Abstract
Background
Patients with type I myotonic muscular dystrophy (DM1) are at risk for sudden death due to atrioventricular conduction block. We sought to characterize the trends and predictors of time dependent electrocardiographic (ECG) variations in patients with DM1.
Methods
Seventy patients with DM1 underwent standard electrocardiography at first evaluation and routine and symptom prompted follow-up. Individual variations in ECG conduction intervals were assessed using spaghetti plots. Clinical predictors of conduction disease progression were assessed using multivariate random effects regression models of panel data clustered by patient and adjusted for heart rate.
Results
Substantial individual variability was noted in time dependent changes in PR, QRS, and QTc intervals of patients with DM1. Changes in the QTc interval were closely associated with prolongation of the QRS interval. Age, the presence of paroxysmal atrial flutter or fibrillation, and the number of cytosine-thymine-guanine (CTG) repeats were independent positive predictors of time dependent PR and QRS prolongation during long-term follow-up. Female sex was negatively associated with PR prolongation but positively associated with QTc prolongation. Lower left ventricular ejection fraction was associated with greater QRS interval progression during long-term follow-up but was not predictive of PR interval progression.
Conclusions
Patients with DM1 can develop rapid changes in cardiac conduction intervals. Paroxysmal atrial flutter or fibrillation, older age, and larger CTG expansions predict greater time dependent PR and QRS interval prolongation and warrant particular attention in the arrhythmic evaluation of this high risk patient subset.
Keywords: electrocardiography, cardiomyopathy, myotonic muscular dystrophy, conduction disease, sudden death
Introduction
Myotonic muscular dystrophy or Steinert’s disease, the most common muscular dystrophy in adults, is an inherited multi-system disorder associated with myotonia, progressive skeletal muscle weakness, and sudden cardiac death.1, 2 Cytosine-thymine-guanine (CTG) repeats on chromosome 19 in the 3′ untranslated region of a serine-threonine protein kinase gene underlie type I myotonic muscular dystrophy (DM1), the more common form of the disease.3
The presence of severe baseline electrocardiographic (ECG) abnormalities are associated with sudden death in adults with DM1,2 and point to progression of atrioventricular block as the predominant mode of cardiac mortality in this population. However, limited data exists regarding longitudinal trends of ECG progression in patients with DM1. We sought to characterize the trends and predictors of time dependent ECG variations in a cohort of patients with DM1.
Methods
Patients and Protocol
The protocol was reviewed and approved by the Johns Hopkins Institutional Review Board. Consecutive DM1 patients referred to the electrophysiology service for arrhythmia risk stratification are enrolled in an open cohort registry. The current retrospective cohort study focused on a sub-sample of the overall cohort, which excluded patients with history of second or third degree atrioventricular block, persistent supra-ventricular arrhythmia, ventricular arrhythmia, or resuscitated sudden death. Seventy patients with DM1 confirmed by genetic testing (69%) or by clinical examination plus genetically proven DM1 in a first-degree family member (31%) were included in this analysis. CTG repeat sequence analysis was ascertained from leukocytes using a commercial assay (Athena Diagnostics, Worcester, MA). Clinical characteristics were recorded by review of history and physical examination notes from the cardiac arrhythmia clinic, and review of medical records. Symptoms of congestive heart failure were graded based upon the New York Heart Association (NYHA) classification system: class I = no limitation of activity; class II = mild limitation of activity; class III = marked limitation of activity; and class IV = unable to perform any activity without dyspnea and discomfort.
Electrocardiography
All patients underwent standard ECG at first evaluation and subject-specific follow-up clinic visits. A single reviewer defined the rhythm and measured intervals on initial and all subsequent ECGs. ECGs with evidence of pacing or supra-ventricular arrhythmia were excluded from analysis. PR, QRS, and QTc intervals were measured and recorded as continuous variables. A mean of 3.6 ECG examinations were performed on each patient.
Statistics
Continuous variables are summarized as mean ± standard deviation. Categorical and dichotomous variables are expressed as percentages. Spaghetti plots, exhibiting each individual patient’s PR, QRS, and QTc interval regression trend during follow-up, were created using subject specific time dependent data. The baseline association of QRS and QTc was assessed using the Spearman’s rank correlation test. Random effects models were fitted to examine associations between patient characteristics and time dependent progression of ECG changes. Multivariate random effects regression models with panel data clustered by subject and adjusted for heart rate were fitted with patient characteristics as dependent variables, and PR, QRS, and QTc intervals respectively, as independent variables. Time dependent QRS duration was added as a dependent variable to the multivariate random effects regression model of QTc prolongation to examine remaining predictors after adjusting for the effect of delayed conduction. P < 0.05 was considered statistically significant. Statistical analyses were performed using Stata software (Version SE 10.1, Stata Corporation, Texas, USA).
Results
Patient Characteristics
Baseline characteristics are summarized in Table 1. Three patients (4.2%) had a pacemaker placed prior to initial evaluation, without evidence of pacing on serial ECGs, and were included in the analysis. Two patients were excluded from analysis due to a paced rhythm. Forty four percent of patients were women. Mean age at initial evaluation was 45 years, and the mean number of CTG repeats was 453. Thirty percent of patients reported a family history of sudden death in a primary or secondary relative. Ninety one percent of patients had minimal or no symptoms of heart failure upon initial evaluation (NYHA class I). Nineteen percent of patients had paroxysmal atrial fibrillation or flutter at baseline. Of 70 patients in the analysis, 59 (84%) underwent echocardiography or cardiac magnetic resonance. In those patients, the mean left ventricular ejection fraction was 58% at baseline presentation.
Table 1.
Baseline characteristics of patients
| Variable (units) | Mean ± Standard Deviation or Percentage |
|---|---|
| Age (years) | 45±15 |
| Female (%) | 44 |
| CTG Repeats | 453±369 |
| Family History of Sudden Death (%) | 30 |
| HR (bpm) | 66±15 |
| PR Interval (ms) | 185±21 |
| QRS Interval (ms) | 113±27 |
| QTc Interval (ms) | 430±34 |
| NYHA Class I (%) | 91 |
| NYHA Class II (%) | 6 |
| NYHA Class III (%) | 3 |
| NYHA Class IV (%) | 0 |
| History of paroxysmal atrial fibrillation or flutter at initial evaluation (%) | 19 |
| History of prophylactic pacemaker implantation at initial evaluation (%) | 4.2 |
| Left Ventricular Ejection Fraction (%) | 58±12 |
Longitudinal Trends in PR Interval
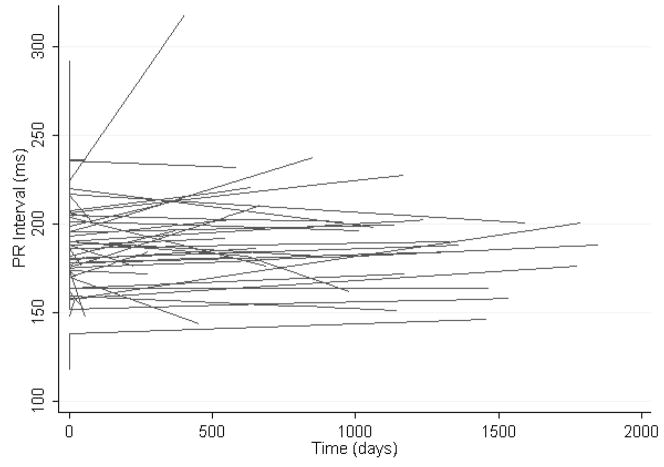
The mean PR interval at baseline was 185 ± 21 msec. After a mean follow-up time of 956 days, the mean PR interval was 191 ± 26 msec. Examination of the spaghetti plot in Figure 1 reveals extensive variability in individual PR interval trends during follow-up.
Figure 1.
The spaghetti plot illustrates heart rate unadjusted longitudinal regression lines exhibiting the time dependent PR interval trend for each individual patient with type I MMD.
Table 2 lists predictor variables in the random effects linear regression model of time dependent changes in PR interval. Time, age, the number of CTG repeats, and paroxysmal atrial flutter or fibrillation were independently and positively associated with PR prolongation during follow-up. In contrast, female sex was negatively associated with PR prolongation.
Table 2.
Predictors of longitudinal PR and QRS interval changes (after adjusting for heart rate) in the multivariate random effects regression model
| PR Interval |
QRS Interval |
|||
|---|---|---|---|---|
| Variable (unit) | Regression Coefficient | P | Regression Coefficient | P |
| Time | +8.7 msec / 1000 days | 0.009 | NS | |
| Age | +6.8 msec / 10 years | 0.001 | +9.3 msec / 10 years | <0.001 |
| Female | −16.5 msec | 0.047 | NS | |
| Number of CTG repeats | +3.3 msec / 100 repeats | 0.021 | +4.7 msec / 100 repeats | <0.001 |
| Family History of Sudden Death | NS | +18.1 msec | 0.006 | |
| NYHA Class | NS | NS | ||
| Paroxysmal atrial fibrillation or flutter | +44.8 msec | <0.001 | +14.9 msec | 0.027 |
| Left Ventricular Ejection Fraction | NS | −16.6 msec/ 10% increase | <0.001 | |
Longitudinal Trends in QRS Interval
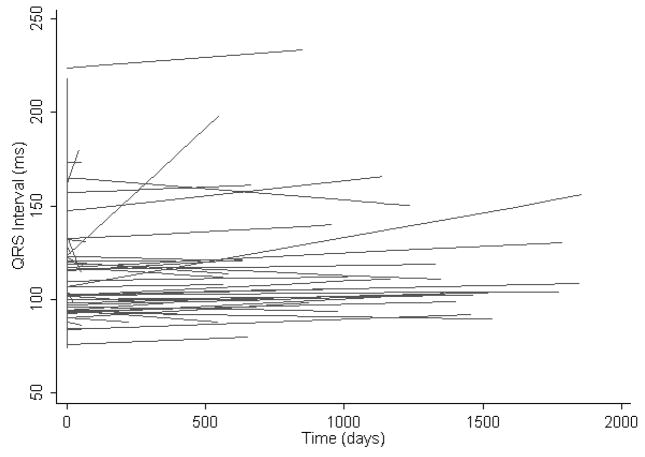
The mean QRS duration at baseline was 113 ± 27 msec. The majority of patients with QRS prolongation exhibited a nonspecific intraventricular conduction delay. Six patients had left bundle branch block, 2 had left anterior fascicular block, and 1 had right bundle branch block. After a mean follow-up time of 956 days, the mean QRS interval was 116 ± 33 msec. The most striking feature of the spaghetti plot in Figure 2 is the presence of select patients with large and rapid increases in QRS interval during follow-up.
Figure 2.
The spaghetti plot illustrates heart rate unadjusted longitudinal regression lines exhibiting the time dependent QRS interval trend for each individual patient with type I MMD.
Table 2 lists predictor variables in the random effects linear regression model of time dependent changes in QRS interval. Age, number of CTG repeats, family history of sudden death, and paroxysmal atrial flutter or fibrillation, were independently and positively associated with QRS prolongation during follow-up. Decreased left ventricular ejection fraction was associated with longer QRS duration.
Longitudinal Trends in QTc Interval
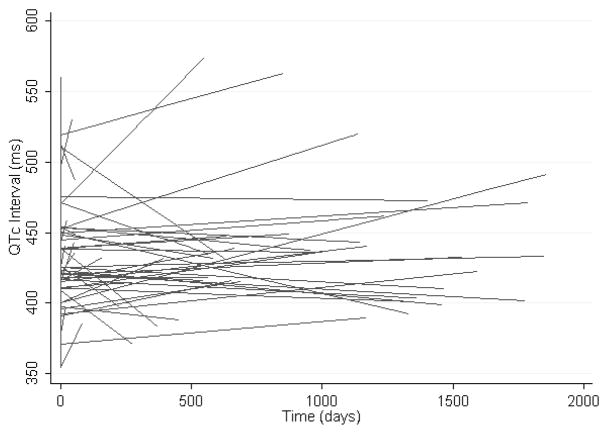
The mean QTc interval at baseline was 430 ± 34 msec. The baseline QTc and QRS were correlated (rho 0.48, P<0.001). After a mean follow-up time of 956 days, the mean QTc interval was 436 ± 44 msec. Examination of the spaghetti plot in Figure 3 reveals extensive variability in QTc interval trends during follow-up. Time dependent QRS prolongation (β=0.85, P<0.001) and female sex (β=28.5, P=0.002) were the only predictive variables in the random effects linear regression model of time dependent QTc prolongation.
Figure 3.
The spaghetti plot illustrates heart rate unadjusted longitudinal regression lines exhibiting the time dependent QTc interval trend for each individual patient with type I MMD.
Clinical Events during Follow-up
Four patients developed atrial fibrillation and 12 patients underwent pacemaker or implantable cardioverter defibrillator (ICD) implantation during follow-up. Of those who underwent device implantation, 1 patient underwent biventricular pacemaker-ICD implantation for development of ischemic cardiomyopathy, congestive heart failure, and left bundle branch block. One patient underwent biventricular pacemaker-ICD implantation for development of non-ischemic cardiomyopathy, congestive heart failure, and left bundle branch block. One patient underwent pacemaker implantation for syncope and progressive conduction system disease. Four patients underwent prophylactic pacemaker implantation for rapid progression of conduction system disease. Five patients underwent electrophysiology study for lightheadedness, syncope, or strong family history of sudden death; three of whom received ICDs for inducible ventricular tachycardia, one of whom received a pacemaker for HV interval prolongation, and one of whom received a pacemaker for prolonged sinus node recovery time. When compared to other patients in the cohort, those who received a pacemaker or ICD had greater PR and QRS prolongation during follow-up (by 15.6 ± 6.1 ms, P=0.013, and 19.2 ± 3.7, P<0.001, respectively) in univariate analyses.
Discussion
The main finding of this study is that clinical characteristics including age, the number of CTG repeats, presence of atrial tachyarrhythmia, and left ventricular ejection fraction appear useful in predicting the rate of conduction disease progression in patients with DM1.
Longitudinal Trends
Conduction abnormalities predominate among cardiac manifestations of DM1. Although overt left ventricular dysfunction is uncommon, sudden cardiac death can occur and is most commonly due to atrioventricular block.4 Previous studies have shown that group mean cardiac conduction intervals in patients with DM1 tend to increase over time.5–7 Our analysis highlights the extent of individual variability about mean population conduction interval trends (Figures 1–3) and underscores the importance of risk stratification for selection of high-risk patients with DM1.
Predictors of Conduction Disease Progression
Previous studies have determined that risk factors such as age > 40 years, ECG abnormalities, and atrial tachyarrhythmias are associated with cardiac events and sudden death in DM1.2, 6 A few studies have explored longitudinal progression of ECG abnormalities via assessment of population means,8 and some assessed the association of predictors such as CTG expansion size with binned mean ECG parameter progression.7 However, to the best of our knowledge, predictors of individual longitudinal ECG trends in DM1 patients have not been examined. Due to the clustered nature of longitudinal data, and to avoid spurious within subject correlations resulting from unobserved between-subject heterogeneity, random effects analysis is ideal for determining the predictors of longitudinal ECG progression in DM1.
Our analysis suggests that age and the presence of paroxysmal atrial flutter or fibrillation are independent positive predictors of PR and QRS prolongation during long term follow-up. Importantly, the presence of atrial tachyarrhythmia was an independent predictor of sudden death in a study of 406 patients with DM1.2 Our finding of the association of atrial tachyarrhythmia with progression of conduction system disease, along with the finding by Groh and colleagues of the association of atrial tachyarrhythmia with sudden death suggest that atrial tachyarrhythmia in DM1 patients acts as a strong surrogate of cardiac involvement and that such patients warrant close follow-up.
In our study, female gender was negatively associated with time dependent PR prolongation. As previously noted by Breton et al, prolongation of the QTc interval is primarily accounted for by QRS duration, likely representing delayed conduction rather than a true repolarization abnormality.9 However, after adjusting for the effect of time dependent QRS duration, female gender was positively associated with QTc prolongation during follow-up. These results suggest that in addition to gender dependent baseline variations in ECG (QTc) intervals, the PR interval is less likely to prolong, and the QTc interval is more likely to prolong during long-term follow-up of women with DM1. Importantly, however, previous large studies have not found an association between gender and clinical events in patients with DM1.2
The number of CTG repeats was positively associated with PR and QRS interval prolongation during long term follow-up of patients with DM1 in our study. These data are consistent with the finding by Clarke et al of a positive correlation between CTG expansion size and group means of the PR and QRS duration at binned follow-up in a cohort of DM1 patients.7 Previous data in a large kindred of DM1 patients also supports the finding that CTG expansion size may predict ECG abnormalities.10 In contrast, other studies including a large number of DM1 patients have not found an association between nucleotide repeat expansion size and conduction disease, and the issue warrants further study.2
Finally, as would be expected from a direct marker of ventricular (infra-Hisian conduction component) myocardial involvement, lower left ventricular ejection fraction was associated with higher QRS interval progression during long term follow-up but was not predictive of PR interval progression.
Study Limitations
ECG intervals reflect not only structural conduction system properties, but also dynamic autonomic states. Therefore, the atrioventricular nodal (reflected in the atrio-His interval) component of the PR interval and the QTc interval can vary substantially depending upon the individual’s autonomic state. Adjustment for heart rate in all statistical models partially corrected for some but not all variability introduced by the autonomic state. However, since autonomic variability is not unidirectional, the level of significance may be lowered but overall effect estimates are not likely to be affected.
The estimated residual intra-class correlation for the PR, QRS, and QTc random effects models were 0.37, 0.43, and 0.37 respectively. Thus 37, 43, and 37 percent, respectively, of the variance in longitudinal PR, QRS, and QTc intervals that are not explained by the covariates are due to time-invariant patient specific characteristics not contained in these models.
Clinical Implications
A significant proportion of sudden deaths in patients with DM1 likely occur due to progression of conduction system disease to complete atrioventricular block. Implantation of permanent pacemakers in DM1 patients with prolonged His-ventricular conduction times has prevented a significant number of brady-arrhythmias, that may have otherwise resulted in sudden death.11 However, invasive electrophysiology studies cannot be performed in all patients with DM1 and at best would provide only a single snapshot of His-ventricular conduction properties. Identification of clinical characteristics that predict the longitudinal rate of conduction disease progression may improve the non-invasive risk stratification of this high-risk patient subset.
Conclusion
Substantial individual variations exist in the development and progression of conduction system disease in patients with DM1. The presence of atrial flutter or fibrillation, older age, and larger CTG expansions are associated with both PR and QRS interval prolongation and warrant close follow-up and consideration of early permanent pacemaker implantation.
Acknowledgments
Funding Sources – Dr. Nazarian is funded by a National Heart, Lung, and Blood Institute Career Development Award (K23HL089333). Dr. Tomaselli is the Michel Mirowski Professor of Cardiology at Johns Hopkins University. The study was also supported by the PJ Schafer Memorial Research Award.
Footnotes
Disclosures – The authors report no conflicts of interest
References
- 1.Ashizawa T, Epstein HF. Ethnic distribution of myotonic dystrophy gene. Lancet. 1991;338(8767):642–3. doi: 10.1016/0140-6736(91)90659-d. [DOI] [PubMed] [Google Scholar]
- 2.Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E, Pourmand R, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358(25):2688–97. doi: 10.1056/NEJMoa062800. [DOI] [PubMed] [Google Scholar]
- 3.Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 4.Moorman JR, Coleman RE, Packer DL, Kisslo JA, Bell J, Hettleman BD, Stajich J, et al. Cardiac involvement in myotonic muscular dystrophy. Medicine (Baltimore) 1985;64(6):371–87. doi: 10.1097/00005792-198511000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Florek RC, Triffon DW, Mann DE, Ringel SP, Reiter MJ. Electrocardiographic abnormalities in patients with myotonic dystrophy. West J Med. 1990;153(1):24–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Colleran JA, Hawley RJ, Pinnow EE, Kokkinos PF, Fletcher RD. Value of the electrocardiogram in determining cardiac events and mortality in myotonic dystrophy. Am J Cardiol. 1997;80(11):1494–7. doi: 10.1016/s0002-9149(97)00742-x. [DOI] [PubMed] [Google Scholar]
- 7.Clarke NR, Kelion AD, Nixon J, Hilton-Jones D, Forfar JC. Does cytosine-thymine-guanine (CTG) expansion size predict cardiac events and electrocardiographic progression in myotonic dystrophy? Heart. 2001;86(4):411–6. doi: 10.1136/heart.86.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fragola PV, Luzi M, Calo L, Antonini G, Borzi M, Frongillo D, Cannata D. Cardiac involvement in myotonic dystrophy. Am J Cardiol. 1994;74(10):1070–2. doi: 10.1016/0002-9149(94)90864-8. [DOI] [PubMed] [Google Scholar]
- 9.Breton R, Mathieu J. Usefulness of clinical and electrocardiographic data for predicting adverse cardiac events in patients with myotonic dystrophy. Can J Cardiol. 2009;25(2):e23–7. doi: 10.1016/s0828-282x(09)70479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokgozoglu LS, Ashizawa T, Pacifico A, Armstrong RM, Epstein HF, Zoghbi WA. Cardiac involvement in a large kindred with myotonic dystrophy. Quantitative assessment and relation to size of CTG repeat expansion. Jama. 1995;274(10):813–9. [PubMed] [Google Scholar]
- 11.Lazarus A, Varin J, Babuty D, Anselme F, Coste J, Duboc D. Long-term follow-up of arrhythmias in patients with myotonic dystrophy treated by pacing: a multicenter diagnostic pacemaker study. J Am Coll Cardiol. 2002;40(9):1645–52. doi: 10.1016/s0735-1097(02)02339-2. [DOI] [PubMed] [Google Scholar]