Abstract
Background/Aim
Brain-gut dysfunction has been implicated in gastrointestinal disorders but a comprehensive test of brain-gut axis is lacking. We developed and tested a novel method for assessing both afferent anorectal-brain function using cortical evoked potentials (CEP), and efferent brain-anorectal function using motor evoked potentials (MEP).
Methods
CEP was assessed following electrical stimulations of anus and rectum with bipolar electrodes in 26 healthy subjects. Anorectal MEPs were recorded following transcranial magnetic stimulation (TMS) over paramedian motor cortices bilaterally. Anal and rectal latencies/amplitudes for CEP and MEP responses and thresholds for first sensation and pain (mA) were analyzed and compared. Reproducibility and interobserver agreement of responses were examined.
Results
Reproducible polyphasic rectal and anal CEPs were recorded in all subjects, without gender differences, and with negative correlation between BMI and CEP amplitude (r −0.66, p=0.001). TMS evoked triphasic rectal and anal MEPs, without gender differences. Reproducibility for CEP and MEP was excellent (CV <10%). The inter-rater CV for anal and rectal MEPs was excellent (ICC 97–99), although there was inter-subject variation.
Conclusions
Combined CEP and MEP studies offer a simple, inexpensive and valid method of examining bidirectional brain-anorectal axes. This comprehensive method could provide mechanistic insights into lower gut disorders.
Keywords: Brain-gut, Anorectal sensation, cortical evoked potentials, transcranial magnetic stimulation
Introduction
The enteric nervous system and the central nervous system play a critical role in the homeostasis of gut peristalsis, sensation and function [1]. The efferent and afferent neuronal pathways between the brain and the gut are intimately involved in mediating sensations and reflexes that govern both anal and rectal function [2,3]. It has been proposed that disturbed interactions between the efferent or afferent pathways and between the brain, rectum and pelvic floor may lead to common disorders of gastrointestinal function and defecation [4].
Neurophysiological tests can provide useful information regarding the integrity of neuronal innervation and neuromuscular function of the gut [1]. During the last few years, several new techniques have emerged that provide information regarding gut-brain communication(s) [5]. For example, brain function in response to visceral stimulation has been studied, using either Positron Emission Tomography (PET) or functional magnetic resonance imaging (fMRI), or magnetic encephalography (MEG) techniques [4,5]. Although useful, these tests are expensive, not widely available and not practical for routine use particularly in gastroenterology laboratories. Moreover, these techniques only assess the afferent gut-brain interactions.
Recently, cortical evoked potentials (CEP) have been introduced as a technique that may provide an objective and quantifiable tool to examine the connections between the afferent tracts, spinal cord, and the cerebral cortex [6]. CEP has advantages over other brain imaging techniques given its relative portability, inexpensiveness, availability, and use in ambulatory clinics. Recent studies have described CEPs from several regions of the gastrointestinal tract [6–10].
In contrast to the studies on afferent gut-brain axis, there is limited information regarding the assessment of the descending brain-gut axis. A novel approach has been to examine the motor evoked potentials (MEPs) of the rectum as well as those of the anal sphincter following magnetic stimulation of the motor cortex (transcranial magnetic stimulation, TMS) [4,5,11–13]. TMS (TMS) activates cortical neurons non-invasively by electromagnetic induction of interneurons and is considered safe, and associated with only minimal discomfort [14]. Originally developed by Barker et al (14), recent reports have described this technique for assessment of anal sphincters [15–19].
Whether CEP and TMS can be performed in the same individual, and whether such a combined assessment can provide information regarding the bidirectional brain-anal-brain and brain-rectal-brain axes is not known. If feasible, such an assessment may provide a simple, non-invasive method of bidirectional assessment of the brain-gut axis as well as provide novel information regarding the neurobiological mechanisms that regulate anal and rectal function.
Our aim was to develop a method for bi-directional assessment of anorectal-brain-anorectal function in humans and generate normative data. We tested whether electrical stimulation of the anus and rectum induces reliable cortical evoked potential (CEP) responses, and whether bihemispheric transcranial magnetic stimulation induces motor evoked potential (MEP) responses in the anus and rectum in healthy subjects. We also examined whether these tests provide reproducible measurements, and quantified the level of inter-observer agreement on the interpretation of CEP and MEP findings, and whether they are influenced by gender or BMI.
MATERIALS AND METHODS
Subjects
We recruited 26 healthy subjects (8 men, 18 women, mean age 39 years, range 19–56) through hospital advertisements. All subjects completed a questionnaire regarding their bowel function. None had gastrointestinal dysfunction, disturbance of defecation, history of previous surgery (except appendectomy) or were taking any medication (except aspirin, oral contraceptive or multivitamins). All participants had normal physical examination and gave written informed consent for a study protocol that was approved by the Institutional Review Board.
Methods
All subjects underwent neurophysiological assessments of both the anal-brain-anal axis and the rectal-brain-rectal axis using the techniques of cortical evoked potentials (CEP) and transcranial magnetic stimulation (TMS).
Rectal and Anal Cortical Evoked Potentials (CEP) after electrical Stimulation
We developed and designed an anorectal probe (Koningsberg, Pasadena, CA) containing two pairs of bipolar steel ring electrodes for the purpose of combined stimulation of the anal and rectal regions. Each pair of electrodes was 1 cm apart. When correctly positioned, the proximal pair was located at 10 cm from the anal verge and was used for rectal stimulation, and the distal pair was located at 1 cm from the anal margin and was used for anal stimulation. The electrical stimulus comprised of a square wave of 200 microseconds duration, which was produced by a constant-current, high-voltage stimulator (Cadwell Sierra II™, Kennewick, WA). Time-locked CEPs were then recorded using silver- silver chloride surface electrodes, with the active electrode positioned at the vertex (Cz) in accordance with the international 10–20 system, and a reference electrode located on the right ear lobe (20). The electrodes were applied using electrode paste and the impedance was kept below 5 kW. CEP data were acquired using a Sierra XP 3.3.3., Cadwell, Kennewick, WA and sampled at a frequency of 2000 Hz, with an epoch duration of 300 ms. The amplifier gain was set at 100,000, with on-line artifact rejection. The bandpass filter was set at 1 and 500 Hz, with a stimulus frequency of 0.2 Hz, and were chosen based on internal pilot studies and previously validated data (21–23).
Furthermore, to assess the role of mucosal electrode contact, we applied a 4 cm long balloon to the tip of the probe in order to enhance contact between the rectal sensor and the rectal wall in a small pilot study (n=3). CEPs were found to be similar with or without rectal balloon distension. Hence, further studies were performed without rectal balloon distension.
Rectal and Anal Motor evoked potentials (MEP) after TMS
We used the aforementioned specially designed anorectal probe to also simultaneously record anal and rectal MEP at rest following transcranial magnetic stimulation. We also recorded the MEP response also at rest from the anterior tibialis muscle using Ag/AgCl cup electrodes to serve as a control. The MEP responses from the anal sphincter, rectum, and anterior tibialis muscle were recorded using the Cadwell system described above, and by using filter settings of 5-2000 Hz, set at a sampling rate of 4–8 kHz.
Transcranial magnetic stimulation (TMS) of the cerebral cortex was performed using a magneticstimulator (Magstim 200; MAGSTIM, Whitland, UK) and either a 70 mm figure of eight coil (type 9790;MAGSTIM) or a Cadwell focal point coil. The coil was positioned over the cranium’s vertex, accordingto the international 10–20 system (20), and the area of the cortex stimulated by the coil was ~ 2 cm 2. The stimulation preferentially activated interneurons of the motor cortex through transsynaptic stimulation (11). Because cortical excitation depends on the coil’s orientation relative to the scalp, the coil was always placed with the anterior edge over the region of interest, with the handle pointing backward (24). The point for stimulation was similar to that described by Turnbull et al (11).
Protocols
Study 1 (Bidirectional assessment of brain-anorectal axis)
After an overnight fast, subjects attended our neurophysiology laboratory. With the subject seated, the location of vertex was marked on the scalp and the electrodes were affixed to the scalp as described previously (20). Next, with the subject lying in the left lateral position, the anorectal probe was inserted per-rectally into the rectum. The rectal pairs of electrodes were located at 10 cm and the anal pair at 1–3 cm from the anal verge. After a 5 minute period, the rectal and anal stimuli were delivered. First, we assessed the thresholds for first sensation and pain by gradually increasing the intensity of electrical stimulation in steps of 1 mA until they reported a first sensation and in steps of 2–4 mA until they reported a sharp or burning painful sensation. The electrical threshold intensity for evoking the CEP was set at 75% of the difference between the thresholds for first sensation and pain (22,23). CEPs were recorded with the patient in a quiet semi-darkened room in the lying position. Four runs of 50 stimuli each were performed for evoking the cortical responses for both the rectal and anal stimulation experiments. A 5-min rest period was allocated between each run, and a 15 minute rest period was allocated between the rectal and anal studies. The order of rectal or anal stimulation was randomized. To enhance the patients’ concentration and decrease artifacts, the subjects were asked to count the number of stimuli they had perceived, wear headphones to reduce extraneous noises, and to lie quietly.
After completing the CEP recordings, the scalp electrodes were removed, and after a 30 minute rest period, subjects were asked to sit on a reclining chair with the feet resting on a stool. Next, we stimulated the scalp by applying the magnetic coil at high intensity to multiple points on and around the vertex, moving the coil in 1cm increments laterally and up to 5cm from the midline, and as well as up to 3cm anterior or posterior to the vertex (11) until a site which evoked consistent and discernable EMG responses in the anus, rectum and tibialis was found. Once found, this was marked on the scalp. In order to determine the threshold intensity, the coil was discharged over this point at subthreshold intensities (50%, 1.1 Teslas) and slowly increased in 5% steps until we recorded an MEP response of >10μV for the anal and rectal responses and 100μV for the tibialis response, with at least 3/6 consecutive trials. This intensity was defined as the threshold intensity ± 20%.
Study 2: Reproducibility study
In order to assess the reproducibility of the CEP and MEP measurements, 6 subjects (3 male, 3 females, mean age 38 years) that underwent a baseline assessment were invited to undergo a second assessment (Test 2) after a 3 month period.
Study 3: Inter-observer agreement analysis
To evaluate the agreement between two independent blinded observers for both the CEP and the MEP recordings, we randomly selected the data from 8 healthy subjects. A total of 24 CEP traces and 48 MEP traces were examined independently by each observer who were asked to mark the onset of P1, N1, P2 and N2 (msec) as well as the amplitude of P1-N1 and P2-N2 (μV) for CEP recordings. Likewise, each observer was asked to mark the traces for the onset and amplitude (μV) of MEP recordings. A third investigator performed the measurements obtained from the two observers’ data.
Definitions of terms and Data analysis
Cortical Evoked Potentials
A typical CEP response comprises 4 wave components that were designated as P1, N1, P2 and N2 using conventional nomenclature (Figure 1). We defined the onset latency as the time interval in ms from triggering the stimulus to the onset of each component. The interpeak latency was defined as the time, in ms, between two consecutive CEP peaks, and the amplitude as the voltage difference in microvolts between two consecutive CEP peaks (7,22,23).
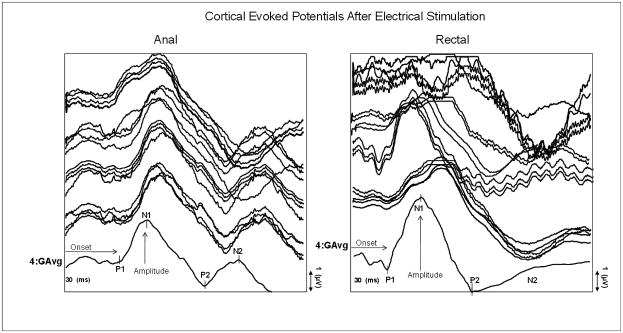
Figure 1.
This figure shows the average raw data for each of the four runs of 50 stimuli and the grand average (4G Avg) for the anal and rectal cortical evoked potential responses in a single subject. The P1, N1, P2, and N2 configuration of the CEP response is shown.
The four runs of CEPs from each subject were averaged to generate a grand average plot, which was then used to measure the CEP responses (Figure 1). The characteristics of the CEP response for both the anal and rectal stimulations were assessed independently by two experienced investigators (JR and KT). The CEP positive peaks were labeled as P1 and P2, negative peaks were labeled as N1 and N2 (Figure 1), and the data were verified by three additional investigators (TY, SR and SH). The latency, inter-peak latency and amplitude for both the anal and the rectal CEP responses from each subject were averaged to obtain the group mean data.
Motor Evoked Potentials after TMS
We defined the MEP latency as the interval from the onset of stimulus to the onset of the individual rectal or anal motor response, expressed in milliseconds (Figure 2). For analysis, we identified the shortest three latencies evoked from each side of the brain and for each recording site (anal and rectal), and the mean of these values were calculated. The amplitude was the peak-to-peak voltage of the MEP response and was expressed in microvolts. A mean amplitude value for each subject was calculated by averaging the three largest responses. Data were analysed by two investigators (AT, KT) who were blinded to the order of stimulations. The data were verified by two investigators (SR and SH) and any discrepancies were reviewed by consensus and expert advice from another investigator (TY).
Figure 2.
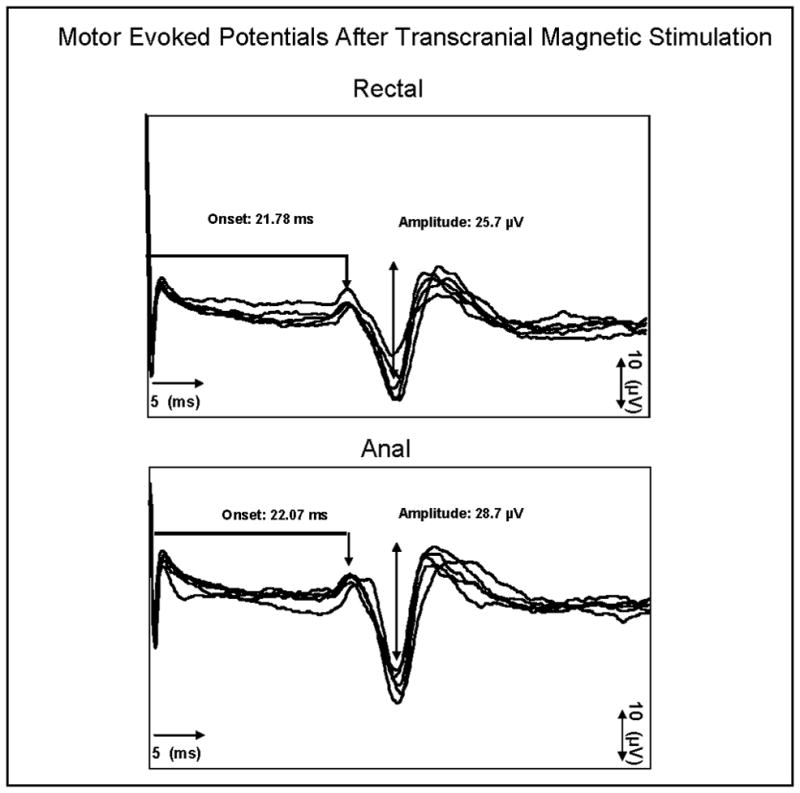
This figure shows the data for five individual motor evoked potential (MEP) responses recorded from the anus and rectum, after transcranial magnetic stimulation of the brain in one healthy subject. The method used for calculating the onset (milliseconds) and amplitude (μV) of responses are also shown.
Statistical Analyses
Data are expressed as mean ± SD, and 95% confidence interval (CI) when appropriate. The latencies and amplitudes for each component of the CEPs and MEPs were compared between men and women using the Mann Whitney U-test. The paired t-test or Wilcoxon signed rank test was used to compare the anal and rectal CEPs (latency and amplitude) and the anal and rectal MEPs (onset latency and amplitude). Pearson or Spearman correlation coefficients were used to examine the relationship between each component of the CEP and MEP its correlation with height, weight and BMI. A p value of < 0.05 was considered statistically significant.
In order to assess reproducibility of the CEP, Bland-Altman plots were performed, and the coefficient of variation (CV) was calculated. The inter-observer data were analyzed by examining the coefficient of variation (CV), and the variation among subjects by examining the intraclass correlation coefficient (ICC).
RESULTS
Study 1
Demographics
Twenty-six subjects, (8 men, 18 women) with a mean age of 39 years (range 19–56) participated. There were no differences between men and women with regard to age (36.25 ± 15 vs. 40.83 ± 12.7 years, p=0.472) or body mass index (BMI) (24.7 ± 2 vs. 24.9 ± 4, p=0.82). Men tended to have higher weight (76.3 ± 8 vs. 67.7± 12 kgs, p=0.07) and were significantly taller (175 ± 6 vs. 164 ± 7 cms, p=0.003) than women.
Rectal and Anal Cortical Sensory Thresholds
The mean electrical stimulus intensity for a first sensation and pain and for both the rectal and anal stimulation are shown in Table 1. Most of the subjects reported a dull and sometimes burning or prickly sensation at lower intensity and a sharp, painful sensation at higher intensity. Occasionally, jerky movements were seen or felt by the subject. The mean stimulus intensity for a first sensation in the anus and in the rectum was similar between men and women (Table 1). Overall the mean sensory threshold for the first sensation in the anal canal was significantly lower (p=0.001) when compared to the first sensation in the rectum. We found no correlation between BMI and rectal (r=−0.12) and anal (r=0.11) pain thresholds.
Table 1.
Sensory Thresholds for first sensation and pain following Rectal and Anal Electrical Stimulation
| Rectal | Anal | |||
|---|---|---|---|---|
| First sensation (mA) | Pain (mA) | First sensation (mA) | Pain (mA) | |
| Men | 24 ± 19.3 (10–35) | 43 ± 28 (10–60) | 7.68 ± 1.7 * (5–10) | 39.6 ± 22.7 (15.5–55) |
| Women | 19.4 ± 3 (8–35) | 51.7 ± 21 (22–88) | 9.36 ± 7.2 * (3–34) | 40.3 ± 21.08 (18–84) |
| Overall | 20 ± 13.2 (8–35) | 49 ± 23.9 (8–88) | 8.8 ± 5.8 * (3–34) | 40.17 ± 21.2 (15.5–84) |
Data expressed as mean ± SD, (95% CI).
p=0.0001 anal versus rectal first sensation in men and women and in combined group.
Rectal and Anal Cortical Evoked Potentials
A reproducible polyphasic CEP pattern was recorded in all subjects both after anal and rectal stimulation. Typical examples of the morphology of CEP response, following anal and rectal stimulation are shown in Figure 1. The latencies and amplitudes of each component of the CEP are shown in Table 2. There were no differences among anal and rectal CEP components between men and women subjects (p>0.20). The amplitude of the P1-N1 wave evoked after rectal stimulation was higher than that evoked after anal stimulation (4.68 ± 3.02 vs. 3.15 ± 2.11, p=0.04).
Table 2.
Latencies and amplitude of each component of the CEP response following Rectal and Anal Stimulation
| Men (n=8) | Women (n=18) | Overall (n=26) | |
|---|---|---|---|
| RECTAL CEP | |||
| P1 latency (ms) | 47.7 ± 14.6 (41.0–56.0) | 54.7 ± 13.13 (33.7–82.1) | 52.6 ± 13.7 (33.7–82.1) |
| N1 latency (ms) | 87.7 ± 26.5 (76.8–110.6) | 90.5 ± 19.5 (71.2–134.5) | 89.6 ± 21.4 (71.2–134.5) |
| P2 latency (ms) | 150.9 ± 47.5 (99–221) | 155.3 ± 44.2 (82.3–235.7) | 154.1 ± 44.2 (82.1–237.5) |
| N2 latency (ms) | 192.1 ± 54.1 (142–299.5) | 201.1 ± 56.2 (99.8–299) | 198.49 ± 54.7 (99.8–299.5) |
| P1-N1 Amplitude (μV) | 4.6 ± 3.3 (1.9–8.5) | 4.7 ± 3 (0.4–11.1) | 4.7 ± 3.1 * (0.5–11.1) |
| P2-N2 Amplitude (μV) | 1.7 ± 1.1 (0.6–7.1) | 2.9 ± 1.8 (0.3–8.4) | 2.5 ± 19.1 (0.3–8.4) |
| ANAL CEP | |||
| P1 latency (ms) | 59.2 ± 30.9 (40.7–83.4) | 61.3 ± 26.4 (37.1–112.1) | 60.6 ± 27.2 (37.1–112.1) |
| N1 latency (ms) | 99.7 ± 39.2 (61.4–126.5) | 104.2 ± 32.1 (60–153.7) | 102.8 ± 33.7 (60–153.7) |
| P2 latency (ms) | 132.8 ± 57.8 (79.2–220.3) | 166.3 ± 58.4 (89.5–282.1) | 156.1 ± 59.1 (79.2–282.2) |
| N2 latency (ms) | 163.6 ± 65.4 (100.8–267.6) | 201.1 ± 57.7 (108.7–299.5) | 189.6 ± 61.2 (100.7–299.5) |
| P1-N1 Amplitude (μV) | 3.4 ± 2.9 (0.6–9.2) | 3.1 ± 1.7 (1.1–6.1) | 3.2 ± 2.1 (0.6–9.2) |
| P2-N2 Amplitude (μV) | 1.7 ± 1.1 (0.4–3.8) | 1.7 ± 1.9 (0.1–6.7) | 1.7 ± 1.1 (0.1–6.7) |
rectal versus anal p=0.04.
There was no correlation between the height of a subject and each individual component of the CEP response. For example the correlation coefficient for height and rectal P1-N1 amplitude was −0.2, p=0.17 and for anal P1-N1 amplitude was −0.06, p=0.7. However, there was a significant negative correlation between BMI and the rectal P1-N1 amplitude (r −0.42, p=0.04) and rectal P2-N2 amplitude (r=−0.66, p=0.001). Also, a negative correlation was observed between BMI and the anal P1-N1 amplitude (r=−0.44, p=0.018).
Motor Evoked Potentials after TMS
In all subjects, cortical stimulation (mean threshold intensity, 1.9 ± 1.1T) evoked a biphasic or triphasic electromyographic response both in the anus and in the rectum. Typical examples of the MEP response are shown in Figure 2. In general, we were able to evoke MEP responses from both muscle groups by stimulating areas within 1–3 cm posterior and lateral to the vertex. The anal and rectal MEP responses could be evoked after stimulation of either cerebral hemisphere, indicating a bilateral representation. In 19 subjects (73%) the rectal MEP were evoked from both hemispheres, in 5 (19%) from the left hemisphere only, and in 2 (8%) from the right hemisphere only. Similarly, in 18 subjects (70%) the anal MEP responses were evoked from both hemispheres, in 4 (15%) from the left hemisphere only, and in 4 (15%) from the right hemisphere only.
The onset latencies and amplitudes (peak to peak) of each component of the MEP response are shown in Table 3. There were no differences between men and women (p>0.20) for either the anal or rectal MEPs. There were no differences in the latency between the anal and the rectal responses.
Table 3.
| Responses from Left Hemisphere | Responses from Right Hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Rectal MEP | Anal MEP | Rectal MEP | Anal MEP | |||||
| Onset (ms) | Amp (μV) | Onset (ms) | Amp (μV) | Onset (ms) | Amp (μV) | Onset (ms) | Amp (μV) | |
| Male | 22.4±1.9 (20.1–24.7) | 25.8 ± 17.1 (10–50) | 22.4 ± 6.2 (16.1–31.7) | 37.0 ± 27.6 (10–70) | 21.8 ± 4.7 (18.4 –30 ) | 21.2 ± 11.2 (10 –49 ) | 21.7 ± 5.14 (13.5 –28.1) | 21.8 ±17.3 (10–40) |
| Female | 20.9 ± 4.8 (11.3–28.2) | 20.6 ± 15.6 (10–60) | 22.2 ± 4.3 (16.83–32.66) | 33.2 ± 63.6 (10–24) | 19.90 ±6.2 (10.8 –33.9 ) | 25.5 ± 30.4 (10 –11 ) | 21.6 ± 4.6 (15.14–29.61) | 21.7 ±14.0 (10 –40 ) |
| Overall | 21.2 ± 4.3 (113–28.2) | 21.9 ± 15.5 (10–60) | 22.3 ±4.8 (16.1–32.6) | 34.4 ± 53.1 (10–24) | 20.5 ± 5.7 (10.8 –33.9 ) | 52.5 ±12.0 (10 – 49 ) | 21.7± 4.6 (13.5 –29.6 ) | 21.7 ±14.6 (10 –40 ) |
MEP=Motor evoked potentials. AMP=Amplitude
The mean bi-hemispheric MEP latencies and amplitudes detected in the anus and rectum. Data are separated for the responses from each cortical hemisphere and also by gender.
There were no correlations between the height of an individual and each component of the MEP response. For example the correlation coefficient for height and rectal MEP amplitude was 0.15, p=0.46 and for anal MEP amplitude was 0.09, p=0.65. However, there was a significant negative correlation between BMI and the rectal MEP amplitude (r= −0.45, p=0.03), but not for the anal MEP amplitude (r=−0.14, p=0.5).
Study 2
Reproducibility of CEP and MEP
Bland-Altman plots showed good agreement between tests 1 and 2 for both the anal and rectal CEP and the MEP latencies in 6 subjects, figure 3. The amplitude of CEP- anal and CEP-rectal responses as well as the amplitude of the anal and rectal MEP responses were well correlated, with all data points falling within 2 standard deviations when plotted on Bland-Altman plots. CEP latency response during study 1 and study 2 was 60.7 ± 15.8ms and 56.9 ± 13.0 ms and for CEP amplitude was 3.5 ± 2.0 μV and 2.7 ± 2.0 μV and for rectal CEP latency was 59.3 ± 7.7ms and 58.7 ± 7.0ms and for rectal CEP amplitude was 4.4 ± 3.6 μV and 4.3 ± 2.9 μV respectively. The mean ± s.d for the left side anal MEP measurements following TMS during study 1 and study 2 were as follows; 21.7 ± 1.3 ms and 21.2 ± 1.7 ms (anal TMS latency), 19.0 ± 8.9 μV and 14.0 ± 5.5 μV (anal TMS amplitude), and 21.5 ± 2.2 ms and 20.9 ± 1.5 ms (rectal TMS latency), and 20.0 ± 7.1μV and 20.0 ± 7.1μV (rectal TMS amplitude). Because data for the right side were similar, only data from the left side is shown here.
Figure 3.
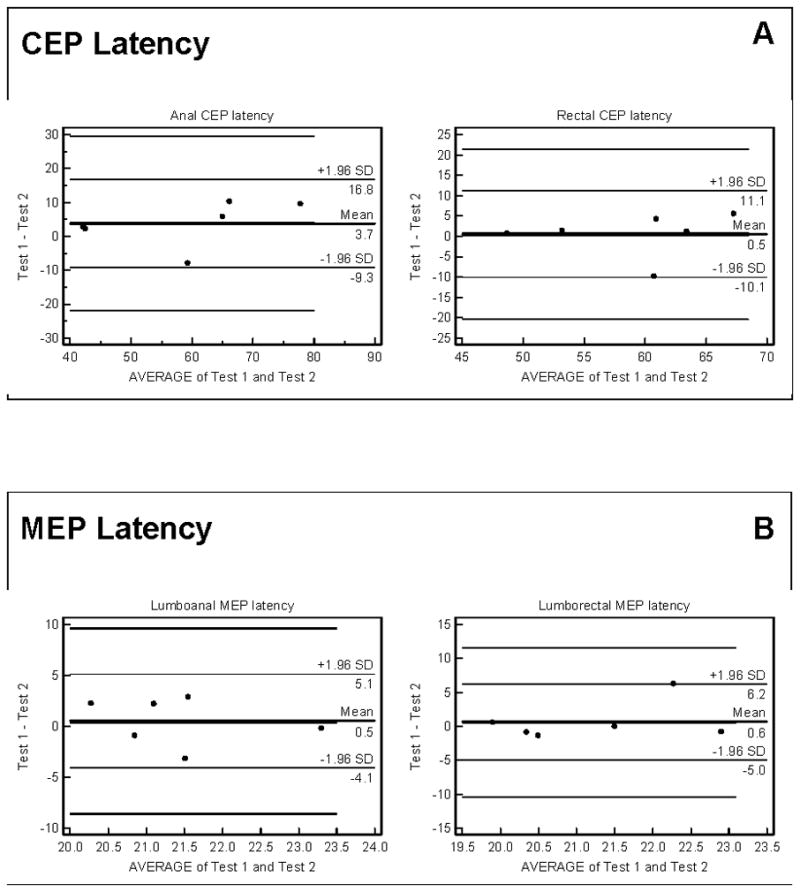
Bland-Altman plots showing the reproducibility of anal and rectal latency of CEP (Panel A) and the latency of the MEP responses following left side transcranial magnetic stimulation (Panel B).
Study 3
Interobserver agreement
A total of 24 sets of CEP and 48 sets of MEP recordings from 8 subjects were assessed. The results of inter-rater standard deviation (inter-rater SD), inter-rater coefficient of variation (inter-rater CV) and intraclass correlation (ICC) are shown in Table 4.
Table 4.
Interobserver analysis for Rectal and Anal CEPs and MEPs
| Anal (mean ± 95%CI) | Rectal (mean ± 95%CI) | ||||||
|---|---|---|---|---|---|---|---|
| Inter-rater SD | Inter-rater CV (%) | ICC | Inter-rater SD | Inter-rater CV (%) | ICC | ||
| P1 onset (ms) | 8.2 (6.1–12.5) | 11.8 (8.8–18.0) | 0.87 | 2.7 (2.0–4.0) | 4.5 (3.4–6.6) | 0.98 | |
| N1 onset (ms) | 5.2 (3.9–7.8) | 4.6 (3.4–6.8) | 0.96 | 3.5 (2.7–5.2) | 3.5 (2.7–5.1) | 0.98 | |
| P1-N1 amplitude (μV | 25 (18–38) | 15.6 (11.6–23.7) | 0.83 | 30 (22–50) | 17.6 (12.6–29.1) | 0.78 | |
| MEP after Left TMS | Onset | 1.0 (0.8–1.2) | 6.4 (5.6–7.7) | 0.66 | 1.2 (1.0–1.4) | 7.7 (6.6–9.0) | 0.83 |
| Amp | 3.6 (3.1–4.3) | 8.7 (7.5–10.4) | 0.99 | 8.0 (6.9–9.5) | 23.3 (20.1–27.7) | 0.93 | |
| MEP after Right TMS | Onset | 1.4 (1.2–1.7) | 9.2 (7.9–11.0) | 0.33 | 1.1 (0.9–1.3) | 7.5 (6.4–9.0) | 0.53 |
| Amp | 8.6 (7.5–10.3) | 19.1 (16.3–23.0) | 0.97 | 6.7 (5.8–8.1) | 18.2 (15.6–21.8) | 0.97 | |
Interpretations for the rectal and anal CEP were similar between observers, but the inter-subject variation between individual data set was relatively high. The inter-rater CV for the anal and rectal MEP was excellent (ICC 97–99).
DISCUSSION
Efferent and afferent neuronal interactions between the brain and gut are intimately involved in mediating sensations, movements and reflexes, and regulating normal anal and rectal function [2,3]. In this study, we have detailed new and validated techniques for performing a comprehensive evaluation of the anorectum-brain-anorectum axis by stimulating and recording both the afferent and efferent evoked potentials in the same subject. Whilst other groups have employed such techniques before in exploring the brain-gut visceral system [5–13,17–19], in this study, we have examined bi-directional brain-anorectal interactions as well as the influence of gender, BMI, reproducibility, and inter-rater differences for the analyses of CEP and MEP responses.
Cortical evoked potentials (CEPs) provide objective data regarding the integrity of the sensory system, are more sensitive than clinical evaluation, because of excellent temporal (millisecond) resolution, and allows characterization of the afferent pathways and cortical processing. Although previous studies have described CEPs after rectal balloon [6,9] or electrical stimulation of anal canal [7,8,26,27,28], here we describe CEP assessments following both rectal and anal stimulations and in the same subject.
In order to facilitate co-localized recordings, we designed a novel probe containing 2 pairs of bipolar steel ring electrodes that were located in the rectum and anal canal. Previous studies using platinum probes required augmentation by an adjacent balloon because of electrical signal loss [7,28]. In our study, we found excellent CEP responses in all individuals without augmentation. Besides CEP, this probe allowed us to evaluate both anal and rectal thresholds for first sensation and pain, after electrical stimulation.
The sensory thresholds for first sensation were significantly lower in the anal canal when compared to the rectum, both in women and men. This difference is possibly due to the more specialized sensory organs, such as the Krause end-bulbs, Golgi–Mazzoni bodies and genital corpuscles, in the upper anal canal as well as better contact with the electrodes within a sphincter zone [2,3]. Although there are no specialized receptors in the rectal mucosa, there is recent evidence that rectal sensation arises from the stimulation of intraganglionic laminar nerve endings [29]. The mean threshold value for first anal perception in our study was similar to another study [26], but different to that reported by other groups [30,31], possibly because of differences in the size of probe, intensity of stimulation [30], and location of sensor emphasizing a need for standardizing these techniques.
The sensory information from the rectum is conveyed via the rectospinal (visceral) afferents that ascend predominantly via parasympathetic pathways to reach the dorsal horn of the spinal cord, whereas the sensory pathway from the anal canal is via the (somatic) pudendal nerves, although there may be some overlap in the transition zone [3]. Both pathways are derived from the sacral plexus (S2, S3 and S4), and hence are closely related. Thus, a comprehensive evaluation of the rectal and anal CEP may allow us to better characterize the afferent anorectal pathway.
A reproducible polyphasic CEP pattern that comprised of 4 wave components, and designated as P1, N1, P2 and N2 were recorded in all subjects, both after anal and rectal stimulation. The CEP morphologies were similar to those observed in previous studies [6,7,8,26–28]. The first CEP latency observed in our study was ~50 milliseconds in the rectum and ~60 milliseconds in anal canal. In previous studies, the onset latencies for the first rectal CEP component have ranged between 34 to 280 milliseconds, and for the first anal CEP component between 43 to 69 milliseconds [6,7,8,26–28]. These differences are most likely due to different protocols and settings among studies, the use of different sites and types of stimulation (electrical or mechanical), and even due to different nomenclature for the wave analysis. However, our findings are consistent with those reported by other groups using similar protocols and after electrical stimulation. It has been argued that electrical stimulation will stimulate all afferents within 200 μseconds, resulting in excellent synchronization between the stimulus and afferent nerve discharge [28]. In contrast, during mechanical stimulation, the balloon inflates over 165 ms resulting in relatively poor synchronization between the stimulus onset, afferent nerve discharge and the onset of CEP averaging. Therefore, CEPs of lower amplitude are recorded [28]. Also, electrical impulses will stimulate all the rectal nerves nonselectively, resulting in a larger signal reaching the central nervous system.
Interestingly, the latency of rectal CEP was shorter than the corresponding latency from the esophagus or duodenum [7]. One reason could be that rectal stimulation activates afferent pathways with faster conduction velocities than other parts of the GI tract. Previous investigators have suggested that electrical stimulation in the rectum activates the faster-conducting A-beta fibers in the pudendal nerve [7,32], leading to a shorter CEP latency than would occur if CEP were mediated via rectal afferent A-delta fibers in the pelvic nerve.
In our study, the onset latencies for the components of the anal and CEP responses were similar, and this could be explained by the fact that sensory information from the rectum and anus are closely related anatomically at the sacral plexus, and they may overlap. However, the amplitude of the CEP response after rectal stimulation was higher (p=0.04) when compared to that evoked after anal stimulation and was similar to that reported previously [6,7,8,26–28]. This could be explained because of differential sensory innervation, of the anal mucosa [2,3] and the rectum [29].
We found no differences between men and women (p>0.20) in anal CEP components confirming a previous study [27]. Likewise, we found no difference in rectal CEP. Although, we did not find any correlation between the height of a subject and individual CEP components, there was a significant negative correlation between BMI and both rectal and anal CEP amplitudes and these are new findings.
Recent reports of TMS [33] have described the conduction velocities and response characteristics of the anal sphincter after diffuse stimulation of the cortex [11–13]. In our study, we not only recorded anal sphincter MEP after cortical TMS, but simultaneously recorded rectal MEP. We confirmed previous observations [11] that onset latencies for anal and rectal MEP were similar. However, unlike previous study, here we assessed them simultaneously and believe that such an evaluation can provide new and integrated information regarding the abnormalities in the complex gut-brain axis. In all subjects, cortical stimulation evoked a biphasic or triphasic electromyographic response both in the anus and rectum. We found that anorectal responses were bilaterally represented on the superior motor cortex of both cerebral hemispheres, although 30% of subjects had some degree of hemispheric predominance.
Previous studies have shown that latencies for MEP and CEP correlate with height suggesting that height is an important variable for defining normality, but mainly in the lower limbs [34,35,36]. This observation suggests that length of the central somatosensory pathway could be proportional to the subject’s height. However, the effect of height on ano-rectal CEP and MEP has not been previously studied. We found that there were no correlations between the height of a subject and each MEP component, but a significant negative correlation between BMI and both CEP and MEP amplitudes.
In a recent study, Deiner et al [37] found that larger BMI is significantly associated with failure to obtain MEP recordings from the lower limbs. This suggests that adiposity may influence the results of neurophysiological testing. BMI is a comprehensive measure of size and is considered to be the best proxy for body fat percentage among ratios of weight and height [38]. In our study, BMI was the only parameter that affected cortical and motor evoked responses suggesting that adiposity influences ano-rectal CEP and MEP values. The exact reason is unclear, but may relate to local population such as greater levels of adipose tissue in the perineum.
Recent studies have also shown that obesity plays an important role in the pathophysiology of functional gastrointestinal disorders [39,40,41]. For example, Delgado-Aros et al [41] found that a high BMI (>25 kg/m2) is associated with decreased colonic compliance and pain sensation; suggesting that obesity may induce colonic dysfunction. However, whether afferent, spinal, and central mechanisms are involved is not known.
It is important to assess the reliability and reproducibility for any new technology. Evaluation in six subjects found that the tests were highly reproducible both for the CEP and MEP latencies and amplitudes. Also, we systematically examined the inter-observer agreement/disagreement with regards to the interpretation of the rectal and anal MEP data. In 72 studies that were independently assessed and analyzed, the inter-rater agreement for almost all parameters was excellent. Previously, it has been reported that the methodology for CEP could be operator-dependent [25]. We did not find this to be the case. However, we found that the inter-subject variation between individual data set was (not surprisingly) high, reflecting normal intrinsic variation among subjects. These observations provide confidence that the measurable data are robust and reproducible.
During the last few years, several new techniques have been developed that provide information regarding the brain-gut axis in humans [4,5]. Although some neurophysiological tests, such as PET, fMRI, and MEG, provide useful information regarding the integrity of the sensorimotor pathways of the anorectum, the clinical utilization of these tests is limited because they are expensive, time consuming, and are not widely available. Moreover, these techniques only assess a part of the gut brain interactions (either the afferent or the efferent) but not the whole tract. Such drawbacks are overcome by the proposed technique.
The equipment for recording CEPs and MEPs are generally available in neurophysiological laboratories and can be adapted for gastrointestinal use. Hence, the proposed methodology represents a significant advance over current techniques and is ideally suited for conducting large-scale clinico-pathophysiological studies that are necessary for a beter understanding of the brain-gut interactions in disorders such as constipation, fecal incontinence or IBS. In addition, this technique could provide an objective way of verifying changes following therapeutic interventions, for example before and after biofeedback therapy.
The limitations of these techniques include the inability to study subjects who have metal devices or prosthesis [42]. Also, TMS is potentially risky in patients with a history of epilepsy or seizures. Also, there is likely to be some experience dependency in applying this technology to patients.
In conclusion, combined CEP and MEP studies offer a simple, inexpensive, reproducible and valid method of examining bidirectional brain-anorectal axes in human subjects. These evaluations can provide useful neurophysiological information regarding normal and abnormal brain-gut interactions.
Supplementary Material
Acknowledgments
Grant Support: This research was supported in part by grant R01DK 57100-03 National Institute of Health and NIH-NCRR 1UL1RR024979, 1KL2RR024980 & 1TL1RR024981 – University of Iowa Clinical and Translational Science Program. Dr Remes-Troche was supported by the AGA, Jon I. Isenberg International Scholar Award. This research is registered with Clinical Trials at www.clinicaltrials.gov and the registration number is NCT00988286. This work was presented at Digestive Disease Week, Chicago, Illinois, 2009 and published as an abstract in Gastroenterology 2009; 136 A778. We sincerely appreciate secretarial support of Mrs. Kimberly Klein and statistical support of Bridget Zimmerman, PhD Institute for Clinical and Translational Science and Carl Kice Brown, M Sc.
Footnotes
Guarantor: Satish SC Rao – Study concept and design, data acquisition, data collection, study recruitment, data analysis and interpretation, manuscript preparation, critical revision, important intellectual content and final approval.
Authors Conflicts of Interests: No conflicts of interest exist
Contributing Authors:
Jose M. Remes-Troche - Study concept and design, data acquisition, data collection, study recruitment, data analysis and interpretation, manuscript preparation, critical revision, important intellectual content and final approval.
Kasaya Tantiphlachiva - Data acquisition, data collection, data analysis, manuscript preparation.
Ashok Attaluri - Data acquisition, data collection, data analysis, manuscript preparation.
Jessica Valestin- Data acquisition, data collection and study recruitment.
Thoru Yamada - Study concept and design, data interpretation, manuscript preparation.
Shaheen Hamdy – Study design, data analysis and interpretation, manuscript preparation, and critical revision.
References
- 1.Remes-Troche JM, Rao SS. Neurophysiological testing in anorectal disorders. Expert Rev Gastroenterol Hepato. 2008;2:323–35. doi: 10.1586/17474124.2.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao SSC. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126:S14–S22. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559–578. doi: 10.1016/s0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- 5.Hobson AR, Aziz Q. Brain imaging and functional gastrointestinal disorders: has it helped our understanding. Gut. 2004;53:1198–1206. doi: 10.1136/gut.2003.035642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loening-Baucke V, Read NW, Yamada T. Cerebral evoked potentials after rectal stimulation. Electroencephalogr Clin Neurophysiol. 1991;80:490–495. doi: 10.1016/0168-5597(91)90130-p. [DOI] [PubMed] [Google Scholar]
- 7.Hobday DI, Hobson AR, Sarkar S, et al. Cortical processing of human gut sensation: an evoked potential study. Am J Physiol Gastrointest Liver Physiol. 2002;283:335–339. doi: 10.1152/ajpgi.00230.2001. [DOI] [PubMed] [Google Scholar]
- 8.Drewes AM, Dimcevski G, Sami SA, et al. The “human visceral homunculus” to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp Brain Res. 2006;174:443–452. doi: 10.1007/s00221-006-0480-0. [DOI] [PubMed] [Google Scholar]
- 9.Chan YK, Herkes GK, Badcock C, et al. Alterations in cerebral potentials evoked by rectal distension in irritable bowel syndrome. Am J Gastro. 2001;96:2413–2417. doi: 10.1111/j.1572-0241.2001.04088.x. [DOI] [PubMed] [Google Scholar]
- 10.Sinhamahapatra P, Saha SP, Chowdhury A, Chakrabarti SK, Ghosh A, Maiti B. Visceral afferent hypersensitivity in irritable bowel syndrome--evaluation by cerebral evoked potential after rectal stimulation. Am J Gastroenterol. 2001;96:2150–2157. doi: 10.1111/j.1572-0241.2001.03952.x. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull G, Hamdy S, Aziz Q, Singh K. The cortical topography of human anorectal musculature. Gastroenterology. 1999;117:32–39. doi: 10.1016/s0016-5085(99)70547-0. [DOI] [PubMed] [Google Scholar]
- 12.Enck P, Herdmann J, Börgermann K, Theisen U, Zacchi P, Lübke HJ. Up and down the spinal cord: afferent and efferent innervations of the external anal sphincter in humans. Neurogastroenterol Motil. 1992;4:271–7. [Google Scholar]
- 13.Pelliccioni G, Scarpino O, Piloni V. Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral stimulation: normative data. J Neurol Sci. 1997;149:69–72. doi: 10.1016/s0022-510x(97)05388-4. [DOI] [PubMed] [Google Scholar]
- 14.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 15.Herdmann J, Enck P, Zacchi-Deutschbein P, Ostermann U. Speed and pressure characteristics of external anal sphincter contractions. Am J Physiol. 1995;269:G225–G231. doi: 10.1152/ajpgi.1995.269.2.G225. [DOI] [PubMed] [Google Scholar]
- 16.Ertekin C, Hansen MV, Larsson LE, Sjödahl R. Examination of the descending pathway to the external anal sphincter and pelvic floor muscles by transcranial cortical stimulation. Electroencephalogr Clin Neurophysiol. 1990;75:500–510. doi: 10.1016/0013-4694(90)90137-9. [DOI] [PubMed] [Google Scholar]
- 17.Herdmann J, Bielefeldt K, Enck P. Quantification of motor pathways to the pelvic floor in humans. Am J Physiol. 1991;260:G720–G723. doi: 10.1152/ajpgi.1991.260.5.G720. [DOI] [PubMed] [Google Scholar]
- 18.Loening-Baucke V, Read NW, Yamada T, Barker AT. Evaluation of the motor and sensory components of the pudendal nerve. Electroencephalogr Clin Neurophysiol. 1994;93:35–41. doi: 10.1016/0168-5597(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 19.Hamdy S, Enck P, Aziz Q, Rothwell JC, Uengoergil S, Hobson A, Thompson DG. Spinal and pudendal nerve modulation of human corticoanal motor pathways. Am J Physiol. 1998;274:G419–G423. doi: 10.1152/ajpgi.1998.274.2.G419. [DOI] [PubMed] [Google Scholar]
- 20.Jasper HH. The 10–20 electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- 21.Hobday DI, Aziz Q, Thacker N, Hollander I, Jackson A, Thompson DG. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124:361–8. doi: 10.1093/brain/124.2.361. [DOI] [PubMed] [Google Scholar]
- 22.Hobson AR, Aziz Q, Firlong PL, Barlow JD, Bancewicz J, Thompson DG. Identification of the optimal parameters for recording cortical evoked potentials to human oesophageal electrical stimulation. Neurogastroenterol Motil. 1998;10:421–30. doi: 10.1046/j.1365-2982.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 23.Harris ML, Hobson AR, Hamdy S, et al. Neurophysiological evaluation of healthy human anorectal sensation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G950–8. doi: 10.1152/ajpgi.00010.2006. [DOI] [PubMed] [Google Scholar]
- 24.Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- 25.Hollerbach S, Tougas G, Frieling T, Enck P, Fitzpatrick D, Upton AR, Kamath MV. Cerebral evoked responses to gastrointestinal stimulation in humans. Crit Rev Biomed Eng. 1997;25:203–42. [PubMed] [Google Scholar]
- 26.Delechenault P, Leroi AM, Bruna T, Denis P, Weber J. Cerebral potentials evoked by electrical stimulation of the anal canal. Dis Colon Rectum. 1993;36:55–60. doi: 10.1007/BF02050302. [DOI] [PubMed] [Google Scholar]
- 27.Leroi AM, Ducrotté P, Bouaniche M, Touchais JY, Weber J, Denis P. Assessment of the reliability of cerebral potentials evoked by electrical stimulation of the anal canal. Int J Colorectal Dis. 1997;12:335–9. doi: 10.1007/s003840050119. [DOI] [PubMed] [Google Scholar]
- 28.Hobday DI, Hobson A, Furlong PL, Thompson DG, Aziz Q. Comparison of cortical potentials evoked by mechanical and electrical stimulation of the rectum. Neurogastroenterol Motil. 2000;12:547–54. doi: 10.1046/j.1365-2982.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- 29.Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G397–406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]
- 30.Vasudevan SP, Scott SM, Gladman MA, Lunniss PJ. Rectal hyposensitivity: evaluation of anal sensation in female patients with refractory constipation with and without faecal incontinence. Neurogastroenterol Motil. 2007;19:660–7. doi: 10.1111/j.1365-2982.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 31.Kamm MA, Lennard-Jones JE. Rectal mucosal electrosensory testing--evidence for a rectal sensory neuropathy in idiopathic constipation. Dis Colon Rectum. 1990;33:419–23. doi: 10.1007/BF02156270. [DOI] [PubMed] [Google Scholar]
- 32.Loening-Baucke V, Read NW, Yamada T. Further evaluation of the afferent nervous pathways from the rectum. Am J Physiol Gastrointest Liver Physiol. 1992;262:G927–G933. doi: 10.1152/ajpgi.1992.262.5.G927. [DOI] [PubMed] [Google Scholar]
- 33.Merton PA, Morton HB, Hill DK, Marsden CD. Scope of a technique for electrical stimulation of the human brain, spinal cord and muscle. Lancet. 1982;2:597–600. doi: 10.1016/s0140-6736(82)90670-5. [DOI] [PubMed] [Google Scholar]
- 34.Chus NS. Motor evoked potentials with magnetic stimulation:correlation with height. Electroencephalogr Clin Neurophysiol. 1989;74:481–5. doi: 10.1016/0168-5597(89)90039-7. [DOI] [PubMed] [Google Scholar]
- 35.Tobimatsu S, Sun SJ, Fukui R, Kato M. Effects of sex, height and age on motor evoked potentials with magnetic stimulation. J Neurol. 1998;245:256–61. doi: 10.1007/s004150050215. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki I, Takada H, Baba M, Matsunaga M. Correlation of somatosensory central conduction time with height. Neurology. 1994;44:1115–9. doi: 10.1212/wnl.44.6.1115. [DOI] [PubMed] [Google Scholar]
- 37.Deiner SG, Kwatra SG, Lin HM, Weisz DJ. Patient characteristics and anesthetic technique are additive but not synergistic predictors of successful motor evoked potential monitoring. Anesth Analg. 2010;111(2):421–5. doi: 10.1213/ANE.0b013e3181e41804. [DOI] [PubMed] [Google Scholar]
- 38.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 39.Teitelbaum JE, Sinha P, Micale M, Yeung S, Jaeger J. Obesity is related to multiple functional abdominal diseases. J Pediatr. 2009;154:444–6. doi: 10.1016/j.jpeds.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 40.Delgado-Aros S, Locke GR, 3rd, Camilleri M, Talley NJ, Fett S, Zinsmeister AR, Melton LJ., 3rd Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99:1801–6. doi: 10.1111/j.1572-0241.2004.30887.x. [DOI] [PubMed] [Google Scholar]
- 41.Delgado-Aros S, Camilleri M, Garcia MA, Burton D, Busciglio I. High body mass alters colonic sensory-motor function and transit in humans. Am J Physiol Gastrointest Liver Physiol. 2008;295:G382–8. doi: 10.1152/ajpgi.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.