a given subset of glycosylated proteins (enrichment) using surface-attached capture agents is of great importance in the systematic identification and quantification of disease-related biomarkers obtained from tissues and circulating cells.1,14 Although interaction analysis between surface-attached boronic acid derivatives and simple saccharides (non-protein-containing) is quite common in the literature,6,15–17 it is surprising to find from an exhaustive survey of the literature that reports on the interaction analysis of surface-immobilized boronic acids with solution-phase proteins—glycosylated and non-glycosylated alike—are limited, at best.3–5,18–19 In two of the extent studies, investigations were performed on colloidal gold possessing a polymer brush of 3-acrylamidophenylboronic acid for determination of glycoprotein presence.4–5 However, the limited number and variety of proteins used did not allow for a thorough probing of protein properties that might have an impact on the feasibility of the Au colloid system in the development of sensors for analysis of glycoproteins. In two other studies, single glycoprotein binding (glycated hemoglobin3 and glycosylated albumin18) on alkanethiol/Au surfaces was investigated, but the elution (regeneration) of the surfaces was not addressed. In the only other study of which we are aware, electrochemical methods were used to study the affinity interactions between electropolymerized boronic acid films on electrodes and a select group of glycoproteins having limited glycan variety.19 Thus, it would be of great benefit to develop surface immobilization chemistries for attachment of a diverse collection of phenylboronic acids and gain knowledge regarding their ability to capture and release different and closely-related glycosylated proteins under a variety of solution conditions.
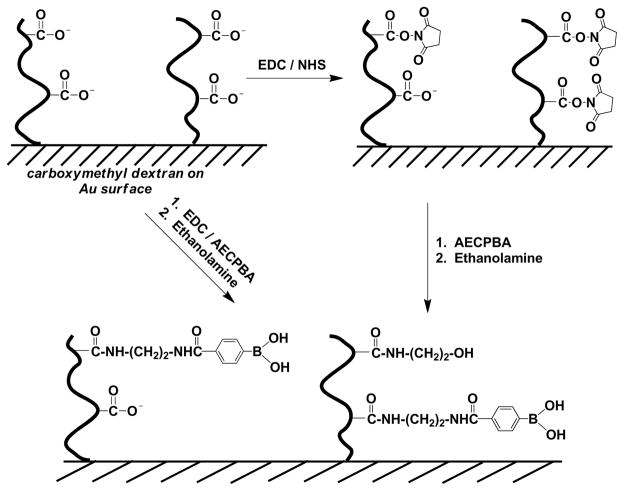
Here we report on evaluation of glycoprotein–surface-attached boronic acid interactions by surface plasmon resonance spectroscopy (SPR). To the best of our knowledge, this is the first time that a boronic acid derivative has been successfully immobilized onto SPR sensor surfaces and then subsequently used to study the interactions between surface boronic acids and solution-phase glycoproteins. We show that SPR can be used to readily follow interactions between surface boronic acids and glycoproteins without complex and laborious surface preparation. In particular, the novel boronic acid derivative 4-[(2-aminoethyl)carbamoyl]phenylboronic acid, AECPBA, is immobilized on carboxymethyl dextran hydrogels using carbodiimide coupling, Scheme 2, and is subsequently employed as the capture element in an SPR device, Scheme 1. We chose AECPBA because a soluble polymer bearing this boronic acid derivative exhibits increased sensitivity to glucose binding under physiological conditions, and the pKa of the boronic acid/boronate pair of AECPBA is lower than other phenylboronic acids, making it attractive for capture of proteins in biological milieu.9 In the work here, a variety of glycosylated and non-glycosylated proteins having various properties was investigated to provide insight into the nature of the interaction between the boronic acid-modified sensor surface and the proteins. Furthermore, the use of immobilized AECPBA as a reversible capture-and-release agent is demonstrated by the quantitative elution of glycoproteins from AECPBA surfaces by borate buffer. Secondary interactions are also discussed in the context of non-specific adsorption to the carboxymethyl dextran matrix and the boronic acid ligand.
Scheme 2.
Preparation of the AECPBA-functionalized carboxymethyl dextran on Au (CM5) sensor surface.
EXPERIMENTAL SECTION
Materials
AECPBA was prepared as previously reported.9 CM5 sensor chips (carboxymethyl dextran on Au) were obtained from Biacore (Uppsala, Sweden). Avidin, ExtrAvidin, fetuin, asialofetuin, RNAse A, RNase B, and human transferrin were purchased from Sigma and were used as received. Other chemicals obtained from Sigma include 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC), 2-(N-morpholino)ethanesulfonic acid (MES), tris(hydroxymethyl)aminomethane (Tris), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), EDTA, glycine, Tween-20, and ethanolamine. N-hydroxysuccinimide (NHS) was obtained from Pierce Biotechnology. Boric acid was obtained from EM Sciences. NaOH and NaCl were purchased from Fisher Scientific. HCl was obtained from VWR. All solutions were prepared in Nanopure water (Barnstead, >18 MΩ·cm). pH 7.40 HBS-EP consisted of 0.01 M HEPES, 0.15 M NaCl, 3.0 × 10−3 M EDTA, and 0.0050% (v/v) Tween-20. All buffers and reagents used were degassed and filtered prior to use in SPR experiments.
Potentiometric Titration
To determine the pKa of AECPBA, 1.5 × 10−2 g of AECPBA was dissolved in 20.00 mL of 0.010 N NaOH. To this solution was added 0.50-mL portions of the titrant (0.010 N HCl containing 0.150 M NaCl) and the pH at each interval was determined using a calibrated glass pH electrode (Denver Instrument). A pKa value of 8.0 was found using this method.
Surface Plasmon Resonance Measurements
Investigations of the interaction of select proteins with AECPBA- and hydroxyl-terminated control surfaces were performed with a Biacore X SPR instrument (Uppsala, Sweden). To prepare the sensor surface, the commercially-available CM5 sensor surface (Biacore) was functionalized with AECPBA either by direct EDC coupling or through EDC/NHS activation, Scheme 2. For direct EDC coupling, 65 μL of a mixture composed of 0.010 M AECPBA and 0.20 M EDC prepared in pH 6.00, 0.025 M MES was injected at a flow rate of 2 μL min−1 after achieving baseline with the same buffer. The unreacted, NHS-activated carboxyl groups were capped by injecting 65 μL of pH 8.50, 1.0 M aqueous ethanolamine, at a flow rate of 10 μL min−1. For the sensor surface modification via EDC/NHS activation, pH 7.40 HBS-EP was used as the running buffer. The surface was activated by injecting 70 μL of a freshly prepared mixture consisting of 0.070 M NHS and 0.20 M EDC at a flow rate of 10 μL min−1. At the same flow rate, several 70 μL-injections of 0.020 M AECPBA prepared in pH 8.50, 0.10 M borate were performed. Finally, the remaining activated esters on the surface were deactivated by injecting 40 μL of pH 8.50, 1.0 M ethanolamine. To examine the binding of select proteins, either 0.050 M Tris buffer or 0.050 M glycine buffer (pH 8.00 and 9.00) containing 0.15 M NaCl were used as running and sample buffers. Protein solutions were passed over the AECPBA-functionalized surfaces at defined concentrations and flow rates. Values reported for the amount of protein bound are the average ± 1 standard deviation from replicate measurements. The AECPBA surface was regenerated following each protein injection with either single or multiple injections of pH 10.00, 0.10 M borate-buffer containing 0.30 M NaCl or a short pulse of 0.050 M NaOH at 10 μL min−1.
RESULTS AND DISCUSSION
Preparation of the AECPBA (Boronic Acid) Sensor Surface
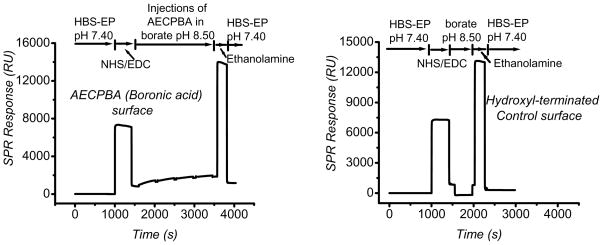
The covalent attachment of AECPBA as followed by SPR, is shown in Figure 1. Activation of the carboxymethyl dextran surface was achieved through injections of EDC and NHS solutions, thereby transforming the carboxylic acid groups into NHS-activated esters. Alternatively, the carboxymethyl dextran surface can be activated directly by the use of EDC only (Figure S-1). Subsequently, solutions of AECPBA in pH 8.50 borate buffer were passed over the surface several times (4× of 70 μL of 0.025 M AECPBA) to maximize the degree of AECPBA attachment. Removal of any non-covalently bound AECPBA and capping of any remaining NHS esters (formation of amide-linked, hydroxyl-terminated regions) was carried out using a solution of ethanolamine. SPR measures the resonance angle at which a minimum of reflected light occurs as a result of a change in the refractive index of the medium near a thin film of metal (Au in this case)—for example, during analyte adsorption. This change in angle is reported in Resonance Units (RU) such that a change of 0.1° is equivalent to 1000 RU.20 The change in SPR response, measured in Resonance Units (RU), for the AECPBA surface was 1200, while hydroxyl-terminated control surfaces resulted in a change of 300 RU. Thus, it can be concluded that the SPR response (~1200 RU) is the result of covalent attachment of AECPBA, a small molecule, throughout the 200-nm thick carboxymethyl dextran hydrogel matrix.21 Although it is desirable to determine the surface density of immobilized AECPBA ligand, this is not possible using the SPR response values. The published conversion factor of 10 RU = 1.0 ng·cm−2 used for proteins20,22 should not be employed, because the refractive index of small molecules can be significantly different from that of proteins.23
Figure 1.
SPR sensorgrams from the preparation of AECPBA (left) and hydroxyl-terminated control (right) surfaces. In each case, the carboxymethyl dextran surfaces were first treated with pH 7.40 HBS-EP (0.010 M HEPES, 0.15 M NaCl, 3.0 × 10−3 M EDTA, 0.0050% v/v Tween-20), then 70 μL of 0.070 M NHS/0.20 M EDC was injected, followed by a minimum of 4 injections of 70 μL of 0.025 M AECPBA in pH 8.50 borate buffer for the AECPBA surface or 70 μL of pH 8.50 borate buffer followed by 40 μL of 1.0 M ethanolamine (pH 8.50) for the hydroxyl-terminated control surface. Capping of any remaining NHS sites on the AECPBA surface was achieved by injecting 40 μL of pH 8.50, 1.0 M ethanolamine.
Model Glycosylated Protein Binding on and Subsequent Elution from AECPBA Surfaces
Initial investigation of the binding capabilities of the AECPBA sensor surface was performed by flowing a solution of the model protein avidin in pH 9.00 glycine-buffered saline. Avidin is a 68 kDa tetrameric protein that consists of 4 identical subunits and contains 10% glycosylation.24 Each subunit contains one glycosylation site at Asn 17;25–26 glycans at this site have been shown by NMR to be heterogeneous in both composition and structure.27 Evidence from that study27 suggests that high mannose and hybrid types make up the oligosaccharide composition, with the latter hybrid type terminated with N-acetylglucosamine and/or galactose.
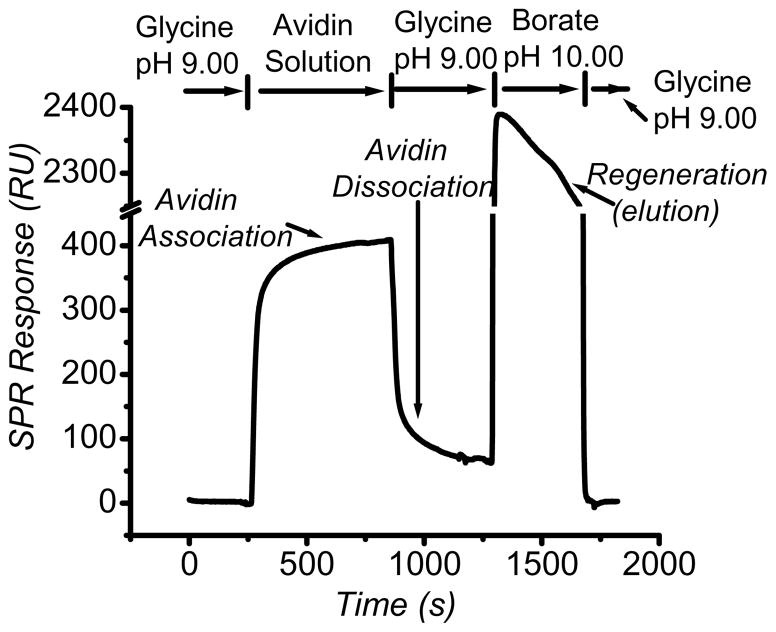
In Figure 2 is shown the SPR response for the AECPBA surface upon exposure to the glycoprotein avidin. The observed interaction beginning at roughly 250 s on the time axis is presumed to result from the specific weak covalent interaction between the active tetrahedral boronate ion and the sugar residues in the glycoprotein, Scheme 1. Although it is tempting to report the association/dissociation constants, the unknown stoichiometry of binding between avidin and AECPBA would render such values suspect.28 However, the amount of surface-bound avidin at [avidin]solution = 5.16 × 10−6 M can be determined. Based on the difference in RU responses of avidin prior to and after its injection, we calculate that roughly 13.1 ± 0.4 ng·cm−2 of avidin is bound to the AECPBA surface, a value that is ~3% of a close-packed avidin monolayer.29 This value did not change upon increasing the number of EDC/NHS or AECPBA injections. The obtained value of 13.1 ± 0.4 ng·cm−2 of avidin was calculated using the established conversion factor of 10 RU = 1.0 ng·cm−2 for proteins from the average SPR response of ~130 RU. The response is measured between the baseline and the RU level after subtraction of the contribution of the bulk refractive index (as caused by any refractive index change due to some differences in the sample and running buffer used). Assuming a Langmuir adsorption process with a close-packed avidin monolayer (440 ng·cm−2),29 and using a typical boronic acid-glycoprotein association constant, such as that found for 3-acrylamidophenylboronic acid avidin complexation (Kassoc = 5.05 × 10−3 M),4 the expected amount of avidin bound to the AECPBA surface with [avidin]solution = 5.16 × 10−6 M is calculated to be 11.2 ng·cm−2, in good agreement with the observed 13.1 ± 0.4 ng·cm−2 value. The limit of detection was not determined in this case. It should be noted that this would be highly dependent upon several factors such as the molecular weight of the analyte, the ligand surface coverage, and the thermodynamic properties elicited by the interaction. However, a similar instrument—Biacore 1000—has been reported to detect as low as ≤1% complete monolayer of bound protein.29 Based on this, the monolayer coverage we obtained was derived from the weak interaction of avidin with the boronic acid groups.
Figure 2.
Representative SPR sensorgram for avidin binding on and elution from the AECPBA surface. The binding experiment was performed with 20 μL of 5.16 × 10−6 M avidin in pH 9.00 glycine-buffered saline (0.050 M glycine, 0.15 M NaCl) at a flowrate of 2 μL min−1, while the elution (regeneration) experiment was performed with 65 μL of pH 10.00 borate-buffered saline (0.10 M borate, 0.30 M NaCl) at a flowrate of 10 μL min−1. The flowrate during the buffer run was kept at 2 μL min−1. The AECPBA surface was prepared through direct EDC coupling.
The covalent interaction between boronate and sugar is a reversible process, and dissociation of the sugar-boronate complex is usually facilitated using an acidic buffer30 or a competing molecule, such as sorbitol.31 However, experiments revealed that an appreciably low pH (e.g. ≤pH 2.00, data not shown) was required to yield effective elution, but with use of such a low pH eluent, SPR responses were found to be inconsistent in-between binding experiments. We attribute this to AECPBA ligand loss from the sensor surface during use of the highly acidic eluent (amide hydrolysis). Similar outcomes have been observed for boronic acids attached via amides on chromatographic supports.30 In the case of sorbitol, its ability to displace diols from the diol-boronate complex is based on its strong interaction with boronate ions.32 Thus, use of sorbitol as an eluent is inappropriate in SPR analyses, because sorbitol binding to the boronate surface would result in misleading interpretation of the SPR baseline following regeneration with sorbitol (inability to establish pre-protein-exposure baseline). In general, sorbitol is a powerful and appropriate eluting agent for select boronic acid sensing systems like those based on electrochemical sensing33 wherein the baseline response is not affected by sorbitol binding on the electrode surface; however, sorbitol should be avoided as an eluent for boronic acid sensing applications possessing a transduction mechanism based on surface mass change, such as SPR and microbalance methods.17
We turned to borate as an eluent34 for the AECPBA-bound glycoprotein avidin (regeneration of the AECPBA surface/release of captured avidin), as the borate acts as a competing molecule for covalent bond formation with the sugar chains of the glycoprotein, resulting in glycoprotein elution from the AECPBA surface. As shown in Figure 2, use of pH 10.00 borate-buffered saline resulted in the apparent complete removal of avidin after the dissociation phase of the experiment (at ~1300 s), as noted by the experimentally observed identical SPR reading before avidin injection (RU = 2) and after borate regeneration of the AECPBA surface (RU = 2). In addition, the AECBPBA surfaces could be treated numerous times (12 times, the maximum attempted) without any measurable impact on the ability of the boronic acid surfaces to bind avidin in subsequent association experiments. In other experiments, multiple injections of borate or a short pulse of NaOH solution35–36 effected regeneration (vide infra). Overall, these outcomes demonstrate the capacity of the AECPBA surface to effectively bind glycoprotein analyte and the ability of borate to act as a mild and simple eluent for AECPBA-bound glycoprotein, thereby providing an avenue for comparative analysis of the binding of chemically distinct glycoproteins to AECPBA surfaces.
Impact of Glycosylated Protein Nature on Binding to AECPBA Surfaces
The relative affinity, measured as surface protein concentration, of several glycosylated proteins examined on the AECPBA surface prepared through the EDC/NHS activation method is summarized in Table 1. The proteins are appropriately chosen so as to exhibit variation in molecular weights, degree of glycosylation, composition of heteroglycan chain and isoelectric points (pI),37–41 to facilitate the determination of the nature of glycoprotein interaction with surface boronic acids. In general, the amount of glycosylated protein bound to the AECPBA surface is significantly greater than for the hydroxyl-terminated control surface. There is no observable general trend in the amount of protein bound with the pI, molecular weight, or degree of glycosylation for this set of proteins. Regeneration (i.e. removal of bound proteins and subsequent achievement of virtually identical baseline before protein injection and after regeneration) was routinely observed on the AECPBA- and hydroxyl-terminated control surfaces for this set of proteins.
Table 1.
Comparison of the amount of proteins bound on the AECPBA surface and the hydroxyl-terminated (ethanolamine-capped) control surface.
| protein properties | amount protein bound (× 102 fmol·cm−2) | ||||
|---|---|---|---|---|---|
| protein [pI] | molecular weight (kDa) | glycosylated ? | degree of glycosylation | AECPBA surface | hydroxyl-terminated control surface |
| fetuin [3.3] | 48.4 | yes | 22% | 4.6 | 2.6 |
| asialofetuin [5.2] | <48.4 | yes | 14% | 16 | 6.0 |
| transferrin [5.6] | 76–81 | yes | 6% | 5.4 | 2.6 |
| RNAse B [9.4] | 14.7 | yes | 9% | 17 | 4.1 |
| RNAse A [9.4] | 13.7 | no | Not applicable | 14 | 4.1 |
Protein concentrations (× 10−4 M) flowed over the AECPBA surfaces were: fetuin, 3.88; asialofetuin, 4.27; transferrin, 4.03; RNAse B, 4.19; and RNAse A, 3.97. 60 μL of protein solution (pH 8.00 glycine-buffered saline) was injected at 15 μL min−1. The AECPBA surface was prepared through EDC/NHS activation. Regeneration was performed with pH 10.00, 0.10 M borate-buffered saline or 0.050 M NaOH solution. The values obtained possess a ±5% experimental error. Information on molecular weight, pI, and degree of glycosylation are provided in references 37–41.
Interestingly, a striking difference in SPR response was observed for fetuin and its desialylated analogue, asialofetuin. The amount of bound fetuin was found to be significantly lower compared to asialofetuin (~25%, Table 1), indicating that the binding constant for asialofetuin, Kassoc, on the AECPBA surface is significantly higher. Structurally, the only difference between the two proteins is the presence of the terminal N-acetylneuraminic acid (a.k.a. sialic acid) group in the six glycan chains of fetuin.37 In a recent investigation of a colloidal gold-carrying, boronic acid polymer brush, the assumption was made that the higher Kassoc observed for ovalbumin compared to avidin was a result of the larger population of hydroxyl groups presented by the mannose-rich ovalbumin over the N-acetylglucosamine-rich avidin.4 If the same case were to hold for fetuin and asialofetuin, the higher number of sugar constituents in fetuin (terminated with 6 sialic acid residues) should result in binding of more fetuin versus asialofetuin on the AECPBA surface; the converse is observed. We propose that the more sugar-rich fetuin (compared to asialofetuin) does not behave similarly to ovalbumin for the following reasons: 1) not all hydroxyl groups will be available to participate in complex formation because some hydroxyl groups are involved in glycosidic linkages between saccharide units; 2) not all hydroxyl groups are oriented in the synperiplanar formation that is a requisite for boronate complexation;42 and 3) steric hindrance43 in the underlying sugars of the glycan chains will preclude binding. It was in fact demonstrated by 11B NMR that the complexation of borates to galactomannan is only through the galactose units attached to and “hanging from” the mannan polymer backbone.44 Evidence from that study44 does not suggest any complexation of borate with the mannose units that are glycosidically-linked together to make up the oligosaccharide backbone. Therefore, based on this, the degree of complex formation is not necessarily dependent on the number of sugar constituents of the glycan chains (% glycosylation) present in the glycoprotein.
With this knowledge in hand, our results with fetuin, asialofetuin, and human transferrin suggest that the identity of the sugar terminus plays a key role in determining the extent of binding of glycoproteins to the AECPBA surface. The six oligosaccharide chains of fetuin are terminated with N-acetylneuraminic acid units (sialic acid), while asialofetuin is essentially fetuin with its oligosaccharide chains terminated with galactose units (resulting from the desialylation procedure used to make it). Although it might be tempting to state that the lower observed AECPBA binding of fetuin is due to the negative charge of the N-acetylneuraminic acid residue (electrostatic repulsion by the boronate ion), this is not necessarily the case. Detailed investigations of N-acetylneuraminic acid binding to boronic/boronate systems revealed that N-acetylneuraminic acid binds more strongly at acidic to neutral pH, contrary to the generally accepted binding of neutral sugars at alkaline pHs.43 It was rationalized that, unlike neutral sugars wherein strong complex formation with boronic acid systems occurs with the tetrahedral boronate ion to create a tetrahedral-formed complex, N-acetylneuraminic acid complexation with boronic acids results from interactions between the glycerin moiety of N-acetylneuraminic acid and the uncharged trigonal boronic acid, resulting in a trigonal complex. The observed increased stability of this trigonal complex at acidic to neutral pH is derived from the intramolecular B–O or B–N interaction created with the neighboring N-acetyl group. Because the boronic acid derivative used here has a pKa of 8.0, and the binding was performed at pH 8.00, complex formation between fetuin and the boronic acid system through the glycerin group is unstable. This is because the larger proportion of tetrahedral boronate ion existing in solution results in tetrahedral-formed complex between the boronate ion and the glycerin moiety without the stabilization from the B–O or B–N interaction, giving way to a weaker binding. This weaker interaction translates into a decreased amount of bound fetuin.
Importantly, we have observed that the amount of human transferrin bound to the AECPBA surface is roughly the same as for fetuin, further suggesting that the degree of glycoprotein binding to the boronic acid surface is heavily influenced by the identity of the terminal sugar of the glycan chains of the glycoprotein. Human transferrin has only two glycan chains, both of which are terminated with N-acetylneuraminic (sialic) acid,45–46 as is the case for the glycan chains of fetuin. In addition, both transferrin and fetuin have galactose units immediately before the sialic acid terminus; the final 5 sugars of their terminal sequences are N-acetylneuraminic acid→galactose→N-acetylglucosamine→ mannose→ mannose.46–48 From Table 1, it is found that the amount of fetuin and transferrin bound on the AECPBA surface is the same within experimental error (~5 × 102 fmol·cm−2). This similarity in the amount of these two proteins bound on the AECPBA surface is striking, as there is a large difference in their degree of glycosylation (fetuin, 22%; transferrin, 6%) and molecular weight (fetuin, 48 kDa; transferrin, ~76 kDa). Thus, our results strongly support the hypothesis that the identity of the sugar terminus plays a key role in determining the extent of binding of glycoproteins to the AECPBA surface. Our observations with fetuin, asialofetuin, and transferrin will be important during the design of systems for the enrichment of glycoproteins from a diverse protein pool.
Non-specific Protein Adsorption on Carboxymethyl Dextran Surfaces
It is interesting to note that SPR responses are evident on the hydroxyl-terminated control surface after glycoprotein solutions were presented to it, albeit the responses are significantly lower than that found on the AECPBA surface for each protein. This hydroxyl-terminated control surface possesses ethanolamine-capped carboxylic acid groups that should result in diminished non-specific binding of proteins.49 We attribute the observed SPR response to non-specific adsorption of proteins on the carboxymethyl dextran surface, similar to what has been observed for some proteins in a previous study.29 Thus, we hypothesize that the observed protein adsorption on the hydroxyl-terminated control surface is electrostatic in nature and may likely be due to the interaction between regions of underivatized surface carboxylic acids and a given protein, as dictated by protein isoelectric point (pI). Alternatively, it can be argued for proteins whose pIs render them negatively charged during the association phase of the experiment, such as fetuin (pI = 3.3) and asialofetuin (pI = 5.2), hydrogen-bonding to the dextran matrix50 is a possible explanation of the observed non-specific adsorption. No matter the cause of the non-specific interactions, it is clear that interaction of the glycoproteins with the AECPBA surface is due to a combination of the specific boronate-sugar complexation and non-specific adsorption to the underlying dextran hydrogel matrix. Based on the higher SPR response on the boronic acid surface, the specific complexation reaction of glycoproteins on the boronic acid surface is dominant.
Glycosylated and Non-glycosylated Protein Binding on AECPBA Surfaces
To determine the specificity of the boronic acid ligand for binding glycosylated proteins versus their non- or deglycosylated counterparts, the non-glycosylated protein RNAse A and deglycosylated ExtrAvidin were investigated and compared to RNAse B (glycosylated) and avidin (glycosylated).
Parallel comparison of RNAse A (non-glycosylated) and RNAse B (glycosylated) is appropriate given that the two ribonucleases are identical in protein structure and only distinguishable at Asn 34, where a high-mannose-oligosaccharide–containing chain resides in RNAse B but not in RNAse A.40,51 In the ideal scenario, any difference between the two in terms of interaction with the boronic acid ligand should be attributable to the presence/absence of the heteroglycan chain at Asn 34. Upon inspection of Table 1, it is found that RNAse A has a considerable degree of interaction with the AECPBA surface, although the amount of RNAse B (glycosylated) binding is 21% greater. Interestingly, the level of interaction of the non-glycosylated RNAse A is even greater than some of the other glycosylated proteins studied. This observation can be rationalized by considering the molecular structure of the AECPBA derivative. This structure can be associated with secondary interactions such as hydrophobic, coulombic, coordination, and hydrogen bonding.11 Therefore, interaction with any material possessing boronic acids is not necessarily limited to the specific boronate/cis-diol ester formation. Conceivably, the properties of the proteins are expected to exert a significant role in the overall interaction process. In the case of the non-glycosylated RNAse A, its interaction with negatively-charged surfaces is well documented in the literature;41,52 this interaction is facilitated by the presence of a positively-charged protein domain that is known to be situated along the longest axis of the RNAse A molecule, thereby affording a large surface area with positive potential. The pI of RNAse A is 9.4,41 and at the binding pH of 8.00 used here, the protein contains a net positive charge, while the boronic acid surface possesses negative charges from the active boronate ion species. It is then reasonable to say that coulombic interaction accounts very well for the high level of SPR response of RNAse A despite it not being glycosylated. As for RNAse B, it can be deduced that both specific and non-specific interactions contribute to the binding observed on the boronic acid surface. It can then be said that these secondary interactions should be capable of providing additional selectivity if they occur in concert with the primary specific interaction, but they become detrimental when they favor non-specific adsorption of non-glycosylated analytes, such as is observed with RNAse A.
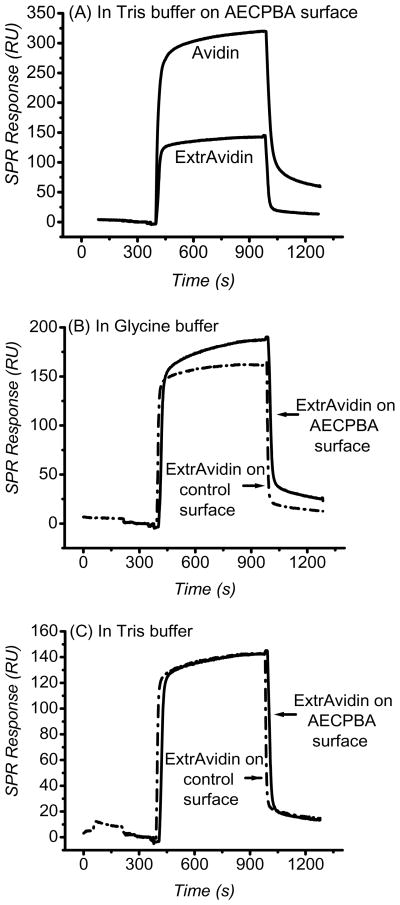
Another comparative analysis was performed with a glycosylated protein and its deglycosylated variant, namely, avidin and ExtrAvidin, on the AECPBA surface prepared from direct EDC coupling. As shown in the representative sensorgrams in Figure 3, the SPR response for avidin is higher (~4-fold) compared to that for ExtrAvidin on the AECPBA surface, with a calculated 9.8 ± 0.5 ng·cm−2 avidin bound and 2.6 ± 0.6 ng·cm−2 ExtrAvidin bound using tris(hydroxymethyl)aminomethane, Tris, buffer. This difference can be viewed as a consequence of the interaction between the heteroglycan chain in avidin and the boronate groups on the sensor surface. In addition, similar to the observation made with RNAse A (non-glycosylated), ExtrAvidin is found to bind to both boronic acid and hydroxyl-terminated control surfaces with the glycine buffer system used (Figure 3B). Importantly, when the buffer was changed to the Tris buffer system, the SPR response for ExtrAvidin was lower than for the glycine buffer case when using the same AECPBA surface (compare Figures 3B and 3C). Note also with the use of Tris that the SPR response for ExtrAvidin is identical on boronic acid and control surfaces (Figure 3C), possibly indicating that non-specific interaction of ExtrAvidin with the carboxymethyl dextran matrix is the sole contributing interaction. It is also possible that the purported single-carbohydrate residue (N-acetylglucosamine) of deglycosylated avidin (e.g. ExtrAvidin) per protein subunit causes some of the interaction on the AECPBA surface.53–56
Figure 3.
Representative SPR sensorgrams during the binding of glycosylated protein avidin and the deglycosylated protein ExtrAvidin. (A) Avidin (5.29 × 10−6 M) versus ExtrAvidin (5.09 × 10−6 M) in pH 9.00 Tris-buffered saline on the AECPBA surface; (B) ExtrAvidin in pH 9.00 glycine-buffered saline and (C) ExtrAvidin in pH 9.00 Tris-buffered saline (right) on AECPBA (solid line) and hydroxyl-terminated control (dash-dot line) surfaces. ExtrAvidin concentrations were 4.94 × 10−6 M and 5.09 × 10−6 M in pH 9.00 glycine-buffered saline and pH 9.00 Tris-buffered saline. Protein binding was performed using 20 μL at 2 μL min−1. The running buffer was kept at a flowrate of 2 μL min−1. The AECPBA surface was prepared through direct EDC coupling.
Typically, it is discouraged to employ buffer systems (e.g. Tris) that can participate in coordination or esterification reactions with the boronate group,11 as this can prove detrimental to sugar binding. However, Mattiasson and co-workers demonstrated that Tris can suppress the interaction of the non-glycosylated protein chymotrypsin with boronate ions.32,57–58 They postulated that Tris has an affinity for the boronate ion that is intermediate to that of sugar diol groups (stronger) and amino acid residues (weaker); thus, Tris acts as a molecular shielder. In other words, Tris, by forming a tridentate complex with the boronate ions, essentially protects the boronates from interacting with amino acid residues of the non-glycosylated proteins but can be competitively displaced by the sugar in the glycoprotein so as to allow glycoprotein binding. In our work, we have observed a similar phenomenon of decreased non-glycosylated protein interaction when glycine buffer was replaced with Tris, even though the structures of ExtrAvidin and chymotrypsin are quite different. It can be surmised that the interaction of ExtrAvidin on the boronic acid surface involves coordination reactions and this interaction is in general likely to occur with any non-glycosylated protein; this should be diminished through use of Tris, as demonstrated here. However, one might suspect that the use of Tris will drastically affect the sensitivity or the responsivity of the surface for glycoprotein binding. As the results suggest, only a mere 30% reduction in the amount of avidin binding was observed. This is likely a consequence of competitive boronic acid binding of Tris versus the sugar chain in avidin. However, as is evident in Figure 3A, the larger SPR response of avidin compared to ExtrAvidin indicates that better specificity is achieved by using Tris buffer.
Outcomes from the systematic comparison between RNAse A and B and avidin and ExtrAvidin made here thus strongly suggest that the selectivity of boronic acids can be diminished by non-specific secondary interactions. In general, we infer that for boronic acid systems to be entirely discriminatory against non-glycosylated proteins, secondary interactions must be taken into consideration and should facilitate in determining experimental conditions that can increase the selectivity of boronic acid systems for glycoprotein analysis. In the work herein, a change in buffer system is found to have a profound impact on the selectivity of the boronic acid capture system.
CONCLUSIONS
Interaction analysis between glycoproteins and the boronic acid derivative, AECPBA, was evaluated using AECPBA-derivatized carboxymethyl dextran-coated gold substrates by surface plasmon resonance spectroscopy. Glycoprotein binding to the boronic acid surface was determined to be a function of the terminal saccharide moiety, information that is potentially useful in the design of surface-capture protein concentration devices and for studies on boronic acid interactions with glycoproteins on cell surfaces. Importantly, glycoproteins that are bound to the AECPBA surfaces can be removed readily using borate buffer at moderately elevated pH. The selectivity of immobilized boronic acids can be affected by non-specific secondary interactions, but these secondary interactions can be identified and decreased, thereby allowing for increased glycoprotein separation capability of boronic acid systems on surfaces.
Supplementary Material
Acknowledgments
The authors thank the US NSF and NIH for financial support of this work and Dr. Subramanian Balamurugan for his assistance with the SPR instrument.
Footnotes
Representative SPR sensorgram for the preparation of AECPBA and hydroxyl-terminated control surfaces by direct EDC coupling method. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Tian Y, Zhang H. Proteomics: Clin Appl. 2010;4:124–132. doi: 10.1002/prca.200900161. [DOI] [PubMed] [Google Scholar]
- 2.Orntoft TF, Vestergaard EM. Electrophoresis. 1999;20:362–371. doi: 10.1002/(SICI)1522-2683(19990201)20:2<362::AID-ELPS362>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Liu JT, Chen LY, Shih MC, Chang Y, Chen WY. Anal Biochem. 2008;375:90–96. doi: 10.1016/j.ab.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Kitano H, Anraku Y, Shinohara H. Biomacromolecules. 2006;7:1065–1071. doi: 10.1021/bm050782u. [DOI] [PubMed] [Google Scholar]
- 5.Anraku Y, Takahashi Y, Kitano H, Hakari M. Colloids Surf, B. 2007;57:61–68. doi: 10.1016/j.colsurfb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Soh N, Sonezaki M, Imato T. Electroanalysis. 2003;15:1281–1290. [Google Scholar]
- 7.Gabai R, Sallacan N, Chegel V, Bourenko T, Katz E, Willner I. J Phys Chem B. 2001;105:8196–8202. [Google Scholar]
- 8.Chen HX, Lee M, Lee J, Kim JH, Gal YS, Hwang YH, An WG, Koh K. Sensors. 2007;7:1480–1495. [Google Scholar]
- 9.Matsumoto A, Ikeda S, Harada A, Kataoka K. Biomacromolecules. 2003;4:1410–1416. doi: 10.1021/bm034139o. [DOI] [PubMed] [Google Scholar]
- 10.Singhal RP, Desilva SSM. Adv Chromatogr. 1992;31:293–335. [PubMed] [Google Scholar]
- 11.Liu X-C. Chin J Chromatogr. 2006;24:73–80. [PubMed] [Google Scholar]
- 12.Lorand JP, Edwards JO. J Org Chem. 1959;24:769–774. [Google Scholar]
- 13.Iwatsuki S, Nakajima S, Inamo M, Takagi HD, Ishihara K. Inorg Chem. 2007;46:354–356. doi: 10.1021/ic0615372. [DOI] [PubMed] [Google Scholar]
- 14.Lijuang Z, Haojie L, Pengyuan Y. Anal Bioanal Chem. 2010;396:199–203. doi: 10.1007/s00216-009-3086-0. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Kim TI, Kim KH, Kim JH, Choi MS, Choi HJ, Koh K. Anal Biochem. 2002;310:163–170. doi: 10.1016/s0003-2697(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Anzai J-I. Langmuir. 2005;21:5102–5107. doi: 10.1021/la050171n. [DOI] [PubMed] [Google Scholar]
- 17.Pribyl J, Skládal P. Anal Chim Acta. 2005;530:75–84. [Google Scholar]
- 18.Fujii E, Shimizu K, Kurokawa Y, Endo A, Sasaki S, Kurihara K, Citterio D, Yamazaki H, Suzuki K. Bunseki Kagaku. 2003;52:311–317. [Google Scholar]
- 19.Liu SQ, Bakovic L, Chen AC. J Electroanal Chem. 2006;591:210–216. [Google Scholar]
- 20.Wilson WD. Science. 2002;295:2103–2105. doi: 10.1126/science.295.5562.2103. [DOI] [PubMed] [Google Scholar]
- 21.Although 2 methods were used to create the AECPBA surfaces, the difference in their performance (in terms of protein binding) was not directly compared and evaluated. Eventhough this is the case, any observations that were compared against each other were obtained under identical conditions (e.g. surface is obtained from one method only).
- 22.Stenberg E, Persson B, Roos H, Urbaniczky C. J Colloid Interface Sci. 1991;143:513–526. [Google Scholar]
- 23.Davis TM, Wilson WD. Anal Biochem. 2000;284:348–353. doi: 10.1006/abio.2000.4726. [DOI] [PubMed] [Google Scholar]
- 24.Green NM, Anfinsen C, Jr, Edsall J, Richards F. Advances in Protein Chemistry. Vol. 29. Academic Press; New York: 1975. pp. 85–133. [DOI] [PubMed] [Google Scholar]
- 25.Delange RJ. J Biol Chem. 1970;245:907–916. [PubMed] [Google Scholar]
- 26.Huang TS, Delange RJ. J Biol Chem. 1971;246:686–697. [PubMed] [Google Scholar]
- 27.Bruch RC, White HB. Biochemistry. 1982;21:5334–5341. doi: 10.1021/bi00264a033. [DOI] [PubMed] [Google Scholar]
- 28.van der Merwe PA, Barclay AN. Curr Opin Immunol. 1996;8:257–261. doi: 10.1016/s0952-7915(96)80065-3. [DOI] [PubMed] [Google Scholar]
- 29.Lahiri J, Isaacs L, Tien J, Whitesides GM. Anal Chem. 1999;71:777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- 30.Koyama T, Terauchi K. J Chromatogr B. 1996;679:31–40. doi: 10.1016/0378-4347(96)00006-0. [DOI] [PubMed] [Google Scholar]
- 31.Bouriotis V, Galpin IJ, Dean PDG. J Chromatogr. 1981;210:267–278. [Google Scholar]
- 32.Li Y, Larsson EL, Jungvid H, Galaev IY, Mattiasson B. J Chromatogr A. 2001;909:137–145. doi: 10.1016/s0021-9673(00)01106-7. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XT, Wu YF, Tu YF, Liu SQ. Analyst. 2008;133:485–492. doi: 10.1039/b714896h. [DOI] [PubMed] [Google Scholar]
- 34.Maestas RR, Prieto JR, Kuehn GD, Hageman JH. J Chromatogr. 1980;189:225–231. [Google Scholar]
- 35.Zou Y, Broughton DL, Bicker KL, Thompson PR, Lavigne JJ. ChemBioChem. 2007;8:2048–2051. doi: 10.1002/cbic.200700221. [DOI] [PubMed] [Google Scholar]
- 36.Biacore Sensor Surface Handbook. Biacore: Uppsala; 2003. [Google Scholar]
- 37.Spiro RG. J Biol Chem. 1960;235:2860–2869. [PubMed] [Google Scholar]
- 38.Seligman PA, Schleicher RB, Allen RH. J Biol Chem. 1979;254:9943–9946. [PubMed] [Google Scholar]
- 39.Hovanessian AG, Awdeh ZL. Eur J Biochem. 1976;68:333–338. doi: 10.1111/j.1432-1033.1976.tb10819.x. [DOI] [PubMed] [Google Scholar]
- 40.Plummer TH, Jr, Hirs CHW. J Biol Chem. 1963;238:1396–1401. [PubMed] [Google Scholar]
- 41.Shang W, Nuffer JH, Dordick JS, Siegel RW. Nano Lett. 2007;7:1991–1995. doi: 10.1021/nl070777r. [DOI] [PubMed] [Google Scholar]
- 42.Nicholls MP, Paul PKC. Org Biomol Chem. 2004;2:1434–1441. doi: 10.1039/b312760e. [DOI] [PubMed] [Google Scholar]
- 43.Otsuka H, Uchimura E, Koshino H, Okano T, Kataoka K. J Am Chem Soc. 2003;125:3493–3502. doi: 10.1021/ja021303r. [DOI] [PubMed] [Google Scholar]
- 44.Pezron E, Ricard A, Lafuma F, Audebert R. Macromolecules. 1988;21:1121–1125. [Google Scholar]
- 45.Seligman PA, Schleicher RB, Allen RH. J Biol Chem. 1979;254:9943–9946. [PubMed] [Google Scholar]
- 46.Satomi Y, Shimonishi Y, Hase T, Takao T. Rapid Commun Mass Spectrom. 2004;18:2983–2988. doi: 10.1002/rcm.1718. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson B, Norden NE, Svensson S. J Biol Chem. 1979;254:4545–4553. [PubMed] [Google Scholar]
- 48.Irimura T, Nicolson GL. Carbohydr Res. 1983;115:209–220. doi: 10.1016/0008-6215(83)88016-1. [DOI] [PubMed] [Google Scholar]
- 49.Bolivar JG, Soper SA, McCarley RL. Anal Chem. 2008;80:9336–9342. doi: 10.1021/ac801750d. [DOI] [PubMed] [Google Scholar]
- 50.Martwiset S, Koh AE, Chen W. Langmuir. 2006;22:8192–8196. doi: 10.1021/la061064b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plummer TH., Jr J Biol Chem. 1968;243:5961–5966. [PubMed] [Google Scholar]
- 52.Lee CS, Belfort G. Proc Nat Acad Sci USA. 1989;86:8392–8396. doi: 10.1073/pnas.86.21.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livnah O, Bayer EA, Wilchek M, Sussman JL. Proc Nat Acad Sci USA. 1993;90:5076–5080. doi: 10.1073/pnas.90.11.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayer EA, Livnah O, Sussman JL, Wilchek M. Glycoconjugate J. 1993;10:276–277. [Google Scholar]
- 55.Bayer EA, Demeester F, Kulik T, Wilchek M. Appl Biochem Biotechnol. 1995;53:1–9. doi: 10.1007/BF02783477. [DOI] [PubMed] [Google Scholar]
- 56.Hiller Y, Gershoni JM, Bayer EA, Wilchek M. Biochem J. 1987;248:167–171. doi: 10.1042/bj2480167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Larsson E, Jungvid H, Galaev I, Mattiasson B. Chromatographia. 2001;54:213–217. [Google Scholar]
- 58.Li Y, Larsson EL, Jungvid H, Galaev IY, Mattiasson B. Bioseparation. 2000;9:315–323. doi: 10.1023/a:1011187724356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.