Abstract
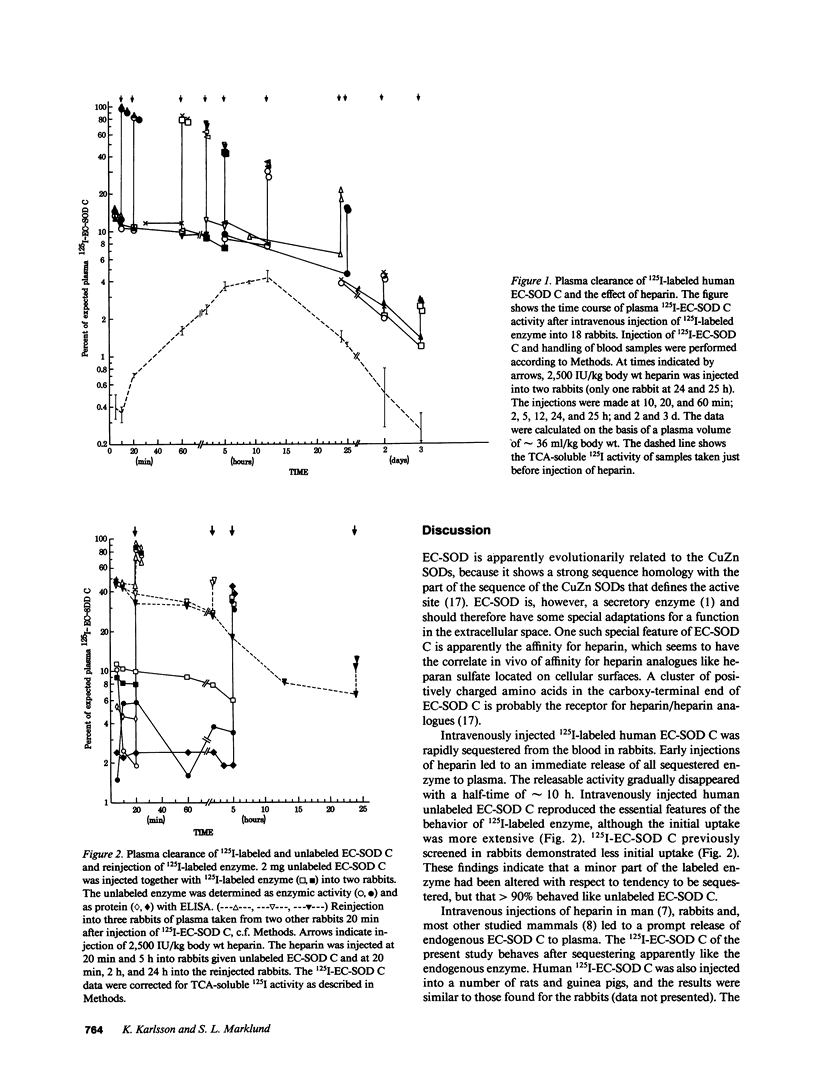
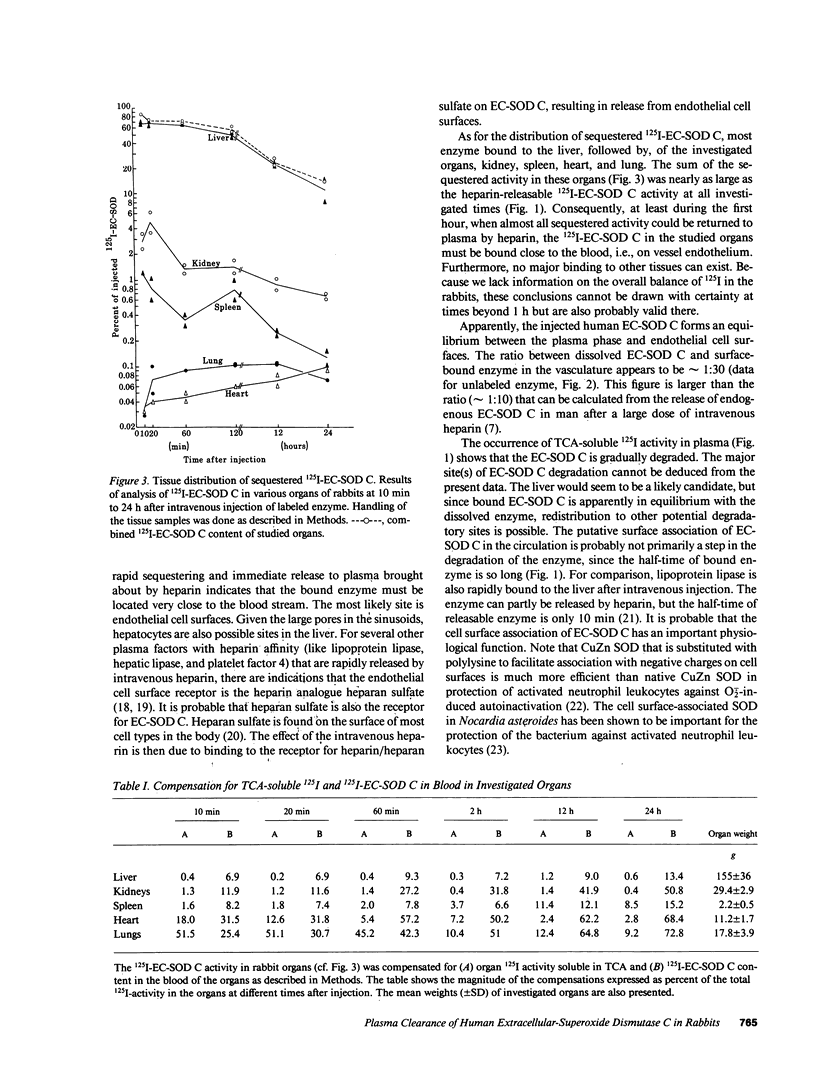
Extracellular-superoxide dismutase (EC-SOD) is heterogenous in the vasculature with regard to heparin affinity and can be separated into three fractions: A, without affinity; B, with weak affinity; and C, with relatively strong heparin affinity. The plasma clearance of intravenously injected 125I-labeled and unlabeled human EC-SOD C was studied in rabbits. About 90% of injected 125I-EC-SOD C was eliminated from the blood within 5-10 min. Injection of heparin after 10 or 20 min led to an immediate release of all sequestered 125I-EC-SOD C back to the blood plasma. Later injections of heparin led to diminished release, although release could still be demonstrated after 72 h. A half-time of approximately 10 h could be calculated for heparin-releasable 125I-EC-SOD C. Unlabeled EC-SOD C, determined as enzymic activity and with ELISA, was likewise sequestered and released to the same degree as 125I-labeled EC-SOD C by heparin as tested at 20 min and 5 h. The immediacy of the heparin-induced release indicates that the sequestered enzyme had been bound to endothelial cell surfaces. The length of the half-time suggests that the putative cell surface binding has a physiological function and is not primarily a step in enzyme degradation. The distribution of sequestered 125I-labeled EC-SOD C to different organs was determined at times between 10 min and 24 h. Of the organs, the liver contained the most 125I-EC-SOD C, followed by kidney, spleen, heart, and lung. At all investigated times, the content in the analyzed organs was nearly as large as the amount that could be promptly released to plasma by intravenous heparin. This indicates that almost all 125I-EC-SOD C in the organs was present on endothelial cell surfaces and was not bound by other tissue cell surfaces, or was present within the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch C., Dawes J., Pepper D. S., Wasteson A. Binding of platelet factor 4 to cultured human umbilical vein endothelial cells. Thromb Res. 1980 Jul 1;19(1-2):129–137. doi: 10.1016/0049-3848(80)90412-0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Bjelle A., Elmqvist L. G. Superoxide dismutase isoenzymes of the synovial fluid in rheumatoid arthritis and in reactive arthritides. Ann Rheum Dis. 1986 Oct;45(10):847–851. doi: 10.1136/ard.45.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984 Oct;74(4):1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Properties of extracellular superoxide dismutase from human lung. Biochem J. 1984 May 15;220(1):269–272. doi: 10.1042/bj2200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. A simple specific method for the determination of the hemoglobin content of tissue homogenates. Clin Chim Acta. 1979 Mar 1;92(2):229–234. doi: 10.1016/0009-8981(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Ohman M., Marklund S. L. Plasma extracellular superoxide dismutase and erythrocyte Cu,Zn-containing superoxide dismutase in alcoholics treated with disulfiram. Clin Sci (Lond) 1986 Apr;70(4):365–369. doi: 10.1042/cs0700365. [DOI] [PubMed] [Google Scholar]
- Robinson-White A., Baylin S. B., Olivecrona T., Beaven M. A. Binding of diamine oxidase activity to rat and guinea pig microvascular endothelial cells. Comparisons with lipoprotein lipase binding. J Clin Invest. 1985 Jul;76(1):93–100. doi: 10.1172/JCI111983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinder L., Bengtsson G., Olivecrona T. Rapid removal to the liver of intravenously injected lipoprotein lipase. Biochim Biophys Acta. 1979 Oct 26;575(1):166–173. doi: 10.1016/0005-2760(79)90142-5. [DOI] [PubMed] [Google Scholar]
- Wallinder L., Peterson J., Olivecrona T., Bengtsson-Olivecrona G. Hepatic and extrahepatic uptake of intravenously injected lipoprotein lipase. Biochim Biophys Acta. 1984 Oct 4;795(3):513–524. doi: 10.1016/0005-2760(84)90181-4. [DOI] [PubMed] [Google Scholar]



