Abstract
Transcranial magnetic stimulation (TMS) can induce masking by interfering with ongoing neural activity in early visual cortex. Previous work has explored the chronometry of occipital involvement in vision by using single pulses of TMS with high temporal resolution. However, conventionally TMS intensities have been high and the only measure used to evaluate masking was objective in nature. Recent studies have begun to incorporate subjective measures of vision, alongside objective ones. The current study goes beyond previous work in two regards. First, we explored both objective vision (an orientation discrimination task) and subjective vision (a stimulus visibility rating on a four-point scale), across a wide range of time windows with high temporal resolution. Second, we used a very sensitive TMS-masking paradigm: stimulation was at relatively low TMS intensities, with a figure-8 coil, and the small stimulus was difficult to discriminate already at baseline level. We hypothesized that this should increase the effective temporal resolution of our paradigm. Perhaps for this reason, we are able to report a rather interesting masking curve. Within the classical-masking time window, previously reported to encompass broad SOAs anywhere between 60 and 120 ms, we report not one, but at least two dips in objective performance, with no masking in-between. The subjective measure of vision did not mirror this pattern. These preliminary data from our exploratory design suggest that, with sensitive TMS masking, we might be able to reveal visual processes in early visual cortex previously unreported.
Keywords: Multiple, Masking, Vision, Suppression, Transcranial, Occipital, Recurrent, Phase
Introduction
Studies into visual awareness often adopt masking paradigms. In such paradigms, a visual target stimulus is presented briefly, and a secondary visual stimulus (mask) is presented either before or after the target stimulus. By using different stimulus onset asynchronies (SOAs) between target and mask, the level of processing of the visual target can be modulated (Breitmeyer and Ogmen 2006). This indicates that there is a temporal organization in visual cortex that underlies the establishment of conscious vision. At certain points in time, this organization should be left unperturbed, or vision will be abolished.
To target the organization in early visual regions specifically, a brain interference method can be used. Transcranial magnetic stimulation (TMS) is ideal in this regard, because it affords a high temporal resolution in brain interference. Using TMS at different SOAs from a visual target stimulus has yielded different time windows at which the organization of stimulated early visual cortex should be left unperturbed (Kammer 2007a). Classically, a time window around 100 ms from visual target stimulus onset was revealed (Amassian et al. 1989). But in addition, earlier time windows have been reported (Corthout et al. 1999; Paulus et al. 1999; Laycock et al. 2007) and TMS effects in later time windows as well (Heinen et al. 2005; Camprodon et al. 2010). When using longer-lasting motion stimuli and relating TMS to stimulus offset, again two masking periods were identified rather than one (Silvanto et al. 2005; Koivisto et al. 2010).
Most TMS-masking studies at any rate identified the ‘classical’ masking effect around 100 ms. However, the precise latency at which masking was strongest, or even present, seems to differ substantially between studies (Kammer 2007a, b). For instance, masking effects have been reported at 60–75 ms (Romei et al. 2007), 80–100 ms (Amassian et al. 1989), and 120 ms (Kammer et al. 2003). Thus, since it seems impossible to pinpoint chronometrically when a TMS pulse has its effect, a window spanning perhaps 60–120 ms is regarded to house a TMS-masking effect. We will continue to refer to this as the ‘classical-masking time window’.
Nearly all previous TMS-masking studies evaluated vision by an objective measure. If the research interest concerns visual awareness, one might argue that a subjective measure is appropriate. After all, a core aspect of visual consciousness is the subjective and reportable nature of it (Boyer et al. 2005; Lau and Passingham 2006; Koivisto et al. 2010). Moreover, the combination of both objective and subjective measures of vision may yield very interesting insights. Boyer et al. (2005) asked their subjects on every trial to discriminate the orientation of a bar and to moreover subjectively report whether they consciously perceived it or not. When evaluating objective discrimination performance on trials that were reported not to be consciously perceived, performance was still far above-chance. This has been dubbed ‘TMS-induced blindsight’. That particular study applied TMS pulses only in the classical-masking time window around 100 ms. This finding raises the question of what happens in other time windows when both subjective and objective measures of vision are implemented. Are dissociations time specific? How does unimpaired objective performance square with previous reports of strong objective TMS masking at this late time window? More generally: does objective visual performance reflect the subjective experience of the visual stimuli? Could both measures be used alternately, or in unison, to investigate visual consciousness? There is to date no study that performed a rigorous high-resolution exploration of the chronometry of TMS masking of subjective and objective vision. This was one goal of the current work.
The second goal was to evaluate what happens to the TMS-induced masking curve, over time, if the TMS-masking paradigm is made to be as sensitive as possible. The purported advantage of single-pulse TMS is the high temporal resolution (Wassermann et al. 2008). However, even if the magnetic field change of a single TMS pulse lasts under a millisecond, it does not mean the neural effects of that pulse are equally brief. In fact, TMS-induced differential effects on neuron behavior can last up to hundreds of milliseconds (Moliadze et al. 2003, 2005). The stronger the TMS pulse is, the longer these neural effects can be expected to last. Traditional TMS-masking studies used circular coils (e.g., Amassian et al. 1989; Beckers and Homberg 1991), and indeed they are yet often used (Boyer et al. 2005). The spatial extent of disturbance may affect how long the neural effects last as well. Indeed, as pointed out, most TMS-masking studies reveal masking in broad time windows around 100 ms, rather than a single peak in one time window (say between 60 and 120 ms, see above). A figure-8 coil can at least provide focal stimulation, addressing this spatial resolution issue. It seems plausible that within a time window of tens of milliseconds early visual cortex is differentially involved in visual processing over this time, rather than occupied in the same way uniformly (see e.g., Foxe and Simpson 2002). The question is whether TMS masking is sensitive enough to reveal putative multiple stages of such involvement. One last factor that may affect the masking curve is the stimulus itself. Larger and clearer stimuli may need higher TMS intensities to yield any masking effect, since the information that needs to be suppressed is more salient. Higher intensities in turn may again decrease temporal resolution. Thus, a more sensitive paradigm might require non-salient stimuli.
Altogether, these considerations lead us to hypothesize that, to obtain the highest effective temporal resolution, one should (1) use difficult and small visual target stimuli, (2) a spatially precise TMS coil, to allow masking with (3) the lowest TMS intensities possible (that still yield masking effects), and (4) high temporal sampling rate (minimizing gaps between measured SOAs). In the current study, we thus implemented such a ‘sensitive TMS paradigm’ and explored its effects on both objective and subjective visual processing.
Methods
Participants
Fourteen participants (four men) volunteered for this study. None had a history of neuropsychiatric disorders, and all had normal or corrected-to-normal vision. The experiment was approved by the local medical-ethical committee, and written informed consent was obtained before participation. An independent medical supervisor screened all participants for TMS experimentation safety prior to measurements, and participants were compensated with gift certificates. Since localization of TMS target site involved elicitation of phosphenes, the four participants in whom phosphenes could not be elicited reliably or in the proper visual field location were not tested in the experiment, leaving a total of ten included participants.
Stimuli and tasks
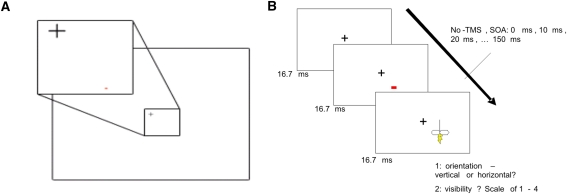
We used stimuli in the form of small rectangular bars (~0.025 × ~0.035 visual degrees), that could be either horizontal or vertical in orientation, and either red or blue in color (for an example, see Fig. 1). Each stimulus was always presented for only 16.7 ms, one frame on a monitor set to 60-Hz refresh rate. Average luminance of the bars was 65 cd/m2, of background 180 cd/m2. The experiment involved a forced-choice discrimination task, and a subjective visibility rating task. In the objective task, subjects were required to indicate, using button presses, whether the orientation of the visual target stimulus was vertical (right index finger) or horizontal (right middle finger). These forced-choice discriminations were made first and constituted the objective measure of visual awareness. After this judgment, participants were asked, in the same trial, to indicate subjectively the visibility of the stimulus. This was done using a 4-point scale, in which value ‘1’ indicated “I didn’t see the orientation at all”; ‘2’ indicated “I don’t think I saw the orientation”; ‘3’ indicated “I think I did see the orientation”; ‘4’ indicated “I saw the orientation clearly”. We indicated that these ratings were a subjective rating of how clearly the stimulus was perceived, not a confidence rating. Moreover, we emphasized that the descriptions were a guideline, but that consistency of the rating throughout the experiment was most important. Subjects were given ample time to practice with both discrimination tasks and subjective ratings.
Fig. 1.
Stimulus and design. a The stimulus was a horizontal or vertical bar. The stimulus was deliberately small and difficult to distinguish, in line with a ‘sensitive TMS-masking paradigm’ (see main text). b Stimuli were presented for 16.7 ms only, after which a TMS pulse at one SOA between 0 and 150 ms (except on no-TMS trials) was administered. Participants were asked to first make a forced-choice judgment about stimulus orientation. Second, they were asked to indicate on a scale of 1–4 how clearly they perceived the orientation of the stimulus
Stimuli were presented on a standard TFT computer monitor (Samsung Syncmaster 931BF), using Presentation software (Neurobehavioral Systems, San Francisco, CA, USA). Viewing distance was 60 cm. The location of the stimuli was 4 degrees visual angle to the lower left or right of fixation—depending on where participants perceived phosphenes more easily in the required eccentricity and polar angle (on the left side in three subjects). Subjects fixated throughout the experiment.
Design and TMS parameters
The experiment consisted of one session per subject. A total of 17 conditions (16 time windows + no-TMS) with 15 trials per time window resulted in 255 orientation task trials per session.1 Each trial took a minimum of 6 s, with subjects otherwise determining the pace by means of button presses—manually indicating readiness to proceed to the next trial. The different TMS SOAs (0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, and 150 ms) and no-TMS trials were randomly interleaved throughout the whole experiment. The different conditions were grouped in 15 task blocks of 17 trials. The order of these blocks was pseudo-randomized. Subjects could take a break whenever and however long they wanted between task blocks, but were asked to at least take a break halfway through the session to rest.
In 4 subjects, a separate ‘NoTMS’ run was added, containing 20 trials. Because of the absence of a TMS pulse in the NoTMS condition during the actual experiment, participants could respond differently due to the expectance of a pulse. Indeed, the average no-TMS performance over all four participants was 0.90 in the separate no-TMS block, while it was 0.78 for these four participants in the no-TMS trials interleaved in the actual experiment. Considering this large difference, we decided not to use no-TMS as baseline, but rather to take a more conservative approach (see section on “Analysis”).
Biphasic TMS pulses were administered with a figure-8 coil (MC-B70), over early occipital cortex. The coil handle was oriented laterally to the right, with initial current direction going away from the handle. Target site localization was based initially on phosphene induction. If a TMS-induced phosphene (nearly) overlapped with the visual field location that corresponded to where visual stimuli would later be presented, the coil was fixed. Stimulation intensity was individually calibrated initially at ~10% above phosphene threshold. When in initial masking trials the participants indicated rarely to subjectively perceive the stimulus orientation, TMS intensity was decreased to the point where participants indicated sometimes to perceive the stimulus, and sometimes not. The average intensity finally used was 58% machine output (MagPro X100).
Since our results show differences between time windows that were 10 ms apart, we were prompted to reconfirm whether our experimental setup was accurate in this range. To this end, we performed calibration measurements. A photodiode was applied to the TFT monitor, measuring luminance changes on an oscilloscope. Simultaneously, the TTL pulse leaving the experimental computer’s parallel port (which otherwise triggers the TMS machine with negligible delay) was measured. Using this setup, we tested latencies of visual stimulus and TTL pulses presented by the stimulation program also used in the experiment, in several neighboring visual stimulus-TMS triggering pulse SOAs (i.e., time window conditions). By these measurements, we could not confirm if all time windows were not shifted by milliseconds, although a stable monitor response delay should have been offset by a programmed TMS discharge delay. These calibration measurements were rather performed to check whether requested increases in TMS SOA were reliable. Indeed, the measurements confirmed that requested 10-ms increases in SOA between stimulus and TMS pulse were perfectly (accurate to the sub-millisecond range) presented by the experimental setup. These calibration measurements thus showed conclusively that whichever temporal pattern of findings we obtained (see “Results”) was not due to technical decisions/limitations.
Analysis
Since this study was exploratory in nature, we included all ten measured participants in the analyses. This conservatively included also participants where individual masking curves showed limited effects (see Fig. 3). In fact, two of the three subjects, where no multiple-dip pattern of results could be observed (see “Results”), had performance across conditions consistently above no-TMS. Thus, masking did not work at all in these participants. Nonetheless, they were included in all analyses—in acknowledgment of the exploratory nature. Note that this conservative procedure across the board makes any and all effects in the final group results probably weaker than necessary. Since our no-TMS baseline was compromised, probably due to an ‘oddball-like’ disruptive effect of the unexpected absence of the TMS pulse in randomly interleaved no-TMS trials, we also took a most conservative baseline to base our statistical tests on. We took the average (performance or visibility rating) of all trials in the experiment to compare fluctuations around this mean to. This we refer to as the ‘all-round mean’. Note that potential TMS effects would already bias this all-round mean downward, making it harder to detect these TMS effects. With 17 conditions, it is not feasible to do statistical correction for multiple comparisons, given TMS effect sizes in general but probably sensitive TMS-masking effect sizes in particular. Thus, we reasoned that taking this conservative all-round mean as reference for each individual time window, combined with several conservative analysis decisions mentioned above, would moderately compensate for the multiple comparisons problem. To thus explore TMS-masking effects, we performed pairwise t test analyses (one-tailed) on each time window separately. The same analysis was performed for both objective orientation discrimination performance and subjective visibility ratings.
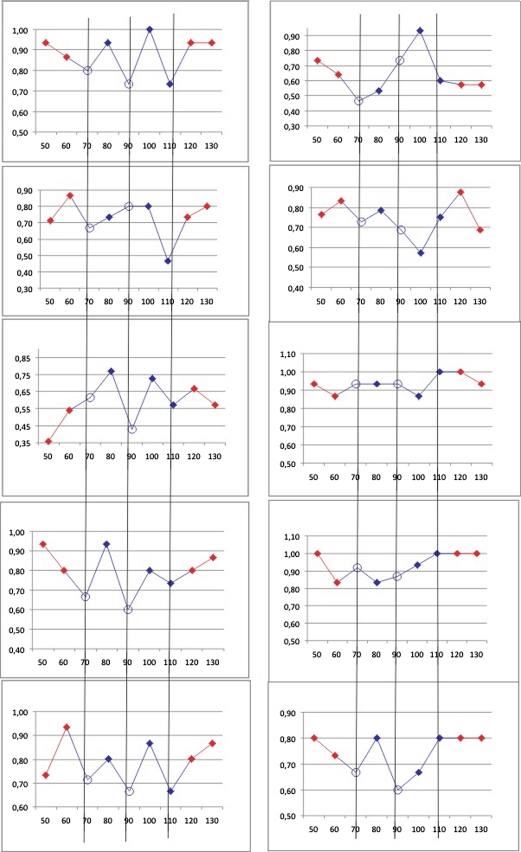
Fig. 3.
Individual masking curves. Focused on the time windows of interest (blue SOAs 70–110), the individual performances over SOAs (between 50 and 130 shown) are presented. Note that proportion correct is on the vertical axis, but that the scale varies across participants. The individual curves are expectedly noisier than the group curve, and not every participant displays the group pattern in his/her individual performance. But the individual curves demonstrate that the group effect is not an artifact of averaging, and indeed many participants display more than one dip
As presented in the Results section, our data required a follow-up analysis to investigate whether objective masking precedes subjective masking. For this analysis, we looked at individual masking curves and extracted peak masking latencies for these two measures. Peak masking was defined as the lowest performance (or visibility rating) over all SOAs. If peak masking occurred at two SOAs with equal masking, the average of these SOAs was taken as peak masking latency value.
Results
In this study, we applied single-pulse TMS over all SOAs between 0 and 150 ms in steps of 10 ms. We measured the effects of TMS pulses on objective visual task performance (an orientation discrimination task) and on subjective orientation visibility rating (on a scale of one to four). Figure 2 shows the resulting data for both measures separately.
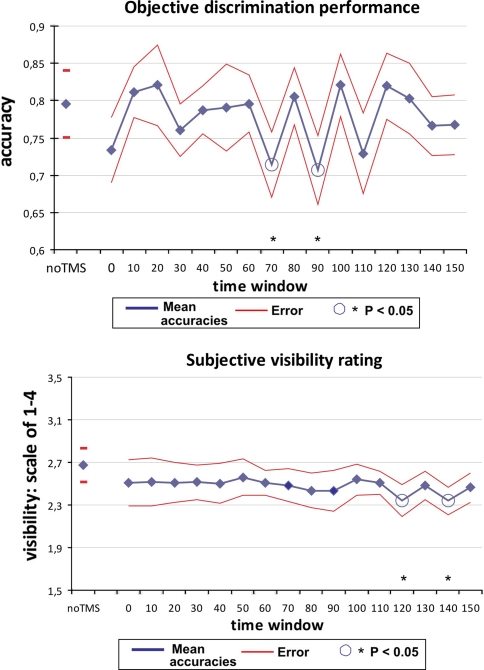
Fig. 2.
Group masking curves. Upper graph the average performance accuracy across participants is shown in blue. SOAs (time between visual stimulus onset and TMS pulse onset) are on the horizontal axis, proportion correct is on the vertical axis. Red curves shading the performance curve represent the standard error of the mean. Open circles reflect a significant decrease from the all-round mean performance. Lower graph the average visibility rating across participants is shown in blue. SOAs are on the horizontal axis, visibility rating between 1 and 4 is on the vertical axis. 2.5 marks the border between ‘seen’ and ‘not seen’. Red curves again reflect standard error of the mean. Open circles reflect a significant decrease from the all-round mean visibility rating
Objective discrimination performance
The upper graph shows the group masking curve of all ten participants, of the objective measure (orientation discrimination task). Whereas classic TMS-masking curves generally display a single broad dip within the classical-masking time window (between 60 and 120 ms, see above), or secondary dips in remote time windows, our data may suggest a different scenario. Orientation discrimination accuracy was decreased in 70 ms, 90 ms, and seemingly in 110 ms, in what seem to be three separate dips in performance. In between these SOAs, at 80 ms and at 100 ms, there seemed to be no masking effect—not a slight decrease of effect, but a real return to baseline. Statistically, the earlier two of these three dips are confirmed to be significant. As explained in the Methods section, we conservatively compared masking effects in individual time windows with the all-round mean. Table 1 reveals the resulting t and P values for these comparisons. Since this was an exploratory study, the number of comparisons was too large to statistically correct for (as discussed in the Methods section). Therefore, results are tentative and future research should aim to replicate and confirm these findings in hypothesis-driven experiments. For the moment, in our comparison to all-round mean, our data suggest that there was a behavioral impairment at 70 ms (t(9) = 2.41; P < 0.05) and at 90 ms (t(9) = 1.93; P < 0.05). Note that time window 0 ms closely approached significance, with (t(9) = 1.80; P = 0.053). In light of previous research (Laycock et al. 2007), this very early dip might also reflect behaviorally relevant processing.
Table 1.
All statistical values from comparison to all-round mean
| Objective performance | Visibility rating | |||
|---|---|---|---|---|
| t value | P value | t value | P value | |
| 0 ms | −1.798 | 0.053 | −0.148 | 0.443 |
| 10 ms | 1.080 | 0.154 | 0.180 | 0.431 |
| 20 ms | 0.630 | 0.272 | −0.300 | 0.386 |
| 30 ms | −0.796 | 0.223 | 0.194 | 0.425 |
| 40 ms | 0.528 | 0.305 | 0.401 | 0.349 |
| 50 ms | 0.383 | 0.355 | 0.998 | 0.172 |
| 60 ms | 0.595 | 0.283 | 0.759 | 0.234 |
| 70 ms | −2.406 | 0.020 | −0.193 | 0.425 |
| 80 ms | 1.170 | 0.136 | −0.601 | 0.281 |
| 90 ms | −1.930 | 0.043 | −0.484 | 0.320 |
| 100 ms | 1.116 | 0.147 | 0.451 | 0.331 |
| 110 ms | −1.114 | 0.147 | 0.313 | 0.381 |
| 120 ms | 1.804 | 0.052 | −3.230 | 0.005 |
| 130 ms | 1.459 | 0.089 | 0.121 | 0.453 |
| 140 ms | −0.234 | 0.410 | −1.839 | 0.050 |
| 150 ms | −0.110 | 0.458 | −0.227 | 0.413 |
Bold values are statistically significant
If different participants have individual masking curves around 100 ms, but slightly displaced temporally, a group masking curve with separate dips could result artifactually from the averaging of these individual curves. To rule out this option, we looked into the individual masking curves. Individual curves were noisier than the group curve, which is to be expected with a total of 15 trials per time window per subject, on which a single accuracy ratio is based. In spite of this, we noticed that many individual participants showed at least two of the three dips (decreases in performance at SOA 70, 90, or 110 compared to the SOAs immediately before and after). To allow inspection of these ‘dips’, we provide all ten individual masking curves (focused on the time windows of interest) in Fig. 3. These include curves of participants in whom masking seemed wholly unsuccessful, but were nonetheless included to be conservative (see “Methods”). With 15 trials per time window, and taking interindividual differences into account, these results seem remarkably consistent. For instance, one of the two participants without any ‘dips’ at the appointed time windows showed two dips at 60 and 80 ms, rather than 70 and 90 ms. Such variability is to be expected, which is why a group curve remains most informative. The individual curves shown in Fig. 3 at any rate do not suggest that the multiple dips in the group map are an averaging artifact based on two or three outliers. Thus, altogether, our data seem to reveal at least two performance dips within the classical-masking time window around 100 ms.
Subjective visibility ratings
Interestingly, the group masking curve on subjective report of visibility did not correspond well to the performance curve (Fig. 2, lower graph). The no-TMS rating was above all TMS pulse time windows, from 0 to 150 ms SOA. When taking, for instance, the visibility rating of time window 0 ms as a reference, there is hardly a deviation across time windows. On a scale of 1–4, in which values 1 and 2 represent ‘I didn’t see it’ and values 3 and 4 represent ‘I did see it’ (to simplify), the average rating was around 2.5—the border between reporting ‘seen’ versus ‘unseen’. This shows that our stimuli really were difficult to perceive, as was our intention in establishing a sensitive TMS-masking paradigm. When performing the statistical comparison of individual time windows versus all-round mean, again two time windows reveal a significant TMS effect; 120 and 140 ms (t(9) = 3.23; P < 0.05, and t(9) = 1.84; P < 0.05, respectively—see Table 1 for all t and P values). It thus seems to make a difference which measure of visual awareness is adopted, when TMS-masking effects seem to occur in a sensitive TMS-masking paradigm. However, we should point out that, although significant, the effects here were exceedingly small. On a scale of 1–4, the differences between the 120 and 140 ms time windows to the average visibility rating were only 0.14 and 0.15, respectively. For comparison, we note that in a different (non-sensitive) TMS-masking study using the same scale (de Graaf et al., in preparation), we found a masking effect on subjective visibility rating around 1.5, which is a full order of magnitude larger. Nonetheless, we acknowledge the statistically significant comparisons. And effect sizes in the objective discrimination task were small as well, compared to masking effects in non-sensitive paradigms. This is to be expected when stimulating at lower intensities than is conventional.
This might lead one to suggest that objective vision is disturbed by TMS before subjective vision (70–90 vs. 120–140 ms, respectively). However, we caution that this is not a valid conclusion based on these results alone. An appropriate analysis to address this question specifically is to look at individual peak masking latencies for objective masking, and for subjective masking, and to compare these directly in a within-subject contrast (see “Methods”). This analysis suggests that the average peak masking latencies for objective and subjective masking curves were 97.5 (SD 27.0) ms and 90.0 (SD 41.4) ms SOA respectively, and not significantly different (t(9) = 0.7; P = 0.50). In our view, it seems that the objective masking curve is interesting and potentially very revealing. The subjective masking curve may not have been sensitive enough to detect these fragile effects.
Discussion
We here present an exploration of TMS-masking effects at different time windows from visual target stimulus onset, using a sensitive TMS-masking paradigm and two measures of visual awareness. We set out to investigate whether a sensitive TMS-masking paradigm might yield different masking curves from those revealed previously. Also, we were interested in whether subjective and objective measures dissociate differentially over time.
Objective versus subjective measures of visual awareness over time
Methodologically, we find that the subjective rating measure of visibility was, in the current study, not sensitive enough to obtain convincing masking effects in the classical-masking window (60–120 ms, see “Introduction”). This is in line with our experiences using the subjective scale, which generally results in the same pattern of masking effects as objective measures of vision, but is less sensitive chronometrically (de Graaf et al. in preparation). At any rate, in this study the behavioral masking effects observed within the classical-masking time window were not mirrored by the subjective measure. The fact that TMS affected discrimination performance but not subjective visibility ratings in time-specific windows is, strictly speaking, a dissociation (de Graaf and Sack 2011). But it is a dissociation opposite to the TMS-induced blindsight that has been reported before (Boyer et al. 2005; Jolij and Lamme 2005). These studies reported that, in the absence of subjective vision, performance was unhampered. In our case, it would seem that with decreased performance, subjective vision was unhampered. But it seems more straightforward to conclude that our measure of subjective vision was less sensitive than the measure of objective vision, than to postulate that objective vision can be disrupted while conscious experience is unchanged. In this regard, it is relevant to keep in mind that the conscious experience of the visual stimuli was always very weak and thus difficult to rate (as is evident from the low visibility rating at baseline). On the other hand, if we take the dips in visibility rating of 120 and 140 ms seriously, then indeed one might speak of a ‘blindsight’-like dissociation, since conscious experience was affected while behavioral processing was not. But this effect size was quite small, so we caution not to stretch interpretations qualitatively where they are supported only slightly quantitatively.
TMS-masking effects in a sensitive paradigm
Our data suggest that, instead of one, there may be at least two dips within the classical-masking time window surrounding 100 ms. We have already emphasized that our experiment was exploratory in nature, and future research should aim to replicate this finding. But the effects are fascinating in our view.
Methodologically, it is promising that our first deliberate attempt to implement a ‘sensitive masking paradigm’ as outlined in the Introduction immediately yields a different pattern of findings from that revealed conventionally. Indeed, we are not aware of previous studies having reported two temporally neighboring masking effects. As outlined in the Introduction, previous TMS-masking studies have reported one dip around 100 ms (starting with Amassian et al. (1989), see Kammer (2007a) for an overview), and sometimes much earlier dips or much later dips (Corthout et al. 1999, 2003; Heinen et al. 2005; Silvanto et al. 2005; Laycock et al. 2007; Camprodon et al. 2010; Koivisto et al. 2010). These secondary dips were, however, always separated from the classical dip by 60–100 ms, whereas we here separated two masking dips only 20 ms apart. Thus, if we for the moment assume that our masking effects do reflect a disruption of early visual cortex processes, the two dips here might reflect a fundamentally different brain process from the alternative dips presented in previous work. The reason previous studies have not identified two dips in such close proximity might be (1) too high TMS intensities, (2) not enough spatial resolution of the used TMS coil (in studies using the round coil), (3) too salient or complex stimuli, or (4) too large gaps between measured SOAs. As hypothesized in the Introduction, we propose that either/all of these paradigmatic factors might serve to effectively decrease temporal resolution; ‘smearing out’ the masking effect over time windows in the masking curve. (Incidentally, we can report that, when asking two participants with multiple dips to return for a high-intensity measurement, we found again only one broad masking dip in the classical masking time window).
Theoretically, we might consider at least two explanations for neighboring dips. Briefly, it might be that there is not one feedforward sweep and one feedback sweep, but rather multiple recurrent processes (e.g., Lamme and Roelfsema 2000). Communications between regions could repeatedly go back and forth (see, e.g., de Graaf et al. 2009) between hierarchical levels, or within—in the form of horizontal recurrent projections. Foxe and Simpson (2002) argued, conceptually and supported by data, that already at the earliest stages of occipital processing, after visual stimulation, recurrent influences may affect ongoing processing. This makes sense ecologically; based on task demands or mere situational context, not all visual input is equally relevant. Indeed, the same authors reported recurrent influences present in the earliest visual evoked potentials (Foxe and Simpson 2002). In our view, the very concept of distributed processing suggests that brain regions must interact intensively and repeatedly, if not continuously, to lead to our complex and yet rapid conscious visual experiences. Thus, multiple recurrent loops may explain multiple, chronometrically neighboring, TMS-masking dips within the broad classical time window. Alternatively, the two dips might correspond to arrival in early visual cortex of M-pathways and P-pathways. Magnocellular (M) pathways are faster than parvocellular (P) pathways (Bullier 2001), and have been hypothesized to arrive at different times, hence possibly allowing TMS to disturb them at different times (see also Paulus et al. 1999). There is no way to distinguish between these options based on our current data.
Conclusion
Future studies might consider further exploring sensitive TMS-masking paradigms to illuminate these issues. Conservatively, we will here conclude that our objective and subjective measures of awareness did not correspond well in a situation of sensitive TMS masking. TMS elicited an interesting pattern for objective performance, which was not mirrored by the subjective masking curve. In the data set here presented, we observed at least two dips of TMS-induced performance decrease, separated by only 20 ms, with no masking in-between. Our new finding of multiple dips within the classical-masking window might be attributed to our sensitive TMS-masking paradigm. We conclude that this pattern of preliminary findings is interesting on both group and individual level and both theoretically and methodologically stimulating for future research.
Acknowledgments
We would like to thank two anonymous reviewers for excellent suggestions that have certainly improved this report. We also acknowledge the Dutch Organization for Scientific Research (NWO) for supporting T.A.G. (personal grant number: 021-002-087) and A.T.S. (personal grant number: 452-06-003).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
In the same session an equal number of trials, using the same stimuli, were obtained using a secondary task for a different experiment involving color judgment: this experiment will not be reported here or elsewhere. The original intentions behind this secondary experiment caused our stimuli to be red on half the orientation trials, and blue on the other half of trials. These color differences were balanced between time windows and of no further influence or import.
References
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Beckers G, Homberg V. Impairment of visual perception and visual short term memory scanning by transcranial magnetic stimulation of occipital cortex. Exp Brain Res. 1991;87:421–432. doi: 10.1007/BF00231859. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Harrison S, Ro T. Unconscious processing of orientation and color without primary visual cortex. Proc Natl Acad Sci USA. 2005;102:16875–16879. doi: 10.1073/pnas.0505332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitmeyer B, Ogmen H. Visual masking: time slices through conscious and unconscious vision. New York: Oxford University press; 2006. [Google Scholar]
- Bullier J. Integrated model of visual processing. Brain Res Brain Res Rev. 2001;36:96–107. doi: 10.1016/S0165-0173(01)00085-6. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Zohary E, Brodbeck V, Pascual-Leone A. Two phases of V1 activity for visual recognition of natural images. J Cogn Neurosci. 2010;22:1262–1269. doi: 10.1162/jocn.2009.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthout E, Uttl B, Walsh V, Hallett M, Cowey A. Timing of activity in early visual cortex as revealed by transcranial magnetic stimulation. Neuroreport. 1999;10:2631–2634. doi: 10.1097/00001756-199908200-00035. [DOI] [PubMed] [Google Scholar]
- Corthout E, Hallett M, Cowey A. Interference with vision by TMS over the occipital pole: a fourth period. Neuroreport. 2003;14:651–655. doi: 10.1097/00001756-200303240-00026. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, Sack AT. Null results in TMS: from absence of evidence to evidence of absence. Neurosci Biobehav Rev. 2011;35:871–877. doi: 10.1016/j.neubiorev.2010.10.006. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, Jacobs C, Roebroeck A, Sack AT. FMRI effective connectivity and TMS chronometry: complementary accounts of causality in the visuospatial judgment network. PLoS One. 2009;4:e8307. doi: 10.1371/journal.pone.0008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp Brain Res. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Heinen K, Jolij J, Lamme VA. Figure-ground segregation requires two distinct periods of activity in V1: a transcranial magnetic stimulation study. Neuroreport. 2005;16:1483–1487. doi: 10.1097/01.wnr.0000175611.26485.c8. [DOI] [PubMed] [Google Scholar]
- Jolij J, Lamme VA. Repression of unconscious information by conscious processing: evidence from affective blindsight induced by transcranial magnetic stimulation. Proc Natl Acad Sci USA. 2005;102:10747–10751. doi: 10.1073/pnas.0500834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer T. Masking visual stimuli by transcranial magnetic stimulation. Psychol Res. 2007;71:659–666. doi: 10.1007/s00426-006-0063-5. [DOI] [PubMed] [Google Scholar]
- Kammer T. Visual masking by transcranial magnetic stimulation in the first 80 milliseconds. Adv Cogn Psychol. 2007;3:177–179. doi: 10.2478/v10053-008-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer T, Scharnowski F, Herzog MH. Combining backward masking and transcranial magnetic stimulation in human observers. Neurosci Lett. 2003;343:171–174. doi: 10.1016/s0304-3940(03)00376-8. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Mantyla T, Silvanto J. The role of early visual cortex (V1/V2) in conscious and unconscious visual perception. Neuroimage. 2010;51:828–834. doi: 10.1016/j.neuroimage.2010.02.042. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/S0166-2236(00)01657-X. [DOI] [PubMed] [Google Scholar]
- Lau HC, Passingham RE. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc Natl Acad Sci USA. 2006;103:18763–18768. doi: 10.1073/pnas.0607716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock R, Crewther DP, Fitzgerald PB, Crewther SG. Evidence for fast signals and later processing in human V1/V2 and V5/MT+: a TMS study of motion perception. J Neurophysiol. 2007;98:1253–1262. doi: 10.1152/jn.00416.2007. [DOI] [PubMed] [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. J Physiol. 2003;553:665–679. doi: 10.1113/jphysiol.2003.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliadze V, Giannikopoulos D, Eysel UT, Funke K. Paired-pulse transcranial magnetic stimulation protocol applied to visual cortex of anaesthetized cat: effects on visually evoked single-unit activity. J Physiol. 2005;566:955–965. doi: 10.1113/jphysiol.2005.086090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W, Korinth S, Wischer S, Tergau F. Differential inhibition of chromatic and achromatic perception by transcranial magnetic stimulation of the human visual cortex. Neuroreport. 1999;10:1245–1248. doi: 10.1097/00001756-199904260-00017. [DOI] [PubMed] [Google Scholar]
- Romei V, Murray MM, Merabet LB, Thut G. Occipital transcranial magnetic stimulation has opposing effects on visual and auditory stimulus detection: implications for multisensory interactions. J Neurosci. 2007;27:11465–11472. doi: 10.1523/JNEUROSCI.2827-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cereb Cortex. 2005;15:1736–1741. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH. The Oxford handbook of transcranial stimulation. New York: Oxford University Press; 2008. [Google Scholar]