Abstract
A metalloprotease, ADAM17, mediates the generation of mature ligands for the epidermal growth factor receptor (EGFR). This is the key signaling step by which angiotensin II (AngII) induces EGFR transactivation leading to hypertrophy and migration of vascular smooth muscle cells (VSMCs). However, the regulatory mechanism of ADAM17 activity remains largely unclear. Here we hypothesized that caveolin-1 (Cav1), the major structural protein of a caveolae, a membrane microdomain, is involved in the regulation of ADAM17. In cultured VSMCs, infection of adenovirus encoding Cav1 markedly inhibited AngII-induced EGFR ligand shedding, EGFR transactivation, ERK activation, hypertrophy and migration, but not intracellular Ca2+ elevation. Methyl-β-cyclodextrin and filipin, reagents that disrupt raft structure, both stimulated an EGFR ligand shedding and EGFR transactivation in VSMCs. In addition, non-detergent sucrose gradient membrane fractionations revealed that ADAM17 cofractionated with Cav1 in lipid rafts. These results suggest that lipid rafts and perhaps caveolae provide a negative regulatory environment for EGFR transactivation linked to vascular remodeling induced by AngII. These novel findings may provide important information to target cardiovascular diseases under the enhanced renin angiotensin system.
Keywords: angiotensin II, caveolin, metalloprotease, vascular smooth muscle cells, migration, hypertrophy
1. Introduction
Angiotensin II (AngII) and the AngII type-1 receptor (AT1) play critical roles in mediating cardiovascular diseases such as hypertension and atherosclerosis [1, 2]. It is widely believed that AngII participates in vascular remodeling associated with these diseases that involve hypertrophy, hyperplasia and migration of vascular smooth muscle cells (VSMCs) [3]. Numerous signal transduction cascades have been implicated in vascular remodeling induced by AngII [4, 5]. We have demonstrated that transactivation of the epidermal growth factor receptor (EGFR) through an EGFR ligand production via a metalloprotease such as ADAM17 is indispensable for hypertrophy and migration of VSMCs induced by AngII [6, 7]. However, the detailed molecular mechanism by which AngII and other G protein coupled receptor agonists activate ADAMs to transactivate EGFR remains largely unclear [8].
Recent increasing bodies of evidence highlight the critical roles of temporal and specific localization of the signaling molecules in mediating signal transduction events rapidly and efficiently [9]. In this regard, lipid rafts which consist of a cholesterol-rich membrane micro-domain, and the membrane invaginations caveolae that are the subsets of rafts, serve as a signaling platform to facilitate the association of signaling complexes as “scaffolds” [10, 11]. Here, we hypothesized that lipid rafts and/or caveolae provide a critical regulatory environment for ADAM17-dependent EGFR transactivation via the AT1 receptor.
To support this hypothesis, it has been reported that many signaling molecules involved in the machinery of EGFR transactivation by AngII are preferentially localized to lipid rafts and/or caveolae [12]. Caveolin-1 (Cav1), the major structural protein in caveolae, interacts directly with the AT1 receptor, a subunit of Gq, Src family kinases and EGFR via a consensus motif (caveolin scaffolding domain) present in cytoplasmic domains of these signaling proteins [12]. Cultured rat aortic VSMCs express Cav1 and have been shown to conserve typical caveolar structure at plasma membrane [13]. Further, Src and Abl seem to be involved in the colocalization of Cav1 and the transactivated EGFR in VSMCs [14]. In addition, recent publications suggest (but do not directly prove) the involvement of lipid raft in regulation of ADAM activities in some model systems [15–18]. These findings suggest the critical role of caveolae/lipid raft that provides an environment to assemble essential signaling components in mediating EGFR transactivation. In the present study, we have tested the above-mentioned hypothesis to explore the novel regulatory mechanism of EGFR transactivation via lipid raft/caveolae and subsequent VSMC growth and migration.
2. Materials and Methods
2.1. Reagents
AngII, methyl-β cyclodextrin (mβCD) and filipin III were purchased from Sigma. For stimulation, 100 nM AngII was used since this concentration had maximal effect on ERK activation in VSMCs [19]. mβCD was used at 10 mM since this concentration has been shown to markedly disrupt caveolar structure in cultured VSMCs without affecting cell viability [13]. Phospho-specific antibody for Tyr1068-phosphorylated EGFR was purchased from Biosource International. Phospho-specific antibody for Tyr204-phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2), and antibodies against EGFR, ERK2 and ADAM17 were purchased from Santa Cruz Biotechnology. Antibodies against Cav1 and paxillin were purchased from BD Transduction Laboratories.
2.2. Cell culture
VSMCs were prepared from thoracic aorta of Sprague-Dawley rats by the explant method as described previously [19]. All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and Temple University. VSMCs were subcultured in DMEM containing 10% fetal bovine serum, penicillin and streptomycin. VSMCs from passage 3 to 12, used in the experiments typically showed more than 99% positive immunostaining of smooth muscle α-actin antibody. Cells at 80~90% confluence in culture wells were made quiescent by incubation with serum-free medium for 2–3 days. To avoid any potential phenotypic selection and genetic alteration, VSMCs have been renewed every 2–3 month and VSMCs from frozen stock were never used. The results were confirmed in at least 2 distinct cell lines.
2.3. Adenoviral vectors and infection
Replication-deficient adenovirus encoding myc epitope-tagged canine Cav1 [20] was a generous gift from Dr William Sessa (Yale University, CT, USA). An adenoviral vector encoding alkaline phosphatase-tagged proHB-EGF (HB-EGF-AP) was created using the pCMV HB-EGF-AP vector [21] as a template. The HB-EGF-AP sequence was amplified by PCR and ligated into the pIRES2-EGFP vector (Clontech) at the EcoRI/SmaI site. The fragment containing the HB-EGF-AP, IRES and EGFP sequence was further ligated into the pENTR4 vector (Invitrogen) at the EcoRI/NotI site and then recombined into pAd/CMV/V5-DEST vector (Invitrogen) by a reaction with LR Clonase II (Invitrogen). The adenovirus titers were determined by Adeno-X™ Rapid Titer Kit (BD Biosciences). VSMCs were infected with adenovirus for 2 days as previously described [22]. The infection efficiency was estimated to be 90–100% as defined by infection with adenovirus (50–100 moi) encoding EGFP.
2.4. Immunoblotting
Immunoblotting was performed as previously described [23]. Quiescent VSMCs grown on 6-well plate (~5x105 cells/well) were stimulated for specified durations. The reaction was terminated by the replacement of medium with 100 µL of 1xSDS sample buffer. Each 40 µL of the cell lysates were subjected to SDS-PAGE gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. The membranes were then exposed to primary antibodies overnight at 4 °C. After incubation with the peroxidase linked secondary antibody for 1 h at room temperature, immunoreactive proteins were visualized by a chemiluminescence reaction kit. The results were quantified by densitometry in the linear range of film exposure using CanoScan N670U (Canon) and Un-Scan-It Gel 5.3 software (Silk Scientific), and the ratios, EGFR-p/EGFR and ERK2-p/ERK2 were calculated. An example of data supporting the linearity has been demonstrated [24]. Unless stated otherwise, results were expressed as % increase in which the response to AngII is defined as 100% because the basal signals are more varied depending on film exposure than the stimulated signals.
2.5. Hypertrophy assays
To assess AngII-induced VSMC hypertrophy directly, we have been utilizing a combination of 3 distinct assays measuring cell protein accumulation, cell volume, and cell proliferation/viability, but not a radiolabeled leucine incorporation assay to avoid unnecessary use of a radioactive compound [25–27]. Consistent with a highly cited past report [28], in VSMCs derived from 12 week-old Sprague-Dawley rats, 72 h AngII incubation in serum-free DMEM resulted in increases in cell protein and volume without any significant change in cell proliferation/viability [25–27]. To measure cell protein accumulation, VSMCs grown on 12-well plates (~2x105 cells/well) were incubated with serum-free DMEM for 1 day and infected with adenovirus in serum-free DMEM for 2 days. The cells were further incubated with or without 100 nM AngII for 3 days. After aspiration of the medium, cells were washed twice with ice-cold Hanks balanced salt solution, and the total amount of cellular protein was measured as previously described [25]. It has been reported that the rat VSMC protein accumulation enhanced by 100 nM AngII was less than 20% for 4days [28]. We have observed 10–20% increases of the VSMC protein by AngII depending on the experiment and believe the variation may reflect differences in the cell lines [26, 27]. To measure cell volume, after the pretreatments described in the protein assay method, VSMCs were washed with Hanks balanced salt solution and trypsinized. The cells were then suspended in PBS and the cell volume was measured by Z2 Coulter Particle Count and Size Analyzer as previously described [25]. To evaluate cell viability and proliferation after the pretreatments described in the protein assay method, the viable cell amount was assessed using a CellTiter 96 Aqueous cell proliferation assay kit (Promega) as previously described [25].
2.6. Migration assay
VSMC migration was measured using a monolayer-wounding protocol in which cells migrated from a confluent area into an area that was mechanically denuded of cells. VSMCs (~5x105 cells/well) infected with adenovirus for 2 days were scraped by a metal dental pick (DenTek) and stimulated by 100 nM AngII for 24 h with 5 mM hydroxyurea. VSMC migration was quantified as previously reported [24].
2.7. Intracellular Ca2+ measurements
Intracellular Ca2+ was measured as described previously by using fura2 as an indicator [29]. VSMCs (~105 cells/well) subcultured on coverslips were loaded with 3 µM fura 2-AM at room temperature for 45 min. The fura 2 fluorescence was acquired at a frequency of 1 Hz and the intracellular Ca2+ values were then obtained as described.
2.8. HB-EGF shedding assay
48 hours after infection of adenovirus encoding HB-EGF-AP together with those encoding Cav1 or the control GFP, VSMCs grown on 12-well plates (~2x105 cells/well) were stimulated with AngII or mβCD for 60 min. The time point was chosen according to a past publication [13]. The HB-EGF-AP secreted into the culture medium was assessed as described previously [29]. We have validated the HB-EGF-AP shedding assay reflecting ADAM17 activity by utilizing dominant-negative mutant of ADAM17 [6] and a metalloprotease inhibitor [29].
2.9. Sucrose density fractionation
Membrane rafts were fractionated from VSMCs as described in our previous work [30]. Briefly, VSMCs (~4x106 cells from two 10 cm dishes) were scraped into an ice-cold, detergent-free Tricene buffer (pH 7.4) and centrifuged to precipitate nuclear material. The resulting supernatant was mixed with 30% Percoll in Tricene buffer and subjected to ultracentifugation for 25 min (77,000 g). The separated plasma membranes were collected, sonicated, and mixed with 60% sucrose (to a final concentration of 40%) before being overlaid with a 35–5% step sucrose gradient and subjected to overnight ultracentrifugation (87,400 g). Fractions were collected from the top sucrose layer, and proteins were precipitated using a solution of 0.1% wt/vol deoxycholic acid in 100% wt/vol trichloroacetic acid. Samples were then subjected to SDS-PAGE and immunoblotted using indicated antibodies.
2.10. Statistical analysis
Data are presented as mean±SEM. Groups were compared using ANOVA followed by Tukey post hoc test, student t test, or paired t test. The null hypothesis was rejected when p<0.05.
3. Results
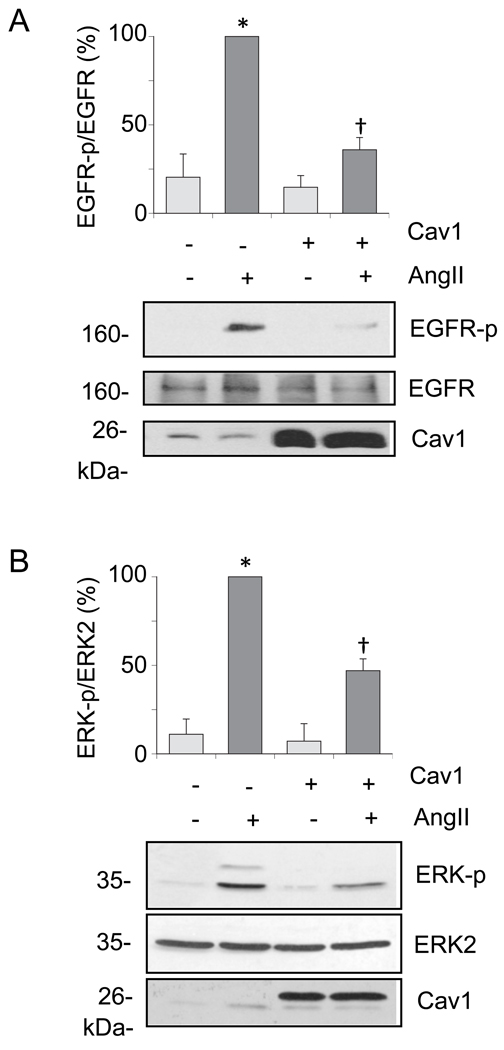
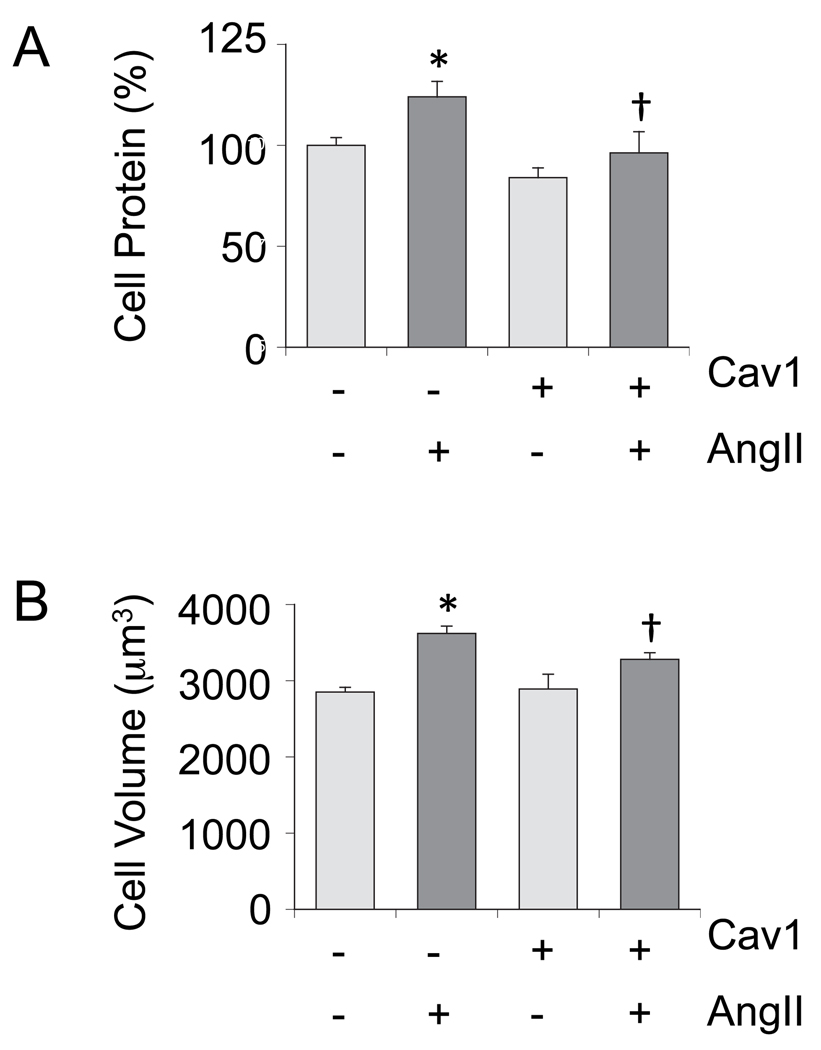
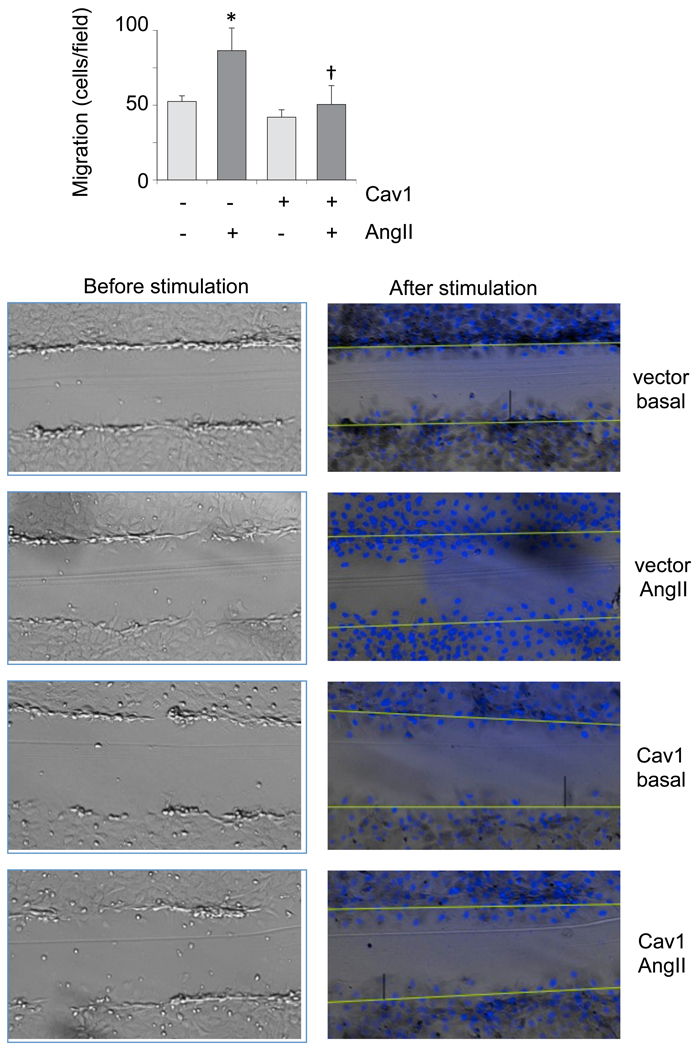
Cav1 gene transfer leads to Cav1 accumulation at the lipid raft fractions associated with enhanced formation of caveolae in cultured VSMCs [31]. To study whether Cav1 and caveolae have any regulatory role in AngII signal transduction linked to vascular remodeling, VSMCs were infected with adenovirus encoding Cav1 or a control vector, and EGFR transactivation and subsequent ERK2 activation induced by AngII was evaluated. As shown in Fig. 1A, Cav1 expression resulted in marked suppression of EGFR transactivation induced by AngII compared to the control. Although to a lesser extent, ERK2 activation by AngII was inhibited significantly (Fig. 1B). Our VSMCs express endogenous Cav1, which is detectable for longer exposure of these blots. The Cav1 gene transfer decreased both basal and AngII-induced VSMC protein accumulation (Figure 2A). The Cav1 gene transfer inhibited AngII-induced cell volume increase but did not affect cell volume at the basal condition (Fig. 2B). It also inhibited VSMC migration induced by AngII as examined in a wound-healing assay (Fig. 3). AngII and or Cav1 had no noticeable effect on cell viability or proliferation in these experimental conditions as assessed by a proliferation assay (data not shown).
Fig. 1.
Cav1 gene delivery inhibits the EGFR/ERK cascade activation by AngII in VSMCs. The cells were infected with adenovirus encoding Cav1 or its control vector (100 moi) for 48 hours and stimulated with 100 nM AngII for 2 min (A) or 10 min (B). Cell lysates were subjected to immunoblot analysis with antibodies as indicated. The bar graphs show quantification of the EGFR and ERK1/2 phosphorylation by densitometry. Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control. †p<0.05 compared with the stimulated control.
Fig. 2.
Cav1 gene delivery attenuates hypertrophy of VSMCs stimulated by AngII. After infection with adenovirus encoding Cav1 or the control vector (100 moi), VSMCs were stimulated by AngII (100 nM) for 3 days. Cell protein accumulation (A) and cell volume (B) were measured. Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control. †p<0.05 compared with the stimulated control.
Fig. 3.
Cav1 gene delivery attenuates migration of VSMCs stimulated by AngII. Confluent VSMCs infected with adenovirus encoding Cav1 or the control vector (100 moi) were scraped by a metal dental pick and stimulated with 100 nM AngII for 24 hours in the presence of 5 mM hydroxyurea to block cell proliferation completely. The nucleus was stained with Hoechst 33342 dye and migrated VSMCs from the wound edge were counted in 4 independent view fields (100x). Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control. †p<0.05 compared with the stimulated control.
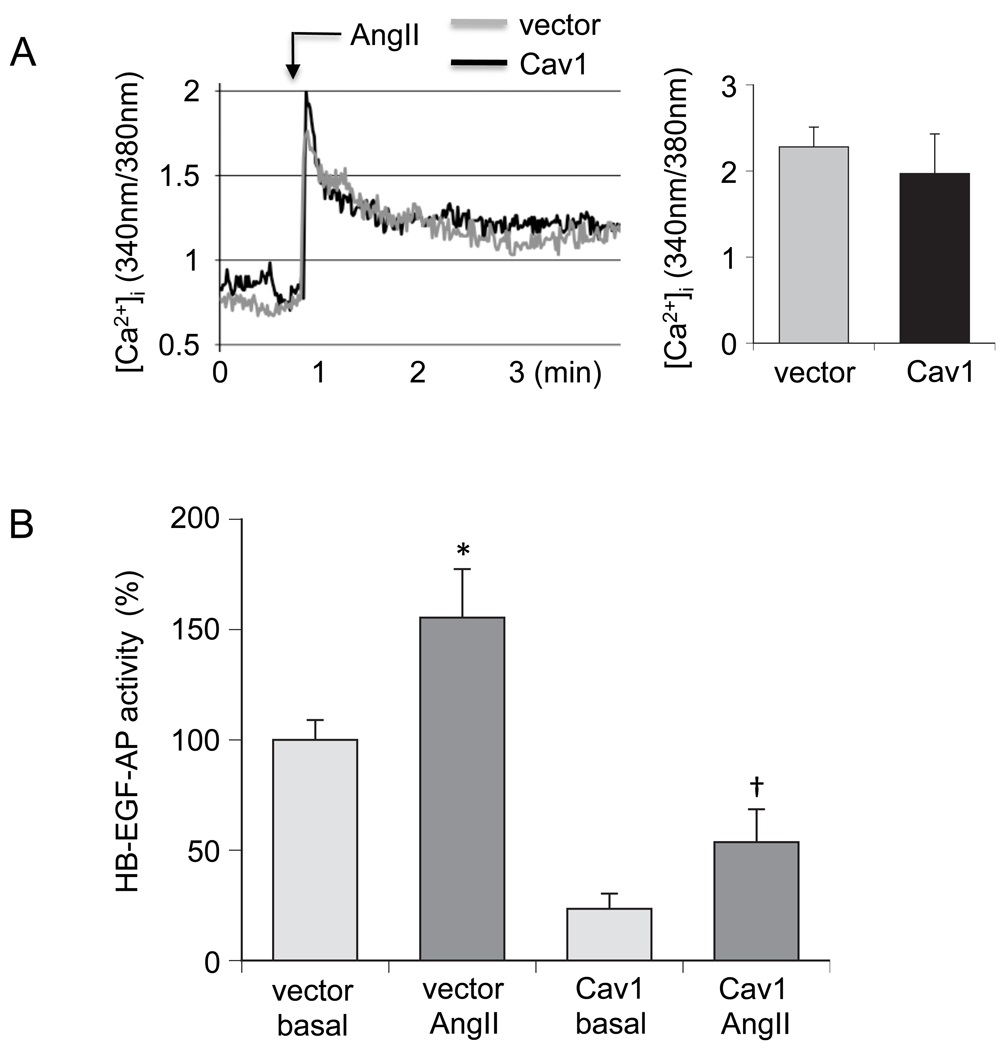
Since the AT1 receptor mediates EGFR transactivation via Gq-coupled intracellular Ca2+ elevation and subsequent HB-EGF shedding in response to AngII in VSMCs [23, 27, 32], we have further examined the effect of Cav1 expression on these signaling events. Although intracellular Ca2+ elevation induced by AngII in VSMCs was not affected, Cav1 expression markedly reduced both basal and AngII-stimulated HB-EGF shedding in VSMCs. While AngII was still able to stimulate HB-EGF shedding beyond the basal control in VSMCs treated with Cav1 adenovirus (Fig. 4), this shedding event remained below the threshold for activation of the EGFR.
Fig. 4.
Effects of the Cav1 gene delivery on intracellular Ca2+ elevation and HB-EGF shedding induced by AngII in VSMCs. A. VSMCs infected with adenovirus encoding Cav1 or its control vector (100 moi) for 48 h were loaded with fura2 and stimulated with 100 nM AngII. Intracellular Ca2+ elevation was measured and the peak stimulations were determined as indicated. B. VSMCs were infected with adenovirus encoding HB-EGF-AP (50 moi) and Cav1 (100 moi) or HB-EGF-AP (50 moi) and the control vector (100 moi) for 48 hours. Cells were then stimulated with 100 nM for 60 min and AP activity in the medium was determined. Data are mean±SEM of 3 experiments. *p<0.05 compared with the basal control. †p<0.05 compared with the stimulated control.
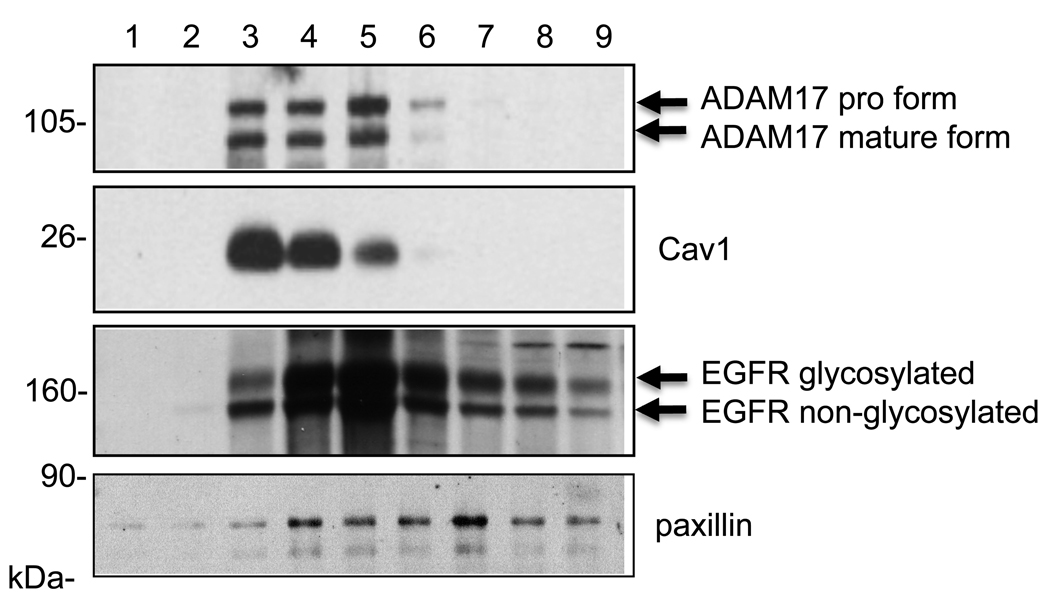
We have demonstrated that a membrane metalloprotease, ADAM17, mediates HB-EGF shedding via the AT1 receptor in VSMCs as well as COS7 cells [6, 29]. Since the above observations indicate a potential regulation of ADAM17 by Cav1, we have tested whether ADAM17 is localized to the Cav1-containing lipid raft domains in VSMCs by a non-detergent sucrose gradient membrane fractionation. Consistent with past findings in cultured endothelial cells [30], Cav1 was localized to those fractions of the plasma membrane corresponding to light buoyant density membrane rafts. Although not completely identical, we found that ADAM17 is cofractionated to the similar plasma membrane compartments as Cav1 (Fig. 5). As previously reported in VSMCs, certain populations of the EGFR were distributed preferentially at the lipid raft fraction. Non-glycosylated EGFR was not separated well from the transactivatable glycosylated EGFR due to the higher percentage of acrylamide utilized (10% for the fractionations versus 7.5% used for all other experiments) [33]. In contrast, paxillin was distributed in heavier fractions than those of Cav1 [34]. However, distributions of EGFR and paxillin were broader than those of Cav1 or ADAM17. In addition, the distribution of ADAM17 in the fractions did not differ regardless of AngII stimulation or the Cav1 gene transfer (data not shown).
Fig. 5.
A sucrose density fractionation of membrane proteins reveals cofractionation of Cav1 and ADAM17 at lipid rafts. Membrane proteins were fractionated from VSMCs as described in the method section. Samples were then subjected to SDS-PAGE and immunoblotted using indicated antibodies. Data shown are representatives from the 3 independent experiments.
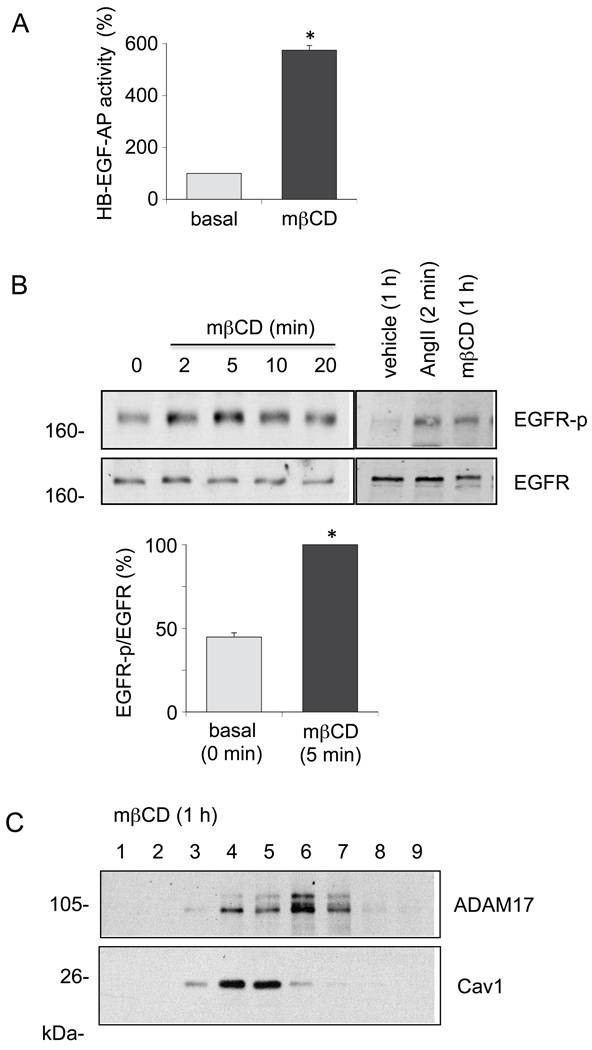
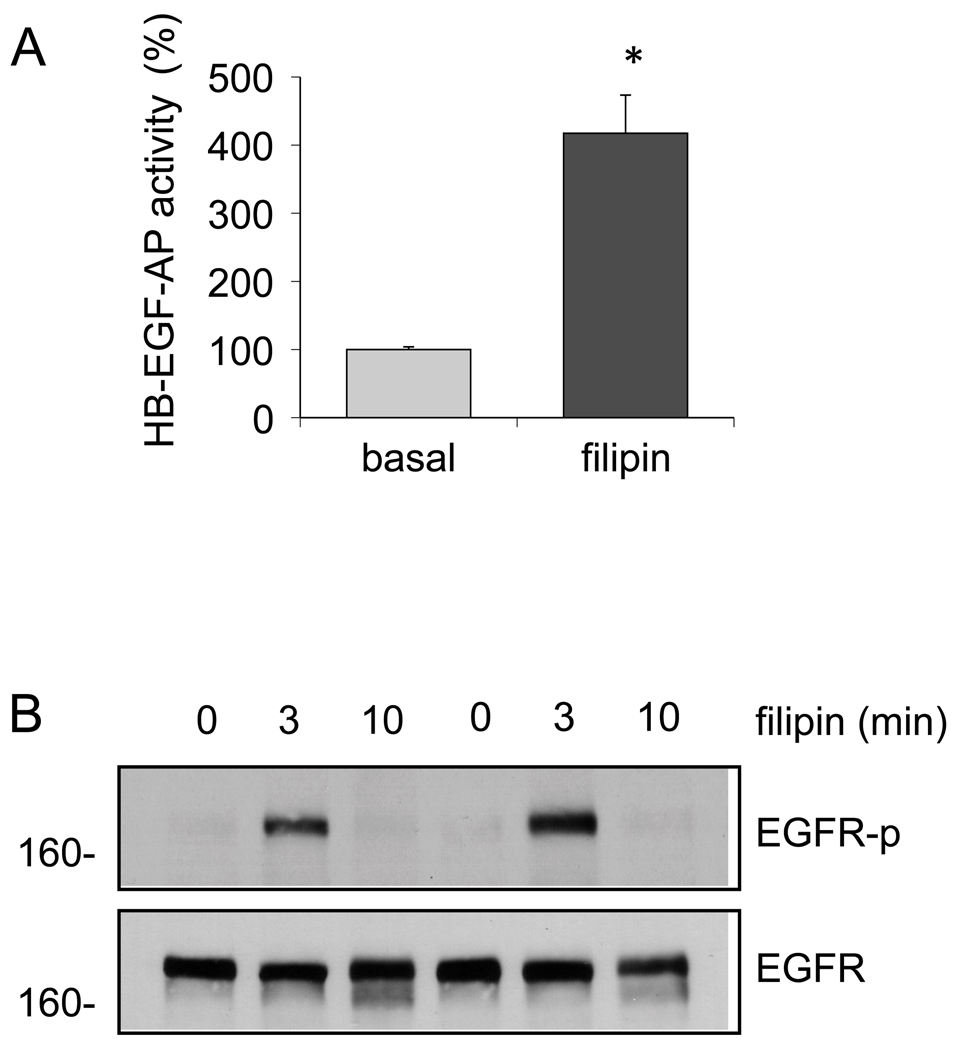
To alternatively test the involvement of Cav1-rich membrane micro-domain in regulation of ADAM17-dependent EGFR transactivation in VSMCs, the cells were treated with mβCD or filipin, reagents frequently utilized to disrupt the lipid raft structures. As shown in Fig. 6, mβCD treatment for 60 min and 2 – 60 min stimulated HB-EGF shedding and EGFR transactivation in VSMCs, respectively. mβCD shifted the ADAM17 distributions to the right in the sucrose gradient fractionation probably due to the lipid raft disruption. Filipin also stimulated HB-EGF shedding and EGFR transactivation (Fig. 7), although the transactivation was transient compared to the mβCD response. Taken together, these data suggest that Cav1-rich membrane domains may provide a negative regulatory environment for ADAM17-dependent EGFR transactivation and subsequent VSMC remodeling induced by AngII.
Fig. 6.
mβCD stimulates HB-EGF shedding and EGFR transactivation in VSMCs. A. VSMCs were infected with adenovirus encoding HB-EGF-AP (50 moi) for 48 hours. Cells were then stimulated with 10 mM mβCD for 60 min and AP activity in the medium was determined. Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control. B. VSMCs were stimulated with 10 mM mβCD for the indicated time periods or with 100 nM AngII for 2 min. Cell lysates were subjected to immunoblot analysis with antibodies as indicated. The bar graphs show quantification of the EGFR phosphorylation by densitometry. Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control. C. VSMCs were incubated with 10 mM mβCD for 1 hour. Membrane proteins were fractionated from VSMCs as described in the method section. Samples were then subjected to SDS-PAGE and immunoblotted using indicated antibodies. Data shown are representatives from the 3 independent experiments.
Fig. 7.
Filipin stimulates HB-EGF shedding and EGFR transactivation in VSMCs. A. VSMCs were infected with adenovirus encoding HB-EGF-AP (50 moi) for 48 hours. Cells were then stimulated with 5 µg/ml filipin for 60 min and AP activity in the medium was determined. Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control. B. VSMCs were stimulated with 5 µg/ml filipin for the indicated time periods. Cell lysates were subjected to immunoblot analysis with antibodies as indicated. Data shown are representatives from the 3 independent experiments.
4. Discussion
The major findings of the present study are that 1) Cav1 expression interferes with the AngII-induced EGFR transactivation cascade between intracellular Ca2+ elevation and ADAM17-dependent HB-EGF shedding, leading to inhibition of AngII-induced VSMC hypertrophy and migration, 2) in quiescent VSMCs, ADAM17 is cofractionated with Cav1 in the lipid raft fractions, and 3) disruptions of the lipid raft structure by 2 distinct pharmacological reagents lead to HB-EGF shedding and EGFR transactivation in VSMCs.
It has been proposed that Cav1 provides a scaffolding role for the association between the AT1 receptor and the EGFR [14, 35] and that non-receptor tyrosine kinases c-Src and cAbl are involved in the trafficking of the receptors in the Cav1 rich lipid raft fractions in VSMCs [14]. In general, our current findings are in line with these past reports in that Cav1-rich plasma membrane is a critical regulatory element of the AT1 receptor signal transduction but also they demonstrate a novel regulatory role of Cav1 to control the activity of ADAM17, a metalloprotease essential for the EGFR transactivation in VSMCs.
Our findings are in contrast to an earlier study reporting that 1 hour-pretreatment of mβCD inhibited AngII-induced EGFR transactivation in VSMCs [13]. This could be explained due to a down-regulation of EGFR by the experimental condition. In fact, our data showed that mβCD stimulated EGFR transactivation in VSMCs. To support our findings that Cav1 expression inhibits EGFR transactivation and subsequent VSMC hypertrophy and migration induced by AngII, it has been reported that loss of Cav1 expression was associated with neointima formation after an arterial injury and Cav1 adenovirus inhibited proliferation in response to platelet-derived growth factor in VSMCs [36]. Moreover, proliferation was enhanced in Cav1 deficient VSMCs both in vitro and in vivo [37, 38]. Although these and our data suggest a therapeutic potential of Cav1 gene transfer against vascular remodeling, silencing Cav1 expression has also been shown to diminish AngII-induced EGFR transactivation in VSMCs [39]. Taken together, there seems to be an appropriate level of Cav1 expression necessary for AngII-induced EGFR transactivation and either deletion or excess expression of Cav1 leads to the inhibition of the EGFR transactivation through distinct mechanisms.
Cofractionation of ADAM17 with Cav1 in the lipid raft fractions suggests that Cav1-rich membrane domains may provide a negative regulatory environment for activity of ADAM17 and its subsequent signal transduction and functions stimulated by AngII. The hypothesis is further supported by the fact that mβCD and filipin, reagents which disrupt the caveolae/lipid rafts structure are able to stimulate shedding of HB-EGF, a well-characterized ADAM17 substrate. Filipin-induced EGFR transactivation was completely abolished by a specific ADAM inhibitor in rat VSMCs (The authors’ unpublished data). In addition, mβCD and filipin have been shown to stimulate ADAM17-dependent shedding of other substrates in some cell systems [15, 18]. However, since AngII was still able to increase HB-EGF shedding and protein accumulation from their reduced basal levels in VSMCs over-expressing Cav1, this mechanism may play a partial role in the activation pathways for ADAM17 or protein synthesis in response to AngII. The discrepancies among the data collected may involve different experimental procedures as well as distinct sensitivities of the assays utilized.
Although many signaling molecules implicated under the regulation at the caveolae interact with Cav1 via a consensus sequence at their cytosolic domains [10–12], ADAM17 does not have such a sequence in its cytosolic domain. Also, we could not confirm the direct interaction with Cav1 and ADAM17 by immunoprecipitation experiments with or without AngII stimulation in VSMCs (unpublished data). ADAM17 has been shown to interact amid a wide array of membrane or membrane associable proteins with signal regulatory capabilities [8, 40]. Therefore, ADAM17 may interact with an unidentified Cav1 binding or caveolae localizing protein in VSMCs, which is critical for ADAM17 regulation by AngII.
For perspective, as demonstrated in the present study, Cav1 represents an important regulator of the vascular AngII signal transduction and its potential therapeutic application is strongly expected. Our findings support a protective role of Cav1 gene transfer to prevent a progression of pathological vascular remodeling associated with the enhanced renin-angiotensin system.
Research Highlights
Caveolin-1 gene transfer interferes with the angiotensin II-induced EGF receptor transactivation cascade including ADAM17 activation.
Caveolin-1 gene transfer inhibited angiotensin II-induced hypertrophy and migration of vascular smooth muscle cells.
ADAM17 colocalizes with Cav1 in the lipid raft fractions.
A disruption of the lipid raft structure leads to HB-EGF shedding and EGF receptor transactivation.
Acknowledgments
We thank Dr. Katherine J. Elliott for critical reading of this manuscript. This work was supported by National Institute of Health Grants, HL076770 (S.E.), HL086551 (V.R.), by American Heart Association Established Investigator Award, 0740042N (S.E.), and by W. W. Smith Charitable Trust Grant, H0605 (S.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None.
References
- 1.Daugherty A, Cassis L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc Med. 2004;14:117–120. doi: 10.1016/j.tcm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Crowley SD, Gurley SB, Coffman TM. AT(1) receptors and control of blood pressure: the kidney and more. Trends Cardiovasc Med. 2007;17:30–34. doi: 10.1016/j.tcm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 4.Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, et al. Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension. 2006;48:534–540. doi: 10.1161/01.HYP.0000237975.90870.eb. [DOI] [PubMed] [Google Scholar]
- 5.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, et al. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–e137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112:417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006 Jul;291(1):C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 9.Smith NJ, Luttrell LM. Signal switching, crosstalk, and arrestin scaffolds: novel G protein-coupled receptor signaling in cardiovascular disease. Hypertension. 2006;48:173–179. doi: 10.1161/01.HYP.0000232641.84521.92. [DOI] [PubMed] [Google Scholar]
- 10.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 11.Callera GE, Montezano AC, Yogi A, Tostes RC, Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens. 2007;16:90–104. doi: 10.1097/MNH.0b013e328040bfbd. [DOI] [PubMed] [Google Scholar]
- 12.Ushio-Fukai M, Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension. 2006;48:797–803. doi: 10.1161/01.HYP.0000242907.70697.5d. [DOI] [PubMed] [Google Scholar]
- 13.Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, et al. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- 14.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res. 2005;97:829–836. doi: 10.1161/01.RES.0000185322.46009.F5. [DOI] [PubMed] [Google Scholar]
- 15.von Tresckow B, Kallen KJ, von Strandmann EP, Borchmann P, Lange H, Engert A, et al. Depletion of cellular cholesterol and lipid rafts increases shedding of CD30. J Immunol. 2004;172:4324–4331. doi: 10.4049/jimmunol.172.7.4324. [DOI] [PubMed] [Google Scholar]
- 16.Thiel KW, Carpenter G. ErbB-4 and TNF-alpha converting enzyme localization to membrane microdomains. Biochem Biophys Res Commun. 2006;350:629–633. doi: 10.1016/j.bbrc.2006.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakatsuki S, Kurisaki T, Sehara-Fujisawa A. Lipid rafts identified as locations of ectodomain shedding mediated by Meltrin beta/ADAM19. J Neurochem. 2004;89:119–123. doi: 10.1046/j.1471-4159.2003.02303.x. [DOI] [PubMed] [Google Scholar]
- 18.Zimina EP, Bruckner-Tuderman L, Franzke CW. Shedding of collagen XVII ectodomain depends on plasma membrane microenvironment. J Biol Chem. 2005;280:34019–34024. doi: 10.1074/jbc.M503751200. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi S, Matsumoto T, Motley ED, Utsunomiya H, Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J Biol Chem. 1996;271:14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- 20.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci U S A. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, et al. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274:36843–36851. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 23.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Kimura K, Shirai H, Eguchi K, Higuchi S, Hinoki A, et al. Endothelial nitric oxide synthase inhibits G12/13 and rho-kinase activated by the angiotensin II type-1 receptor: implication in vascular migration. Arterioscler Thromb Vasc Biol. 2009;29:217–224. doi: 10.1161/ATVBAHA.108.181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, Eguchi K, Ohtsu H, Higuchi S, Dhobale S, Frank GD, et al. Activation of Endothelial Nitric Oxide Synthase by the Angiotensin II Type-1 Receptor. Endocrinology. 2006;147:5914–5920. doi: 10.1210/en.2006-0834. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima H, Frank GD, Shirai H, Hinoki A, Higuchi S, Ohtsu H, et al. Novel role of protein kinase C delta Tyr311 phosphorylation in vascular smooth muscle cell hypertrophy by angiotensin II. Hypertension. 2008;51:232–238. doi: 10.1161/HYPERTENSIONAHA.107.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, et al. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149:3569–3575. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 29.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, et al. G protein coupling and second messenger generation are indispensable for a metalloprotease-dependent HB-EGF shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 30.Carlile Klusacek M, Rizzo V. Endothelial cytoskeletal reorganization response to protease activated receptor-1 (PAR1) stimulation is mediated by membrane rafts but not caveolae. Am J Physiol Heart Circ Physiol. 2007;293:H366-H75. doi: 10.1152/ajpheart.01044.2006. [DOI] [PubMed] [Google Scholar]
- 31.Ingueneau C, Huynh-Do U, Thiers JC, Negre-Salvayre A, Salvayre R, Vindis C. Caveolin-1 sensitizes vascular smooth muscle cells to mildly oxidized LDL-induced apoptosis. Biochem Biophys Res Commun. 2008;369:889–893. doi: 10.1016/j.bbrc.2008.02.134. [DOI] [PubMed] [Google Scholar]
- 32.Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem. 2001;276:7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- 33.Konishi A, Berk BC. Epidermal growth factor receptor transactivation is regulated by glucose in vascular smooth muscle cells. J Biol Chem. 2003;278:35049–35056. doi: 10.1074/jbc.M304913200. [DOI] [PubMed] [Google Scholar]
- 34.Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, et al. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res. 2010;107:787–799. doi: 10.1161/CIRCRESAHA.110.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivares-Reyes JA, Shah BH, Hernandez-Aranda J, Garcia-Caballero A, Farshori MP, Garcia-Sainz JA, et al. Agonist-induced interactions between angiotensin AT1 and epidermal growth factor receptors. Molecular Pharmacology. 2005;68:356–364. doi: 10.1124/mol.104.010637. [DOI] [PubMed] [Google Scholar]
- 36.Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, et al. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003;23:1521–1527. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- 37.Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004;43:8312–8321. doi: 10.1021/bi049609t. [DOI] [PubMed] [Google Scholar]
- 38.Hassan GS, Williams TM, Frank PG, Lisanti MP. Caveolin-1-deficient aortic smooth muscle cells show cell autonomous abnormalities in proliferation, migration, and endothelin-based signal transduction. Am J Physiol Heart Circ Physiol. 2006;290:H2393–H2401. doi: 10.1152/ajpheart.01161.2005. [DOI] [PubMed] [Google Scholar]
- 39.Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]
- 40.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]