Abstract
Aromatase, estrone sulfatase, and 17β-hydroxysteroid dehydrogenase type 1 are involved in the key steps of 17β-estradiol biosynthesis. Structure-function studies of aromatase, estrone sulfatase and 17β-hydroxysteroid dehydrogenase type 1 are important to evaluate the molecular basis of the interaction between these enzymes and their inhibitors. Selective and potent inhibitors of the three enzymes have been developed as antiproliferative agents in hormone-dependent breast carcinoma. New treatment strategies for hormone-dependent breast cancer are discussed.
Keywords: Aromatase, estrone sulfatase, 17β-hydroxysteroid dehydrogenase, crystal structure, inhibitor, breast cancer
1. Introduction
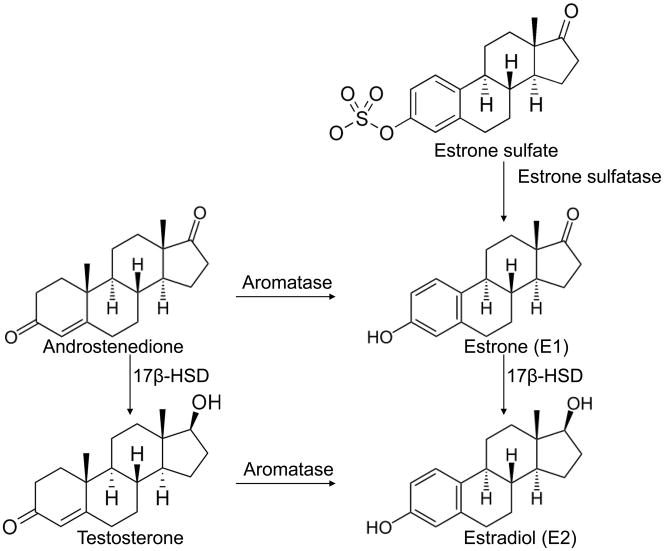
The great majority of breast cancers are hormone-dependent, and it is widely accepted that estrogen plays an important role in the genesis and evolution of breast tumors. It is well established that the concentration of 17β-estradiol (E2), the most biologically active estrogen in breast tumors, can be as much as ten-fold higher than that in plasma in postmenopausal women (van Landeghem et al. 1985). Human breast cancer tissues contain the essential enzymes aromatase, estrone sulfatase, and 17β-hydroxysteroid dehydrogenase (17β-HSD), involved in the key steps of E2 biosynthesis (Figure 1). Two principle pathways are implicated in the formation of E2 in breast cancer tissues: the ‘aromatase pathway’, which converts androgens (androstenedione and testosterone) into estrogens (estrone and E2), and the ‘sulfatase pathway’, which converts estrone sulfate into estrone by estrone sulfatase. Another important step is the conversion of the functionally less active estrone to the biologically potent E2 by the action of 17β-HSDs. It has been proposed that intracrine biosynthesis of estrogens by aromatase, estrone sulfatase, and 17β-HSDs in the breast accounts for most of the estrogens in postmenopausal women. Because E2 has a stimulatory effect on the proliferation of breast cancer cells, blocking its formation by the inhibition of these enzymes should be of paramount importance for the control of breast tumor growth. Selective and potent inhibitors of these enzymes have been developed and have shown promise as antiproliferative agents in hormone-dependent breast carcinoma. For example, the third-generation aromatase inhibitors (AIs) (i.e., anastrozole, letrozole and exemestane) have been approved by the FDA for the treatment of hormone-dependent breast cancer in postmenopausal women. Dr. Mike Reed made important contributions in the demonstration of the functional importance of the three enzymes, as well as in the development of potent estrone sulfatase inhibitors and dual aromatase-sulfatase inhibitors to be potential drugs for estrogen-dependent breast cancer. To recognize Dr. Reed’s contributions in these areas, his and other investigators’ findings on structure-function studies of aromatase, estrone sulfatase, and 17β-HSDs and inhibitors of these enzymes are reviewed in this paper.
Figure 1.
The last steps of E2 biosynthesis by aromatase, estrone sulfatase, and 17β-HSD.
2. Aromatase
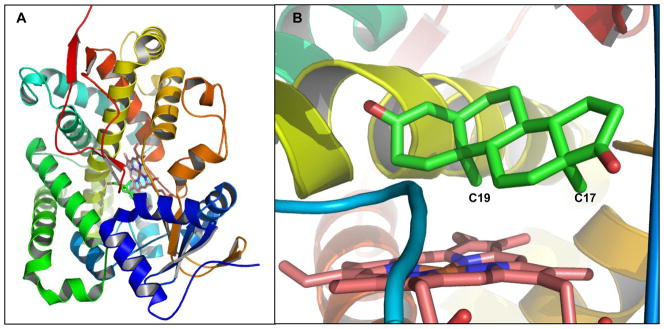
Aromatase is a cytochrome P450 (CYP450) and is the rate-limiting enzyme in estrogen biosynthesis. Through interaction with NADPH-cytochrome P450 reductase (CPR), aromatase catalyzes three steps of hydroxylation to convert androgen to estrogen. Significant efforts from a number of laboratories were made to study the mechanisms of aromatization (Akhtar et al. 1982; Brodie et al. 1969; Hackett et al. 2005; Hahn and Fishman 1984; Miyairi and Fishman 1985; Morand et al. 1975; Numazawa et al. 1994). To understand the structure-function relationship of aromatase, molecular characterization of purified aromatase (Hong et al. 2007; Kagawa et al. 2004; Yoshida and Osawa 1991), site-directed mutagenesis (Auvray et al. 2002; Hong et al. 2008; Hong et al. 2007; Kadohama et al. 1993; Kao et al. 1996; Kao et al. 2001; Kao et al. 1998), and structural modeling analysis (Favia et al. 2006; Graham-Lorence et al. 1995; Hong et al. 2007; Laughton et al. 1993) have been carried out. The crystal structure of full-length aromatase in complex with androstenedione solved at 2.9 Å resolution marks a major milestone in structure determination of CYP450s (Ghosh et al. 2009), as this is the first crystal structure of full-length transmembrane CYP450, although the structure of the N-terminal transmembrane domain was not well defined (Figure 2A). The active-site cleft of the complex is relatively small (< 400 Å3) when compared with other CYP450s, thus an androstenedione molecule fits snugly into this androgen-specific cleft (Figure 2B). This crystal structure confirms several key active site residues predicted from previous site-directed mutagenesis and structure modeling, including D309 and T310 (I helix), F134 (B-C loop), S478 (β-4 sheet), and V370-M374 (3′-flanking loop of the K helix) (Hong et al. 2007), and suggests additional active site residues F221, W224, M447, and S470.
Figure 2.
A. The crystal structure of human placental aromatase cytochrome P450 complexed with androstenedione (PDB# 3EQM) (Ghosh et al. 2009). B. The active site of human aromatase. The substrate androstenedione is shown in green.
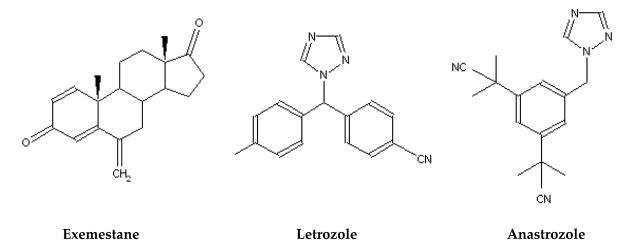
Third-generation AIs (Figure 3) including two triazole derivatives, anastrozole (Arimidex) (Plourde et al. 1995) and letrozole (Femara) (Lipton et al. 1995), and one steroid analogue, exemestane (Aromasin) (Evans et al. 1992), are currently used clinically for the endocrine treatment of hormone-dependent breast cancer in postmenopausal patients (Bajetta et al. 2000; Plourde et al. 1995; Smith and Dowsett 2003). The x-ray crystal structure of aromatase in the presence of the substrate androstenedione helps us to understand better how the steroidal inhibitor exemestane interacts with the enzyme (Ghosh et al. 2009). However, the current information is not sufficient to explain the molecular basis of the mechanism-based inhibition by exemestane. Two nonsteroidal inhibitors, letrozole and anastrozole, which don’t resemble the chemical structure of the androgen substrate, bind to aromatase with high affinity and specificity. Letrozole, the most potent aromatase inhibitor reported so far, has a Ki value of 0.1 to 1 nM (Choate and Resko 1996). Without a crystal structure of aromatase with bound letrozole, results from aromatase site-directed mutagenesis experiments (Kao et al. 1996; Kao et al. 2001) have been used to develop a model to explain how nonsteroidal inhibitors bind to aromatase (Hong et al. 2009b).
Figure 3.
Structures of aromatase inhibitors exemestane, Letrozole, and anastrozole.
CPR is essential for aromatase to catalyze the formation of estrogen. Enzyme kinetic analysis from our laboratory demonstrates that during estrogen synthesis, CPR binds strongly to aromatase with a Km value of 0.5 nM (Hong et al. 2009a), which supports three simultaneous hydroxylation steps in converting androgen to estrogen. The site-directed mutagenesis experiments also provided evidence that the electrostatic interactions between residues N175/T177 of CPR and residue K108 of aromatase play an important role in their association (Hong et al. 2009a). Our findings suggest that the site-specific interaction between aromatase and CPR is critical for effective estrogen synthesis, while CPR attaches aromatase at regions away from the active site where androgen is converted to estrogen, and where AIs bind.
In addition to synthetic AIs, a number of phytochemicals and environmental chemicals act as competitive inhibitors of aromatase (Adams and Chen 2009; Brueggemeier et al. 2001; Hong and Chen 2006). Most of these chemicals inhibit aromatase with Ki values in the micromolar range. The binding nature of these chemicals has been reported by our laboratory using site-directed mutagenesis and computer modeling analysis (Hong et al. 2008; Kao et al. 1998).
3. Estrone sulfatase
Estrone sulfatase, along with aromatase and 17β-HSD, is responsible for maintaining high levels of E2 in tumor cells. After estrone is synthesized by aromatase, it can be converted to estrone sulfate in the liver by the catalysis of estrogen sulfotransferase (Tseng et al. 1983). Through circulation, estrone sulfate can be then stored in tissues, including breast tumors. Estrone sulfatase catalyzes the hydrolysis of estrone sulfate to estrone, which is subsequently reduced to E2 by 17β-HSD. In breast tumor tissue, especially in postmenopausal women, the concentration of estrogen sulfate is several times higher than that found in the plasma or in the area of the breast considered as normal (Edery et al. 1981; Millington 1975; Pasqualini et al. 1996; van Landeghem et al. 1985). It is now widely recognized that this reservoir of estrogen sulfates provides an important source of estrogens in tumors via the action of estrone sulfatase. Previous studies have reported that, in human breast tumors, the sulfatase pathway predominates over the aromatase pathway (Gunnarsson et al. 2001; Vihko et al. 2006). Thus, estrone sulfatase has been considered to be an attractive target for therapeutic intervention.
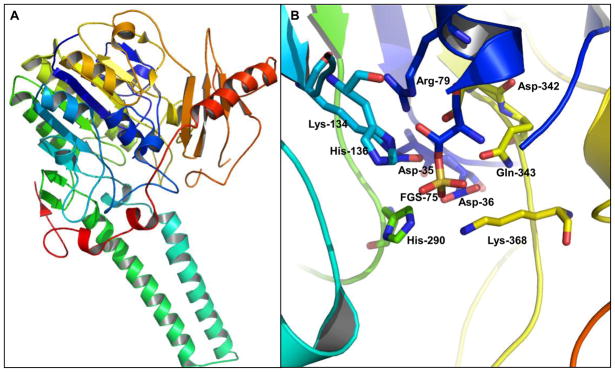
The structure of estrone sulfatase, purified from the microsomal fraction of human placenta, was determined at 2.6 Å by x-ray crystallography (Figure 4A) (Hernandez-Guzman et al. 2003). The catalytic amino acid is created by post-translational modification of a highly conserved cysteine residue to hydroxylformylglycine (FG), followed by a covalent linkage to a sulfate moiety as a sulfate ester of FG (FGS) (Bond et al. 1997). The residue FGS75 is involved in stabilizing the calcium ion and the sulfate ester in the active site (Bond et al. 1997). The calcium ion is located at the center of the catalytic site near the residue FGS75 and is required for estrone sulfatase activity (Hernandez-Guzman et al. 2003). The posttranslational conversion of this cysteine residue was also well studied by several other groups, demonstrating that the conversion is required for generating catalytically active sulfatases (Dierks et al. 1997; Schmidt et al. 1995). The active site residues of estrone sulfatase were identified to be D35, D36, FGS75, R79, K134, H136, H290, D342, Q343, and K368 (Figure 4B).
Figure 4.
A. The crystal structure of human estrone sulfatase (PDB# 1P49) (Hernandez-Guzman et al. 2003). B. The active site of estrone sulfatase. The substrate binding site is located at the pocket underneath the residues D36, FGS75, H290, and K368
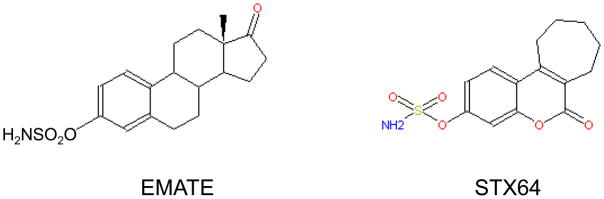
Several classes of estrone sulfatase inhibitors (Figure 5) have been reported, mainly by Dr. M. Reed and his colleagues in the past 10 years. Estrone-3-sulfamate is the first potent estrone sulfatase inhibitor that causes a time- and concentration-dependent irreversible inactivation of the enzyme (Howarth et al. 1994; Purohit et al. 1995). Sulfamoyloxy-substituted 2-phenylindoles have been developed as antiestrogen-based inhibitors of estrone sulfatase (Golob et al. 2002). Compound STX64 (also known as 667 Coumate and BN83495), which has an IC50 value of 8 nM in placental microsomes (Foster et al. 2008), has become the first estrone sulfatase inhibitor to enter clinical trail for postmenopausal patients with advanced hormone-dependent breast cancer and has shown encouraging results (Stanway et al. 2007).
Figure 5.
Structures of estrone sulfatase inhibitors estrone-3-sulfamate (EMATE) and STX64.
Since aromatase is needed for the synthesis of estrogen that is then converted to estrogen sulfate by estrogen sulfotransferase, hormone-dependent breast cancer may be more effectively treated by dual inhibition of aromatase and steroid sulfatase. A new design strategy was explored that involves introducing the aromatase inhibitory pharmacophore into a template that has been designed primarily for sulfatase inhibition (Woo et al. 2010). A series of compounds that can inhibit both aromatase and sulfatase has been developed based on the structure of estrone 3-sulfamate, a typical estrone sulfatase inhibitor (Numazawa et al. 2006). In addition, a series of dual aromatase-sulfatase inhibitors that are sulfamate derivatives of nonsteroidal AIs, including letrozole and anastrozole, has been successfully developed (Woo et al. 2007; Woo et al. 2008; Woo et al. 2003). The design of these dual aromatase-sulfatase inhibitors shares a common strategy; that is, to engender the sulfatase inhibitory pharmacophore into an established aromatase inhibitor with minimal structural change incurred to the original scaffold in order to retain and maximize aromatase inhibition.
4. 17β-HSDs
17β-HSDs are a group of enzymes that catalyze dehydrogenation of 17-hydroxysteroids in steroidogenesis. Most studies on the 17β-HSDs have primarily assessed type 1. 17β-HSD1 is an important enzyme for E2 production because it can use estrone as a substrate generated by both aromatase and sulfatase pathways, and it principally reduces the 17β-keto group of estrone to 17β-hydroxyl group using NADPH as a cofactor (Aka et al. 2010). This enzyme has been demonstrated to be involved in maintaining high E2 levels in breast tumors of postmenopausal women (Miyoshi et al. 2001; Poutanen et al. 1995). In certain breast cancer patients, intratumoral E2 was produced by the 17β-HSD1-mediated reduction of estrone produced by aromatase (Sasano et al. 2008; Sasano et al. 2006). Higher expression of 17β-HSD1 mRNA has been demonstrated in almost 50% of breast cancer tissues (Vihko et al. 2004). The expression and activity of 17β-HSD1 are significantly higher in breast cancer than in normal breast tissue (Pasqualini 2004), and it has been suggested that this higher expression could explain the elevated E2 concentration in breast tumors (Vermeulen et al. 1986). A high level of 17β-HSD1 correlates with an increased risk of developing a late relapse of breast cancer in ER-positive breast cancer patients (Day et al. 2006; Vihko and Apter 1989). In addition, 17β-HSD2 and 17β-HSD7 have been suggested to play a secondary role in the balance between estrone and E2 (Dunbier et al. 2010; Haynes et al. 2010; Lonning et al. 2009).
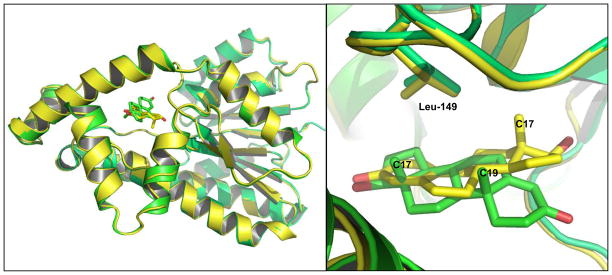
The first diffraction-quality crystal of 17β-HSD1 was obtained in the early 1990s (Zhu et al. 1994). A dozen different complex structures have been solved to date (Azzi et al. 1996; Breton et al. 1996; Gangloff et al. 2003; Lin et al. 2000; Lin et al. 1996; Persson et al. 2003; Puranen et al. 1997; Qiu et al. 2002; Sawicki et al. 1999; Shi and Lin 2004). The crystal structures of 17β-HSD1 complexed with testosterone or E2 are shown in Figure 6A. Both testosterone and E2 bind in the narrow hydrophobic tunnel of 17β-HSD1 with a high degree of complementarity (Figure 6B). However, testosterone is bound in an alternative orientation to 17β-HSD1 compared with E2. The residue L149 plays an important role in the discrimination between C19 androgen and C18 estrogen (Gangloff et al. 2003).
Figure 6.
A. The crystal structures of human 17β-HSD1 complexed with testosterone (PDB# 1JTV, green), or estradiol (PDB# 1IOL, yellow)(Azzi et al. 1996). B. The substrate binding site of 17β-HSD1: testosterone (green), estradiol (yellow).
When testosterone is positioned in the same binding mode as E2, a steric clash between the 19-methyl group and the residue L149 is observed. This steric hindrance may compel testosterone to bind in a reverse binding mode (Gangloff et al. 2003), which results in a much lower binding affinity of C19 androgen when compared to C18 estrogen (Labrie et al. 2000).
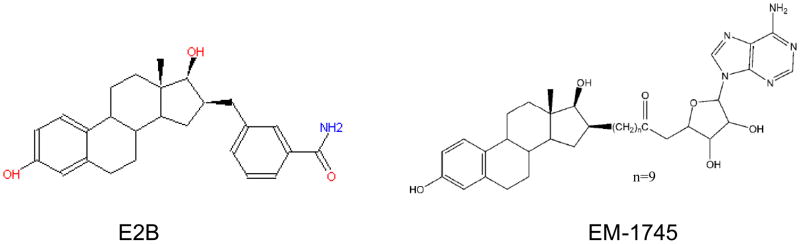
Inhibitors of 17β-HSDs constitute a growing interest in biomedical research, and new compounds have been developed in recent years (Brozic et al. 2008; Day et al. 2008; Laplante et al. 2008; Poirier 2003; Poirier et al. 2005). To develop new compounds capable of inhibiting 17β-HSD1, a series of structure-function studies were conducted in several laboratories focusing on the understanding of steroid binding to the enzyme (Azzi et al. 1996; Gangloff et al. 2003; Mazumdar et al. 2009; Miyoshi et al. 2001; Poutanen et al. 1995; Qiu et al. 2007; Shi and Lin 2004). A hybrid inhibitor having both the substrate and cofactor parts, EM-1745 (Figure 7), competed with the substrate as well as the cofactor cores (Qiu et al. 2002). The E2-adenosine hybrid compound EM-1745 revealed key interactions with two different enzyme-binding sites, namely the substrate- and the cofactor-binding sites. In addition, the IC50 value for a new inhibitor E2B was determined to be 42 nM in T47D cells (Mazumdar et al. 2009). A kinetic study demonstrated that E2B inhibits the conversion of estrone to E2, with a Ki of 0.9 ± 0.15 nM (Mazumdar et al. 2009). Such strong inhibition is in agreement with the effective interaction of E2B with the enzyme, suggesting its potential as a lead compound for breast cancer therapy.
Figure 7.
Structures of 17β-HSD1 inhibitors E2B and EM-1745.
5. Discussion
While aromatase, estrone sulfatase, and 17βHSD1 all use sex steroids as substrates, with the information we have so far it is difficult to compare the similarity among the substrate binding sites of these three enzymes. This is not unexpected, as they are different types of enzymes with distinct catalytic mechanisms. The dissimilarity in the active site regions of these enzymes allows the development of specific inhibitors for each enzyme. As reviewed in this paper, a majority of the inhibitors of were developed through structure-activity studies and biochemical analysis. The three-dimensional structures of these enzymes have not been shown to be useful in drug design, but they provide valuable information to help understand the catalytic mechanisms and molecular basis of inhibitor binding. The x-ray structures are certainly valuable to verify the importance of key amino acid residues in the active sites and in the binding pockets for substrates and inhibitors.
Among the inhibitors of aromatase, estrone sulfatase, and 17β-HSDs, AIs are the best-developed, and clinically effective for hormone-dependent breast cancer. Letrozole, anastrozole, and exemestane have shown to be superior in terms of relapse-free survival and recurrence in the endocrine treatment of hormone-dependent breast cancer in postmenopausal patients. Although these three inhibitors are efficacious drugs for hormone-dependent breast cancer, there are concerns for acquired resistance and side effects associated with estrogen deprivation. It is essential to consider new therapeutics to reduce side effects and overcome resistance to current AIs. Inhibitors that target estrone sulfatase or 17β-HSDs may not be very effective for monotherapy because aromatase is the only enzyme that catalyzes the synthesis of estrogen from androgen. Although estrone is less active than E2, it is still an active estrogen. However, there will be a benefit for combination therapies using inhibitors of estrone sulfatase or 17β-HSDs with an AI, which could lower the dose of the AI, thus reduce side effects created by the AI, and delay the development of resistance to either drug. Considering that the expression levels of estrone sulfatase and 17β-HSD1 are significantly higher in breast tumor than in normal breast tissue, the use of inhibitors of these two enzymes together with AIs may also enhance tumor-selective reduction of E2 levels. New treatment strategies combining inhibitors of estrone sulfatase or 17β-HSDs with AI could also extend the period of time of endocrine therapy, thereby disease progression and the need for chemotherapy may be significantly delayed. Preclinical, translational and clinical studies to evaluate such treatment options are critically needed.
Dr. Mike Reed demonstrated his vision in the endocrine therapy of breast cancer by the design and characterization of dual aromatase-sulfatase inhibitors. These inhibitors could be the new generation of drugs to treat hormone-dependent breast cancer. His contributions in hormonal carcinogenesis and endocrine therapy will be greatly missed by all of us.
Abbreviations
- AI
aromatase inhibitor
- CPR
NADPH-cytochrome P450 reductase
- CYP450
cytochrome P450
- E2
17β-estradiol
- 17β-HSD
17β-hydroxysteroid dehydrogenase
- 17β-HSD1
17β-hydroxysteroid dehydrogenase type 1
- ER
estrogen receptor
- FG
hydroxylformylglycine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams LS, Chen S. Phytochemicals for breast cancer prevention by targeting aromatase. Front Biosci. 2009;14:3846–3863. doi: 10.2741/3493. [DOI] [PubMed] [Google Scholar]
- Aka JA, Mazumdar M, Chen CQ, Poirier D, Lin SX. 17beta-hydroxysteroid dehydrogenase type 1 stimulates breast cancer by dihydrotestosterone inactivation in addition to estradiol production. Mol Endocrinol. 2010;24(4):832–845. doi: 10.1210/me.2009-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M, Calder MR, Corina DL, Wright JN. Mechanistic studies on C-19 demethylation in oestrogen biosynthesis. Biochem J. 1982;201(3):569–580. doi: 10.1042/bj2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvray P, Nativelle C, Bureau R, Dallemagne P, Seralini GE, Sourdaine P. Study of substrate specificity of human aromatase by site directed mutagenesis. Eur J Biochem. 2002;269(5):1393–1405. doi: 10.1046/j.1432-1033.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- Azzi A, Rehse PH, Zhu DW, Campbell RL, Labrie F, Lin SX. Crystal structure of human estrogenic 17 beta-hydroxysteroid dehydrogenase complexed with 17 beta-estradiol. Nature structural biology. 1996;3(8):665–668. doi: 10.1038/nsb0896-665. [DOI] [PubMed] [Google Scholar]
- Bajetta E, Zilembo N, Bichisao E, Martinetti A, Buzzoni R, Pozzi P, Bidoli P, Ferrari L, Celio L. Tumor response and estrogen suppression in breast cancer patients treated with aromatase inhibitors. Ann Oncol. 2000;11(8):1017–1022. doi: 10.1023/a:1008388823113. [DOI] [PubMed] [Google Scholar]
- Bond CS, Clements PR, Ashby SJ, Collyer CA, Harrop SJ, Hopwood JJ, Guss JM. Structure of a human lysosomal sulfatase. Structure. 1997;5(2):277–289. doi: 10.1016/s0969-2126(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Breton R, Housset D, Mazza C, Fontecilla-Camps JC. The structure of a complex of human 17beta-hydroxysteroid dehydrogenase with estradiol and NADP+ identifies two principal targets for the design of inhibitors. Structure. 1996;4(8):905–915. doi: 10.1016/s0969-2126(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Brodie HJ, Kripalani KJ, Possanza G. Studies on the mechanism of estrogen biosynthesis. VI. The stereochemistry of hydrogen elimination at C-2 during aromatization. J Am Chem Soc. 1969;91(5):1241–1242. doi: 10.1021/ja01033a050. [DOI] [PubMed] [Google Scholar]
- Brozic P, Lanisnik Risner T, Gobec S. Inhibitors of 17beta-hydroxysteroid dehydrogenase type 1. Current medicinal chemistry. 2008;15(2):137–150. doi: 10.2174/092986708783330629. [DOI] [PubMed] [Google Scholar]
- Brueggemeier RW, Gu X, Mobley JA, Joomprabutra S, Bhat AS, Whetstone JL. Effects of phytoestrogens and synthetic combinatorial libraries on aromatase, estrogen biosynthesis, and metabolism. Ann N Y Acad Sci. 2001;948:51–66. doi: 10.1111/j.1749-6632.2001.tb03986.x. [DOI] [PubMed] [Google Scholar]
- Choate JV, Resko JA. Paradoxical effect of an aromatase inhibitor, CGS 20267, on aromatase activity in guinea pig brain. J Steroid Biochem Mol Biol. 1996;58(4):411–415. doi: 10.1016/0960-0760(96)00047-7. [DOI] [PubMed] [Google Scholar]
- Day JM, Foster PA, Tutill HJ, Parsons MF, Newman SP, Chander SK, Allan GM, Lawrence HR, Vicker N, Potter BV, et al. 17beta-hydroxysteroid dehydrogenase Type 1, and not Type 12, is a target for endocrine therapy of hormone-dependent breast cancer. International journal of cancer. 2008;122(9):1931–1940. doi: 10.1002/ijc.23350. [DOI] [PubMed] [Google Scholar]
- Day JM, Tutill HJ, Newman SP, Purohit A, Lawrence HR, Vicker N, Potter BV, Reed MJ. 17Beta-hydroxysteroid dehydrogenase Type 1 and Type 2: association between mRNA expression and activity in cell lines. Mol Cell Endocrinol. 2006;248(1–2):246–249. doi: 10.1016/j.mce.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Dierks T, Schmidt B, von Figura K. Conversion of cysteine to formylglycine: a protein modification in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1997;94(22):11963–11968. doi: 10.1073/pnas.94.22.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbier AK, Anderson H, Ghazoui Z, Folkerd EJ, A’Hern R, Crowder RJ, Hoog J, Smith IE, Osin P, Nerurkar A, et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J Clin Oncol. 2010;28(7):1161–1167. doi: 10.1200/JCO.2009.23.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery M, Goussard J, Dehennin L, Scholler R, Reiffsteck J, Drosdowsky MA. Endogenous oestradiol-17beta concentration in breast tumours determined by mass fragmentography and by radioimmunoassay: relationship to receptor content. Eur J Cancer. 1981;17(1):115–120. doi: 10.1016/0014-2964(81)90220-6. [DOI] [PubMed] [Google Scholar]
- Evans TR, Di Salle E, Ornati G, Lassus M, Benedetti MS, Pianezzola E, Coombes RC. Phase I and endocrine study of exemestane (FCE 24304), a new aromatase inhibitor, in postmenopausal women. Cancer Res. 1992;52(21):5933–5939. [PubMed] [Google Scholar]
- Favia AD, Cavalli A, Masetti M, Carotti A, Recanatini M. Three-dimensional model of the human aromatase enzyme and density functional parameterization of the iron-containing protoporphyrin IX for a molecular dynamics study of heme-cysteinato cytochromes. Proteins. 2006;62(4):1074–1087. doi: 10.1002/prot.20829. [DOI] [PubMed] [Google Scholar]
- Foster PA, Woo LW, Potter BV, Reed MJ, Purohit A. The use of steroid sulfatase inhibitors as a novel therapeutic strategy against hormone-dependent endometrial cancer. Endocrinology. 2008;149(8):4035–4042. doi: 10.1210/en.2008-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff A, Shi R, Nahoum V, Lin SX. Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17beta-hydroxysteroid dehydrogenase. Faseb J. 2003;17(2):274–276. doi: 10.1096/fj.02-0397fje. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457(7226):219–223. doi: 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob T, Liebl R, von Angerer E. Sulfamoyloxy-substituted 2-phenylindoles: antiestrogen-based inhibitors of the steroid sulfatase in human breast cancer cells. Bioorganic & medicinal chemistry. 2002;10(12):3941–3953. doi: 10.1016/s0968-0896(02)00306-1. [DOI] [PubMed] [Google Scholar]
- Graham-Lorence S, Amarneh B, White RE, Peterson JA, Simpson ER. A three-dimensional model of aromatase cytochrome P450. Protein Sci. 1995;4(6):1065–1080. doi: 10.1002/pro.5560040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson C, Olsson BM, Stal O. Abnormal expression of 17beta-hydroxysteroid dehydrogenases in breast cancer predicts late recurrence. Cancer Res. 2001;61(23):8448–8451. [PubMed] [Google Scholar]
- Hackett JC, Brueggemeier RW, Hadad CM. The final catalytic step of cytochrome p450 aromatase: a density functional theory study. J Am Chem Soc. 2005;127(14):5224–5237. doi: 10.1021/ja044716w. [DOI] [PubMed] [Google Scholar]
- Hahn EF, Fishman J. Immunological probe of estrogen biosynthesis. Evidence for the 2 beta-hydroxylative pathway in aromatization of androgens. J Biol Chem. 1984;259(3):1689–1694. [PubMed] [Google Scholar]
- Haynes BP, Straume AH, Geisler J, A’Hern R, Helle H, Smith IE, Lonning PE, Dowsett M. Intratumoral estrogen disposition in breast cancer. Clin Cancer Res. 2010;16(6):1790–1801. doi: 10.1158/1078-0432.CCR-09-2481. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guzman FG, Higashiyama T, Pangborn W, Osawa Y, Ghosh D. Structure of human estrone sulfatase suggests functional roles of membrane association. J Biol Chem. 2003;278(25):22989–22997. doi: 10.1074/jbc.M211497200. [DOI] [PubMed] [Google Scholar]
- Hong Y, Chen S. Aromatase inhibitors: structural features and biochemical characterization. Ann N Y Acad Sci. 2006;1089:237–251. doi: 10.1196/annals.1386.022. [DOI] [PubMed] [Google Scholar]
- Hong Y, Cho M, Yuan YC, Chen S. Molecular basis for the interaction of four different classes of substrates and inhibitors with human aromatase. Biochem Pharmacol. 2008;75(5):1161–1169. doi: 10.1016/j.bcp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Hong Y, Li H, Ye J, Miki Y, Yuan YC, Sasano H, Evans DB, Chen S. Epitope characterization of an aromatase monoclonal antibody suitable for the assessment of intratumoral aromatase activity. PloS one. 2009a;4(11):e8050. doi: 10.1371/journal.pone.0008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Li H, Yuan YC, Chen S. Molecular characterization of aromatase. Ann N Y Acad Sci. 2009b;1155:112–120. doi: 10.1111/j.1749-6632.2009.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Yu B, Sherman M, Yuan YC, Zhou D, Chen S. Molecular basis for the aromatization reaction and exemestane-mediated irreversible inhibition of human aromatase. Mol Endocrinol. 2007;21(2):401–414. doi: 10.1210/me.2006-0281. [DOI] [PubMed] [Google Scholar]
- Howarth NM, Purohit A, Reed MJ, Potter BV. Estrone sulfamates: potent inhibitors of estrone sulfatase with therapeutic potential. J Med Chem. 1994;37(2):219–221. doi: 10.1021/jm00028a002. [DOI] [PubMed] [Google Scholar]
- Kadohama N, Zhou D, Chen S, Osawa Y. Catalytic efficiency of expressed aromatase following site-directed mutagenesis. Biochim Biophys Acta. 1993;1163(2):195–200. doi: 10.1016/0167-4838(93)90181-p. [DOI] [PubMed] [Google Scholar]
- Kagawa N, Hori H, Waterman MR, Yoshioka S. Characterization of stable human aromatase expressed in E. coli. Steroids. 2004;69(4):235–243. doi: 10.1016/j.steroids.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Kao YC, Cam LL, Laughton CA, Zhou D, Chen S. Binding characteristics of seven inhibitors of human aromatase: a site-directed mutagenesis study. Cancer Res. 1996;56(15):3451–3460. [PubMed] [Google Scholar]
- Kao YC, Korzekwa KR, Laughton CA, Chen S. Evaluation of the mechanism of aromatase cytochrome P450. A site-directed mutagenesis study. Eur J Biochem. 2001;268(2):243–251. doi: 10.1046/j.1432-1033.2001.01886.x. [DOI] [PubMed] [Google Scholar]
- Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ Health Perspect. 1998;106(2):85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Simard J, Labrie C, El-Alfy M, Pelletier G, Belanger A. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. Journal of molecular endocrinology. 2000;25(1):1–16. doi: 10.1677/jme.0.0250001. [DOI] [PubMed] [Google Scholar]
- Laplante Y, Cadot C, Fournier MA, Poirier D. Estradiol and estrone C-16 derivatives as inhibitors of type 1 17beta-hydroxysteroid dehydrogenase: blocking of ER+ breast cancer cell proliferation induced by estrone. Bioorganic & medicinal chemistry. 2008;16(4):1849–1860. doi: 10.1016/j.bmc.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Laughton CA, Zvelebil MJ, Neidle S. A detailed molecular model for human aromatase. J Steroid Biochem Mol Biol. 1993;44(4–6):399–407. doi: 10.1016/0960-0760(93)90243-p. [DOI] [PubMed] [Google Scholar]
- Lin SX, Han Q, Azzi A, Zhu D, Gangloff A, Campbell RL. 3D-structure of human estrogenic 17beta-HSD1: binding with various steroids. J Steroid Biochem Mol Biol. 2000;73(3–4):183. doi: 10.1016/s0960-0760(00)00072-8. [DOI] [PubMed] [Google Scholar]
- Lin SX, Zhu DW, Azzi A, Campbell RL, Breton R, Labrie F, Ghosh D, Pletnev V, Duax WL, Pangborn W. Studies on the three-dimensional structure of estrogenic 17 beta-hydroxysteroid dehydrogenase. J Endocrinol. 1996;150(Suppl):S13–20. [PubMed] [Google Scholar]
- Lipton A, Demers LM, Harvey HA, Kambic KB, Grossberg H, Brady C, Adlercruetz H, Trunet PF, Santen RJ. Letrozole (CGS 20267). A phase I study of a new potent oral aromatase inhibitor of breast cancer. Cancer. 1995;75(8):2132–2138. doi: 10.1002/1097-0142(19950415)75:8<2132::aid-cncr2820750816>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Lonning PE, Helle H, Duong NK, Ekse D, Aas T, Geisler J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol. 2009;117(1–3):31–41. doi: 10.1016/j.jsbmb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Mazumdar M, Fournier D, Zhu DW, Cadot C, Poirier D, Lin SX. Binary and ternary crystal structure analyses of a novel inhibitor with 17beta-HSD type 1: a lead compound for breast cancer therapy. Biochem J. 2009;424(3):357–366. doi: 10.1042/BJ20091020. [DOI] [PubMed] [Google Scholar]
- Millington DS. Determination of hormonal steroid concentrations in biological extracts by high resolution mass fragmentography. J Steroid Biochem. 1975;6(3–4):239–245. doi: 10.1016/0022-4731(75)90139-9. [DOI] [PubMed] [Google Scholar]
- Miyairi S, Fishman J. Radiometric analysis of oxidative reactions in aromatization by placental microsomes. Presence of differential isotope effects. J Biol Chem. 1985;260(1):320–325. [PubMed] [Google Scholar]
- Miyoshi Y, Ando A, Shiba E, Taguchi T, Tamaki Y, Noguchi S. Involvement of up-regulation of 17beta-hydroxysteroid dehydrogenase type 1 in maintenance of intratumoral high estradiol levels in postmenopausal breast cancers. International journal of cancer. 2001;94(5):685–689. doi: 10.1002/ijc.1525. [DOI] [PubMed] [Google Scholar]
- Morand P, Williamson DG, Layne DS, Lompa-Krzymien L, Salvador J. Conversion of an androgen epoxide into 17beta-estradiol by human placental microsomes. Biochemistry. 1975;14(3):635–638. doi: 10.1021/bi00674a027. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Midzuhashi K, Nagaoka M. Metabolic aspects of the 1 beta-proton and the 19-methyl group of androst-4-ene-3,6,17-trione during aromatization by placental microsomes and inactivation of aromatase. Biochem Pharmacol. 1994;47(4):717–726. doi: 10.1016/0006-2952(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Tominaga T, Watari Y, Tada Y. Inhibition of estrone sulfatase by aromatase inhibitor-based estrogen 3-sulfamates. Steroids. 2006;71(5):371–379. doi: 10.1016/j.steroids.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Pasqualini JR. The selective estrogen enzyme modulators in breast cancer: a review. Biochim Biophys Acta. 2004;1654(2):123–143. doi: 10.1016/j.bbcan.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab. 1996;81(4):1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- Persson B, Kallberg Y, Oppermann U, Jornvall H. Coenzyme-based functional assignments of short-chain dehydrogenases/reductases (SDRs) Chem Biol Interact. 2003;143–144:271–278. doi: 10.1016/s0009-2797(02)00223-5. [DOI] [PubMed] [Google Scholar]
- Plourde PV, Dyroff M, Dowsett M, Demers L, Yates R, Webster A. ARIMIDEX: a new oral, once-a-day aromatase inhibitor. J Steroid Biochem Mol Biol. 1995;53(1–6):175–179. doi: 10.1016/0960-0760(95)00045-2. [DOI] [PubMed] [Google Scholar]
- Poirier D. Inhibitors of 17 beta-hydroxysteroid dehydrogenases. Current medicinal chemistry. 2003;10(6):453–477. doi: 10.2174/0929867033368222. [DOI] [PubMed] [Google Scholar]
- Poirier D, Boivin RP, Tremblay MR, Berube M, Qiu W, Lin SX. Estradiol-adenosine hybrid compounds designed to inhibit type 1 17beta-hydroxysteroid dehydrogenase. J Med Chem. 2005;48(26):8134–8147. doi: 10.1021/jm058235e. [DOI] [PubMed] [Google Scholar]
- Poutanen M, Isomaa V, Peltoketo H, Vihko R. Role of 17 beta-hydroxysteroid dehydrogenase type 1 in endocrine and intracrine estradiol biosynthesis. J Steroid Biochem Mol Biol. 1995;55(5–6):525–532. doi: 10.1016/0960-0760(95)00201-4. [DOI] [PubMed] [Google Scholar]
- Puranen T, Poutanen M, Ghosh D, Vihko R, Vihko P. Origin of substrate specificity of human and rat 17beta-hydroxysteroid dehydrogenase type 1, using chimeric enzymes and site-directed substitutions. Endocrinology. 1997;138(8):3532–3539. doi: 10.1210/endo.138.8.5303. [DOI] [PubMed] [Google Scholar]
- Purohit A, Williams GJ, Howarth NM, Potter BV, Reed MJ. Inactivation of steroid sulfatase by an active site-directed inhibitor, estrone-3-O-sulfamate. Biochemistry. 1995;34(36):11508–11514. doi: 10.1021/bi00036a025. [DOI] [PubMed] [Google Scholar]
- Qiu W, Campbell RL, Gangloff A, Dupuis P, Boivin RP, Tremblay MR, Poirier D, Lin SX. A concerted, rational design of type 1 17beta-hydroxysteroid dehydrogenase inhibitors: estradiol-adenosine hybrids with high affinity. Faseb J. 2002;16(13):1829–1831. doi: 10.1096/fj.02-0026fje. [DOI] [PubMed] [Google Scholar]
- Qiu W, Zhou M, Mazumdar M, Azzi A, Ghanmi D, Luu-The V, Labrie F, Lin SX. Structure-based inhibitor design for an enzyme that binds different steroids: a potent inhibitor for human type 5 17beta-hydroxysteroid dehydrogenase. J Biol Chem. 2007;282(11):8368–8379. doi: 10.1074/jbc.M606784200. [DOI] [PubMed] [Google Scholar]
- Sasano H, Suzuki T, Miki Y, Moriya T. Intracrinology of estrogens and androgens in breast carcinoma. J Steroid Biochem Mol Biol. 2008;108(3–5):181–185. doi: 10.1016/j.jsbmb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Sasano H, Suzuki T, Nakata T, Moriya T. New development in intracrinology of breast carcinoma. Breast cancer (Tokyo, Japan) 2006;13(2):129–136. doi: 10.2325/jbcs.13.129. [DOI] [PubMed] [Google Scholar]
- Sawicki MW, Erman M, Puranen T, Vihko P, Ghosh D. Structure of the ternary complex of human 17beta-hydroxysteroid dehydrogenase type 1 with 3-hydroxyestra-1,3,5,7-tetraen-17-one (equilin) and NADP+ Proc Natl Acad Sci U S A. 1999;96(3):840–845. doi: 10.1073/pnas.96.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Selmer T, Ingendoh A, von Figura K. A novel amino acid modification in sulfatases that is defective in multiple sulfatase deficiency. Cell. 1995;82(2):271–278. doi: 10.1016/0092-8674(95)90314-3. [DOI] [PubMed] [Google Scholar]
- Shi R, Lin SX. Cofactor hydrogen bonding onto the protein main chain is conserved in the short chain dehydrogenase/reductase family and contributes to nicotinamide orientation. J Biol Chem. 2004;279(16):16778–16785. doi: 10.1074/jbc.M313156200. [DOI] [PubMed] [Google Scholar]
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348(24):2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- Stanway SJ, Delavault P, Purohit A, Woo LW, Thurieau C, Potter BV, Reed MJ. Steroid sulfatase: a new target for the endocrine therapy of breast cancer. Oncologist. 2007;12(4):370–374. doi: 10.1634/theoncologist.12-4-370. [DOI] [PubMed] [Google Scholar]
- Tseng L, Mazella J, Lee LY, Stone ML. Estrogen sulfatase and estrogen sulfotransferase in human primary mammary carcinoma. J Steroid Biochem. 1983;19(4):1413–1417. doi: 10.1016/0022-4731(83)91116-0. [DOI] [PubMed] [Google Scholar]
- van Landeghem AA, Poortman J, Nabuurs M, Thijssen JH. Endogenous concentration and subcellular distribution of androgens in normal and malignant human breast tissue. Cancer Res. 1985;45(6):2907–2912. [PubMed] [Google Scholar]
- Vermeulen A, Deslypere JP, Paridaens R. Steroid dynamics in the normal and carcinomatous mammary gland. J Steroid Biochem. 1986;25(5B):799–802. doi: 10.1016/0022-4731(86)90311-0. [DOI] [PubMed] [Google Scholar]
- Vihko P, Harkonen P, Soronen P, Torn S, Herrala A, Kurkela R, Pulkka A, Oduwole O, Isomaa V. 17 beta-hydroxysteroid dehydrogenases--their role in pathophysiology. Mol Cell Endocrinol. 2004;215(1–2):83–88. doi: 10.1016/j.mce.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Vihko P, Herrala A, Harkonen P, Isomaa V, Kaija H, Kurkela R, Pulkka A. Control of cell proliferation by steroids: the role of 17HSDs. Mol Cell Endocrinol. 2006;248(1–2):141–148. doi: 10.1016/j.mce.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Vihko R, Apter D. Endogenous steroids in the pathophysiology of breast cancer. Critical reviews in oncology/hematology. 1989;9(1):1–16. doi: 10.1016/s1040-8428(89)80012-5. [DOI] [PubMed] [Google Scholar]
- Woo LW, Bubert C, Sutcliffe OB, Smith A, Chander SK, Mahon MF, Purohit A, Reed MJ, Potter BV. Dual aromatase-steroid sulfatase inhibitors. J Med Chem. 2007;50(15):3540–3560. doi: 10.1021/jm061462b. [DOI] [PubMed] [Google Scholar]
- Woo LW, Fischer DS, Sharland CM, Trusselle M, Foster PA, Chander SK, Di Fiore A, Supuran CT, De Simone G, Purohit A, et al. Anticancer steroid sulfatase inhibitors: synthesis of a potent fluorinated second-generation agent, in vitro and in vivo activities, molecular modeling, and protein crystallography. Molecular cancer therapeutics. 2008;7(8):2435–2444. doi: 10.1158/1535-7163.MCT-08-0195. [DOI] [PubMed] [Google Scholar]
- Woo LW, Jackson T, Putey A, Cozier G, Leonard P, Acharya KR, Chander SK, Purohit A, Reed MJ, Potter BV. Highly potent first examples of dual aromatase-steroid sulfatase inhibitors based on a biphenyl template. J Med Chem. 2010;53(5):2155–2170. doi: 10.1021/jm901705h. [DOI] [PubMed] [Google Scholar]
- Woo LW, Sutcliffe OB, Bubert C, Grasso A, Chander SK, Purohit A, Reed MJ, Potter BV. First dual aromatase-steroid sulfatase inhibitors. J Med Chem. 2003;46(15):3193–3196. doi: 10.1021/jm034033b. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Osawa Y. Purification of human placental aromatase cytochrome P-450 with monoclonal antibody and its characterization. Biochemistry. 1991;30(12):3003–3010. doi: 10.1021/bi00226a004. [DOI] [PubMed] [Google Scholar]
- Zhu DW, Lee X, Labrie F, Lin SX. Crystal growth of human estrogenic 17beta-hydroxysteroid dehydrogenase. Acta crystallographica. 1994;50(Pt 4):550–555. doi: 10.1107/S0907444994001307. [DOI] [PubMed] [Google Scholar]