Abstract
Neurons that produce gonadotropin-releasing hormone (GnRH) are the final common pathway by which the brain regulates reproduction. GnRH neurons are regulated by an afferent network of kisspeptin-producing neurons. Kisspeptin binds to its cognate receptor on GnRH neurons and stimulates their activity, which in turn provides an obligatory signal for GnRH secretion, thus gating down-stream events supporting reproduction. We have developed kisspeptin antagonists to facilitate the direct determination of the role of kisspeptin neurons in the neuroendocrine regulation of reproduction. In vitro and in vivo studies of analogues of kisspeptin-10 with amino substitutions have identified several potent and specific antagonists. A selected antagonist was shown to inhibit the firing of GnRH neurons in the brain of the mouse and to reduce pulsatile GnRH secretion in female pubertal monkeys; the later supporting a key role of kisspeptin in puberty onset. This analog also inhibited the kisspeptin-induced release of luteinizing hormone (LH) in rats and mice and blocked the postcastration rise in LH in sheep, rats, and mice, suggesting that kisspeptin neurons mediate the negative feedback effect of sex steroids on gonadotropin secretion in mammals. The development of kisspeptin antagonists provides a valuable tool for investigating the physiological and pathophysiological roles of kisspeptin in the regulation of reproduction and could offer a unique therapeutic agent for treating hormone-dependent disorders of reproduction, including precocious puberty, endometriosis, and metastatic prostate cancer.
Introduction
The production of gonadotropin-releasing hormone (GnRH) by neurons that reside in the forebrain is the primary effector of gonadotropin secretion, which is critical for downstream regulation of gamete and sex steroid hormone production by the testes and ovaries. Neurons that produce GnRH are modulated by feedback actions of sex steroids and a host of other factors, including photoperiod, metabolic signals, and stress (Porkka-Heiskanen et al., 1997; Richardson et al., 2004; Centeno et al., 2007). However, the afferent inputs transducing these effects into changes in GnRH secretion have remained elusive. A key candidate transducer emerged in 2003 through genetically linked mutations in a G-protein-coupled receptor (gpr-54) (de Roux et al., 2003; Seminara et al., 2003). gpr-54 was known to be the cognate receptor for neuropeptides, called kisspeptins, which are differentially processed from a precursor protein encoded by the KiSS-1 gene (Kotani et al., 2001), originally identified as a metastasis inhibitor (Lee et al., 1996). Kisspeptin/gpr-54 signaling has since emerged as a linchpin in the neuroendocrine regulation of reproduction (Popa et al., 2008). Subsequently, it was learned that kisspeptin-producing neurons directly innervate GnRH neurons and that kisspeptins are potent secretagogues for GnRH and luteinizing hormone (LH) (Gottsch et al., 2004; Irwig et al., 2004; Popa et al., 2008). Moreover, kisspeptin neurons have been implicated as conduits that relay humoral and environmental signals to GnRH neurons (Castellano et al., 2005; Smith et al., 2005; Estrada et al., 2006; Revel et al., 2006; Greives et al., 2007). Thus, kisspeptin/gpr-54 signaling represents a nodal point in the neuroendocrine regulation of reproduction and a potential therapeutic target.
Kisspeptin and gpr-54 are expressed in a number of other tissues, but clear functions have not been established. Kisspeptin immunoreactivity is markedly elevated in pregnancy (Horikoshi et al., 2003), produced by trophoblast cells (Janneau et al., 2002) and inhibits trophoblast invasion (Bilban et al., 2004). Kisspeptins have also been reported to stimulate vasoconstriction (Mead et al., 2007), effect synaptic transmission (Arai et al., 2005) and insulin secretion (Hauge-Evans et al., 2006).
Through their modulation of the hypothalamic–pituitary–gonadal (HPG) axis, GnRH analogues are extensively used to treat hormone-dependent diseases including prostatic, breast and ovarian cancers, endometriosis, uterine fibroids, and precocious puberty, as well as to induce ovulation for infertility and in vitro fertilization (Conn and Crowley, 1994; Millar et al., 2004). Because kisspeptin exerts such a powerful stimulatory effect on GnRH and gonadotropin secretion, it is plausible that intervention at the level of the kisspeptin/gpr-54 signaling may have potential for treatment of these conditions, possibly with more subtly regulated control than achieved with GnRH analogues.
The development of kisspeptin antagonists would, therefore, provide the wherewithal to elucidate the role of kisspeptin in reproductive development and puberty, in positive and negative gonadal feedback, as well as in metabolic and stress effects on the reproductive system.
We report on a systematic structure-activity study of kisspeptin analogs and the development of potent and specific antagonists, and present a detailed in vivo analysis of the effects in rodents, sheep, and monkeys. The studies reveal a pivotal role for kisspeptin in controlling puberty and in steroid hormone feedback on gonadotropin secretion underlining the potential wide utility of these antagonists for research and clinical applications.
Materials and Methods
Peptides
Materials.
Human kisspeptin-10 and peptide analogues 186–191, 200–203, 206–213, and 228–248 were custom synthesized by EZBiolabs. Peptide analogues for preliminary studies were synthesized to a purity of >80% (range, 83–99% measured by HPLC) and peptide 234, which was selected for detailed studies was synthesized at a purity of >95%. The authenticity of peptides was confirmed by mass spectrometry. The source of all other reagents is indicated in the text.
In vitro assays for antagonist activity
Cell culture.
Chinese hamster ovary (CHO) cells stably expressing the human gpr-54 receptor (CHO/gpr-54) were obtained from Prof. G. Vassart (University of Brussels, Brussels, Belgium). The cells were maintained in DMEM (Sigma) supplemented with 10% fetal calf serum, 2% glutamine, and 1% penicillin (10,000 units/ml)/streptomycin (10,000 mg/ml) at 37°C in a humidified 5% CO2 atmosphere.
Whole-cell receptor binding assay.
Assays were performed as described previously (Lu et al., 2007; Coetsee et al., 2008). Briefly, kisspeptin-10 was prepared at 1:100 dilution in HEPES modified DMEM supplemented with 0.1% bovine serum albumin and 125I-labeled kisspeptin-10 (100,000 cpm/0.5 ml). Cell monolayers were placed on ice and exposed to 0.5 ml peptide (10 pm–10 μm); the cells were then incubated at 4°C for 3 h. After 3 h, cells were washed twice with ice-cold Dulbecco's PBS (DPBS; with calcium and magnesium) and then 0.5 ml 0.1 m sodium hydroxide (NaOH) were added to cells for 20 min while shaking. Lysates were transferred to plastic tubes and bound radioactivity counted on the Wallac 1470 Wizard gamma counter (PerkinElmer Life and Analytical Sciences).
Inositol phosphate stimulation assay.
Before stimulation, CHO/gpr-54 cells were washed twice with DPBS (without calcium or magnesium) then incubated overnight with 3H-myoinositol, labeled HEPES-modified DMEM with 1% penicillin/streptomycin at 37°C. HEPES-modified DMEM supplemented with 1% penicillin/streptomycin and 1% lithium chloride (0.5 ml) was added to cells for 30 min at 37°C to block inositol phosphate (IP) hydrolysis. Cells were then stimulated with 0.5 ml kisspeptin-10 and analogues (10 pm–1 μm) diluted at 1:100 in the above media for 1 h at 37°C, then with 10 mm formic acid at 4°C for 1 h to lyse cells. Lysates were transferred to plastic tubes containing 0.5 ml Dowex resin to bind the radioactive IP, and the resin was then washed with 1 ml water. The resin was next washed with 60 mm ammonium formate/5 mm sodium tetraborate followed by 1 m ammonium formate/0.1 m formic acid to release the bound radiation. Then, 800 μl of the radioactive solution were transferred to scintillation vials containing 2.5 ml scintillation fluid and radioactivity counted on a beta counter for 60 s. Experiments were repeated 3–5 times. IP production was plotted as mean values ± SEM and analyzed by using a two-way ANOVA followed by Bonferroni post hoc test (p ≥ 0.05).
IP antagonism assay.
CHO/gpr-54 cell monolayers were stimulated with 0.25 ml kisspeptin (10 nm) alone or in combination with 0.25 ml peptide analogues (100 pm–1 μm), to investigate the inhibition of kisspeptin stimulation of IP production. Experiments were repeated 3–5 times. IP production was plotted as mean values ± SEM and analyzed using a two-way ANOVA followed by Bonferroni post hoc test (p ≥ 0.05).
GnRH neuron firing
Animals.
Firing of GnRH neurons were recorded in brain slices from transgenic female mice in which green fluorescent protein (GFP) is genetically targeted to GnRH neurons (Suter et al., 2000). Mice were housed on a 14 h light/10 h dark cycle, with lights off at 4:30 P.M., and were maintained on Harlan 2916 rodent chow and water ad libitum. All procedures were approved by the Animal Care and Use Committee of University of Virginia and were conducted within the guidelines of the National Research Council's Guide for the Care and Use of Laboratory Animals. Adult female GnRH–GFP mice were ovariectomized (OVX) under isoflurane (Abbott Laboratories) anesthesia. Postoperative analgesia was provided by a long-acting local anesthetic (0.25% bupivacaine; 7.5 μl/site; Abbott Laboratories). At the time of surgery, mice received SILASTIC (Dow Corning) capsules containing 0.625 μg estradiol in sesame oil. Recordings were done 2–4 d after surgery in the A.M., when estradiol has been demonstrated to have a negative feedback effect (Christian et al., 2005). No more than four cells from a single animal were recorded, all in different slices.
Brain slice preparation.
Brain slices were prepared using a previously described method (Nunemaker et al., 2002). Briefly, all solutions were bubbled with a 95% O2/5% CO2 mixture throughout the experiments and for at least 15 min before exposure to the tissue. The brain was rapidly removed and placed in ice-cold, high-sucrose saline solution containing 250 mm sucrose, 3.5 mm KCl, 26 mm NaHCO3, 10 mm glucose, 1.25 mm Na2HPO4, 1.2 mm MgSO4, and 2.5 mm MgCl2. Coronal 300 μm brain slices were cut with a Vibratome 3000 (Technical Products, International). Slices were incubated for 30 min at 30–32°C in a solution of 50% high-sucrose saline and 50% normal saline (NS) containing 135 mm NaCl, 3.5 mm KCl, 10 mm glucose, 1.3 mm Na2HPO4, 1.2 mm MgSO4, and 2.5 mm CaCl2 and then were transferred to a solution of 100% NS at room temperature and kept at least 30 min and no more than 6 h before recording.
Electrophysiological recordings.
Targeted extracellular recordings (also known as loose-patch) were used to study effects of kisspeptin-10 and peptide 234 on GnRH neuron firing activity (Pielecka and Moenter, 2006). Because low resistance seals (<50 MΩ) do not influence the cell membrane, this approach is a minimally invasive method for monitoring the endogenous electrical activity of a single cell. Although these events are not action potentials per se, they accurately reflect changes in the action potential firing rate. For simplicity, we have used the phrases firing rate, firing pattern, and/or firing activity to refer to these events.
Individual brain slices were placed in a recording chamber continuously superfused with oxygenated NS solution and kept at 29–31°C. Cells were visualized with an Olympus BX50WI upright fluorescent microscope with infrared differential interference contrast (Opelco). GnRH neurons were identified by brief illumination at 470 nm to visualize the GFP signal. Patch borosilicate pipettes (World Precision Instruments), which ranged from 1.5–3 ΩM were filled with normal HEPES-buffered solution containing 150 mm NaCl, 10 mm HEPES, 10 mm glucose, 2.5 mm CaCl2, 1.3 mm MgCl2, and 3.5 mm KCl. Pipettes were placed in contact with the GnRH neurons using an MP-285 micromanipulator (Sutter Instruments). Seal resistances ranged from 8 MΩ and either remained stable or increased during recording up to as high as 50 MΩ. Current traces were obtained using an EPC-8 amplifier (HEKA) with the PulseControl XOP (Instrutech) running in Igor Pro (Wavemetrics) on the G4 Macintosh computer (Apple Computer) to acquire data. A voltage-clamp mode with a pipette holding potential of 0 mV, filtering at 10 kHz, digitized with an ITC-18 acquisition interface (Instrutech), was used for the recordings.
Experimental design.
Human kisspeptin-10, 1 nm (Phoenix Pharmaceuticals) was applied via the incubation bath. Different doses (1, 10, 100 nm) of peptide 234 were used to examine its antagonistic action on kisspeptin-10 activation of gpr-54. Positive control cells were recorded for a 10-min stable baseline and then treated with kisspeptin-10 for 5 min, followed by a 15-min washout. Experimental cells were recorded for a 5-min stable baseline in NS, followed by 10 min in peptide 234 (1, 10, or 100 nm), then 5 min in kisspeptin-10 plus the same dose of antagonist, followed by a wash in NS. Washing with a solution containing antagonist yielded similar results (1 nm peptide 234; n = 5 cells; data not shown). One cell in the 10 nm peptide 234 group showed no activity; addition 15 mm KCl confirmed cell viability and recording integrity.
Data analysis.
Using programs written for Igor Pro, extracellularly recorded events were counted and binned at 1-min intervals to identify changes in firing rate of GnRH neurons. Binned event data were analyzed using Microsoft Excel for the mean firing rate before kisspeptin-10 treatment (baseline, note this is during peptide 234 treatment in cells in the antagonist treatment groups) during kisspeptin-10 application (treatment), and during washout. For cells in the antagonist treatment group, firing rates in NS versus antagonist before kisspeptin-10 application were also compared. Mean firing rate was determined by dividing the total number of events detected by the duration of recording in each condition; 2 min were skipped after drug changes to eliminate transition periods. Because GnRH neuron firing activity changes with time (Nunemaker et al., 2002; Pielecka and Moenter, 2006), the fold change for each treatment group was calculated. Groups were compared using ANOVA followed by Bonferroni post hoc test.
Effects of peptide 234 on GnRH release in female rhesus monkeys
Animals.
Four female rhesus monkeys, born and raised in the Wisconsin National Primate Research Center, were used in this study. They were housed in pairs (cages, 172 × 86 × 86 cm) in rooms with 12 h of light/12 h of darkness and controlled temperature (22°C). The animals were fed a standard diet of Harlan 20% Protein Primate Diet twice each day and supplemented with fresh fruit several times per week. Water was available ad libitum. The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the National Institutes of Health (NIH) and United States Department of Agriculture.
Experimental design.
The effects of peptide 234 on GnRH release were examined using the microdialysis method. This method allows collection of dialysate samples for GnRH measurement while peptide 234 was infused through the semipermeable membrane, as described previously (23,24). Peptide 234 dissolved in CNS perfusion fluid was infused in the stalk median eminence region through the microdialysis probe for 30 min, whereas dialysates were continuously collected. For a control, vehicle was similarly infused. Each animal was examined with peptide 234 (10 nm concentration) or vehicle at a random order in the same experiment, a 2-h minimum interval between two challenges. Two of the four animals were examined twice in separate experiments totaling six experiments. During the entire experiment, monkeys were placed in proximity to a companion monkey, given constant access to food and water, and were provided frequently with fruit, cereal, raisins, and other snacks. The mean age of sampling was 33.1 ± 0.4 months.
Cranial pedestal implantation.
For collection of perfusates in the stalk-median eminence region, we used the microdialysis method, as described previously (Keen et al., 2008). Before experiments, monkeys at 29.1 ± 1.2 months of age (body weight, 3.6 ± 0.1 kg) were well adapted to the primate chair, experimental environment, and investigator. They were implanted with a cranial pedestal under isoflurane anesthesia, similar to those described previously for push/pull perfusion method (Gearing and Terasawa, 1988). Animals were allowed to recover for at least 1 month before experimentation.
Microdialysis methods.
On the day of experiment, the monkey was placed in the stereotaxic apparatus under ketamine [15 mg/kg, body weight (b.w.)] and medetomidine (0.03–0.05 mg/kg, b.w.) anesthesia. The custom-made guide cannula (CMA 12) consisted of a stainless steel shaft [76.0 mm in length, 0.91 mm outer diameter (o.d.)] and a removable stainless steel stylet (96.0 mm in length, 0.6 mm o.d.), which extruded 20 mm from the guide cannula tip. It was inserted into the skull 5 mm above the stalk median eminence (S-ME) with a hydraulic microdrive unit (MO95-B). The microdrive unit allowed for accurate three-dimensional adjustment of the tip location. The x, y, and z coordinates for the S-ME were calculated using ventriculographs and the final radiographs taken during cranial pedestal implantation surgery. Cannula placement was confirmed with radiographic visualization. After placement of the guide cannula, the monkey was removed from the stereotaxic apparatus and placed into a primate chair. After proper placement in the chair, the inner stylet was removed from the guide cannula, and a custom-made microdialysis probe (a stainless steel shaft 96.0 mm in length, 0.6 mm o.d.), fitted with a membrane (5 mm in length, 0.5 mm o.d.), was inserted into the S-ME through the guide cannula as described previously (Frost et al., 2008; Keen et al., 2008). To reverse the effects of medetomidine, atipamazole (0.15–0.25 mg/kg) was injected (intravenously) into the animal. The animal was fully awake within 1 h after probe insertion.
A CNS perfusion fluid consisting of 147 mm NaCl, 2.7 mm KCl, 1.2 mm CaCl2, 0.85 mm MgCl2, purchased from CMA/Microdialysis with bacitracin (4 U/ml) added, was infused through the inflow tubing at 2 μl/min with the CMA/102 microdialysis pump outfitted with a 1 or 2.5 ml Hamilton gas tight syringe (Reno). Perfusates were continuously collected at 10-min intervals for up to 12 h through the outflow tubing into 12 × 75 mm borosilicate tube, on ice with a fraction collector (Model FC203B). The perfusate samples were immediately frozen on dry ice and stored at −80°C.
GnRH RIA.
RIA was conducted with the antiserum R42 kindly provided by Dr. Terry Nett (Colorado State University, Fort Collins, CO), as described previously (Gearing and Terasawa, 1988). Assay sensitivity was 0.02 pg/tube and intraassay and interassay coefficients of variation were 8.1 and 11.3%, respectively.
Data analysis.
Peaks of GnRH release were identified using the PULSAR algorithm as described previously (Merriam and Wachter, 1982). The mean value of a 30-min period of GnRH collection before and after peptide 234 (or vehicle) challenge was calculated in each experiment. Subsequently, the means (±SEM) of all experiments during each time period were calculated for statistical comparison. The difference between before, during, and after the treatment (within treatment), as well as between treatments (peptide 234 vs vehicle), was examined using two-way ANOVA followed by Tukey's multiple comparison test. Differences were considered significant at p < 0.05.
Dynamic studies on peptide 234 inhibition of LH in male rats
Experimental design.
Adult male rats (body weight, 280–310 g) bred in the vivarium of the University of Córdoba were used. The animals were maintained under constant conditions of light (14 h of light, from 7:00 A.M.) and temperature (22°C), and, before cannula implantation, were kept in groups of four rats per cage with ad libitum access to pelleted food and tap water. Experimental procedures were approved by the Córdoba University Ethical Committee for animal experimentation and were conducted in accordance with the European Union normative for care and use of experimental animals.
The animals were implanted with intracerebroventricular cannulae under light ether anesthesia and caged individually thereafter. To allow delivery of compounds into the lateral cerebral ventricle, the cannulae were lowered to a depth of 4 mm beneath the surface of the skull; the insert point was 1 mm posterior and 1.2 mm lateral to bregma. Functional tests were conducted at least 24–48 h after cannula implantation.
Pharmacological tests in intact males involved 5 μl injections at 60 min intervals (intracerebroventricularly) of 1 nmol doses of the peptide 234. The last injection was accompanied by the injection of an effective (but submaximal) dose of 100 pmol kisspeptin-10 (Phoenix Pharmaceuticals). Animals injected with vehicle (physiological saline) served as controls. Blood samples (250 μl) were taken by jugular venipuncture at 15 and 60 min after each intracerebroventricular injection. In addition, blood samples were taken immediately before initiation of the experiment (time, 0 min) and at 120 min after last injection (time, 240 min). Experimental procedures and kisspeptin doses were selected on the basis of previous studies (Castellano et al., 2005; Navarro et al., 2005; Adachi et al., 2007). For each time point, 10–12 samples were taken per group.
In addition, repeated intracerebroventricular injection of the peptide 234 was also conducted in orchidectomized (ORX) rats. The animals were subjected to bilateral ORX via the scrotal route and 1 week after surgery were subjected to cannula implantation as described above. The test involved three intracerebral injections of the antagonist (1 nmol; at 0, 60, and 120 min of the procedure) and blood sampling by jugular venipuncture in basal conditions (0 min) and 15 min after each intracerebroventricular injection. Additional blood samples were obtained at 180 and 240 min after central administration of peptide 234. For each time point, 10–12 samples were taken per group.
Hormone measurements.
Serum LH levels were measured in a volume of 50 μl using a double-antibody method and radioimmunoassay kits supplied by the NIH (Dr. A. F. Parlow, The National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD). Rat LH-I-10 was labeled with 125I using IODO-GEN precoated tubes, following the instructions of the manufacturer (Pierce), and hormone concentrations were expressed using reference preparation LH-RP-3 as standard. Intraassay and interassay coefficients of variation were below 8 and 10%, respectively. The sensitivity of the assay was 5 pg/tube. In addition, serum testosterone measurements were selectively conducted in blood samples taken 60 min after coadministration of kp-10 and peptide 234 (time, 180 min). To this end, a commercial kit from MP Biomedicals was used, following the instructions of the manufacturer. The sensitivity of the assay was 0.1 ng/tube, and the intraassay coefficient of variation was 4.5%. For each hormone, all samples were measured in the same assay. Accuracy of hormone determinations was confirmed by assessment of rat serum samples of known hormone concentrations used as external controls.
Data analysis.
Hormonal determinations were conducted in duplicate, with a minimal total number of 10 samples per group. When appropriate, in addition to individual time point measurements, integrated LH secretory responses were calculated as the area under the curve (AUC), following the trapezoidal rule. Hormonal data are presented as mean ± SEM. Results were analyzed for statistically significant differences using Student's t test or ANOVA followed by Student–Newman–Keuls multiple range test (Sigma Stat 2.0). p ≤ 0.05 was considered significant.
Effects of peptide 234 on LH levels in male mice
Animals.
Adult male mice (7–8 weeks old) were purchased from The Jackson Laboratory and housed on a 14 h light/10 h dark cycle (lights off at 6:00 P.M.) in groups of 3–5 mice/cage. Rodent chow (Harlan Teklad 7912) and water were available ad libitum. All procedures were approved by the Animal Care and Use Committee of the University of Washington.
Experimental design.
The effects of peptide 234 on the elevated LH secretion of castrated animals were determined. Adult male mice were either castrated under isoflurane anesthesia or left intact. One week later, mice were given two intracerebroventricular freehand infusions (3 μl each) of either vehicle (20% propylene glycol, 13% DMSO) or peptide 234 (15 nmol or 1 nmol) into the lateral ventricle, as described previously (Laursen and Belknap, 1986; Hohmann et al., 2000; Krasnow et al., 2003). The two infusions were identical for each animal and were administered 60 min apart, each under light isoflurane anesthesia. Blood was collected retro-orbitally 30 min after the second infusion and centrifuged for 6 min. Plasma was collected and stored at −20°C until assay for LH. All infusions were performed between 8:00 A.M. and 12:00 P.M. Groups contained n = 6–8 animals.
Having found a strong antagonistic effect of the higher dose (15 nmol) of peptide 234, an additional dose–response study was then performed to better specify effective doses of the analog. Following the same procedure as before, a new cohort of castrated male mice was given two intracerebroventricular infusions of either vehicle or one of three doses of peptide 234 (1, 5, 15 nmol) and blood collected 30 min after the second infusion. Groups contained n = 6–8 animals.
In the next set of experiments, we determined whether a single infusion of peptide 234 would have inhibitory effects on LH secretion and whether the antagonistic effects of peptide 234 were specific to kisspeptin signaling. First, castrated male mice (and a control group of intact males) were given a single intracerebroventricular infusion (3 μl) of either vehicle or peptide 234 (5, 15 nmol), and blood was collected retro-orbitally 30 min later. Next, a separate cohort of intact males were treated first with a 3 μl i.c.v. infusion of vehicle or peptide 234 and then, 5 min later, administered a second 3 μl i.c.v. infusion of either kisspeptin (Phoenix Pharmaceuticals) or artificial CSF (aCSF). Blood was collected 30 min after the second infusion and assayed for LH. The dose of kisspeptin was 100 fmol (previously shown to be effective in eliciting an LH response in mice), and the dose of peptide 234 was 100 pmol. Groups contained n = 6–8 animals.
Hormone measurements and statistical analyses.
For all experiments, serum LH levels were measured via radioimmunoassay by the University of Virginia Ligand Assay Core Laboratory. Samples were assayed in duplicate, and the intraassay and interassay coefficients of variation were below 6.1 and 6.7%, respectively. The sensitivity of the assay was 0.04 ng/ml. For each experiment, all samples were measured in the same assay. Values are presented as group means ± SE, and differences between groups were determined using one-way ANOVA, with post hoc analysis determined by Fisher's protected least significant difference (PLSD) (Statview 5.0.1). In all cases, statistical significance level was set at p < 0.05.
Effect of peptide 234 on LH in ovariectomized ewes
Experimental procedure.
All experimental procedures were conducted under a protocol approved by the Monash School of Biomedical Sciences Animal Ethics Committee. Adult Corriedale ewes were housed under natural lighting and were bilaterally OVX at least 1 month before any experimental manipulations. Permanent indwelling third cerebral ventricular (3V) cannulae were implanted in a subsequent surgical procedure as described previously (Barker-Gibb et al., 1995). Approximately 2 weeks after 3V surgery, one external jugular vein was cannulated for blood sampling and animals housed in single pens; cannulae were kept patent with heparinized saline. Ewes were assigned to two treatment groups (four per group); peptide 234 (diluted in aCSF; 150 mm NaCl, 1.2 mm CaCl2, 1 mm MgCl2, 2.8 mm KCl) or control (aCSF only). The following day, infusion lines were connected to 3V cannulae, and blood sampling commenced at 7:00 A.M. Samples were collected every 10 min. After 3 h of sampling, peptide 234 (or control) was infused into the 3V at a dose of 40 μg/h for 1 h, with an initial loading dose of 10 μg. Both peptide 234 and vehicle were infused at 200 μl/h using Graseby MS16A infusion pumps (Smith Medical Australasia). After infusion, 3V lines remained in place, and blood sampling continued for a further 2 h (total of 6 h). Plasma was harvested immediately from samples and frozen at −20°C until assayed.
Plasma LH concentrations were measured in duplicate, with NIH-oLH-S18 as standard (Barker-Gibb et al., 1995). Assay sensitivity was 0.1 ng/ml, and the intraassay coefficient of variation was <10% over the range of 0.3–12.8 ng/ml. Pulse analysis of the plasma LH data was as described previously (Clarke, 1993).
Plasma prolactin concentrations were measured in duplicate using Sigma, Lot 114F-0558, NOL-7135 as standard (Thomas et al., 1990). The sensitivity of the assay was 1 ng/ml. Intraassay coefficient variant (CV) was <10% between 1 and 22 ng/ml.
Plasma cortisol concentrations were assayed in duplicate using antiserum number 3368 (Bioquest) and 125I-labeled cortisol (GE Healthcare). The sensitivity of the assay was 3 ng/ml. Intraassay CV was <10% between 3 and 18 ng/ml, and the interassay coefficient variant (CV) was 15%.
Data analysis.
For data analysis of LH, prolactin, and cortisol, the mean plasma value in each ewe was calculated for the period before (0–180 min), during (180–240 min), and after (240–360 min) the infusion. In addition, the mean LH pulse amplitude for each ewe was also calculated before, during, and after the infusion. Repeated measures ANOVAs were used to determine the effect of peptide 234 treatment on hormone levels over each period and where appropriate one-way ANOVAs were used to assess specific difference within each period.
Results
Effects of substitutions of amino acids within kisspeptin-10 on stimulation of IP release in CHO cells stably expressing human gpr-54
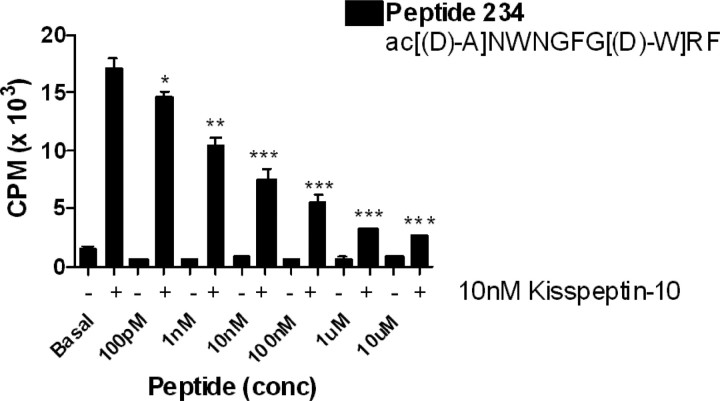
As kisspeptin-10 is the minimal sequence required for full receptor binding and activation, we explored the effect of systematic substitution of amino acids in this region monitoring the inhibition of 10 nm kisspeptin-10 stimulation of IP production in cells stably expressing the human gpr-54. Because the C-terminal sequence RF.NH2 is conserved in this large and ancient peptide family, we reasoned that this is essential for receptor binding and focused our attention on the preceding 8 aa. We truncated the N-terminal 5 aa and introduced various substitutions with d-amino acids. This resulted in a loss of efficacy in IP production and a weak and incomplete ability to inhibit IP stimulation by 10 nm kisspeptin-10 [see supporting information (SI), supplemental Table S1, available at www.jneurosci.org as supplemental material]. This indicated the first 5 aa are important for receptor activation. We then substituted residues alone or in combination in the full 10 aa peptide and monitored the effect this had on intrinsic IP stimulation and any inhibitory effects these substitutions had on IP stimulation by 10 nm kisspeptin-10. This systematic substitution of amino acids allowed us to develop a range of analogues with partial agonistic and antagonistic properties at gpr-54 (see supplemental Table S1, available at www.jneurosci.org as supplemental material). Many of these analogues incorporated a glycine substitution for Ser5 in combination with a d-amino acid (tryptophan or leucine) at Leu8. Although these substitutions alone did significantly (p < 0.01) inhibit 10 nm kisspeptin-10 stimulation of IP, they generally did not reduce this stimulation to basal levels (see supplemental Table S1, available at www.jneurosci.org as supplemental material).
To develop full antagonists, further d-amino acid substitutions were made at Tyr1, Asn2, and Trp3. The most effective antagonists with substitutions in these positions were peptides 230, 232, 233, 234, 235, and 236, which alone had little or no stimulation of IP, but had IC50s of <10−8 m and a maximal inhibition of 66–93% of kisspeptin stimulation of IP (see supplemental Table S1, available at www.jneurosci.org as supplemental material). The most complete inhibition was achieved with d-Ala1 substitution [see supplemental Table S1, available at www.jneurosci.org as supplemental material (234)] (Fig. 1). This combination of substitutions inhibited 10 nm kisspeptin-10 stimulation of IP by 93%, with an IC50 of 7 nm and had no intrinsic IP stimulation, signifying high antagonist activity. The studies have also highlighted the significance of the glycine substitution at Ser5 and d-tryptophan at Leu8 combined with appropriate substitutions in the N terminus. The active analogs displayed high binding affinities in competition with 125I-kisspeptin-10. Most decamer analogues tested bound with an EC50 similar to kisspeptin, for example peptide 234, had a binding affinity of 2.7 nm (see supplemental Fig. S1, available at www.jneurosci.org as supplemental material). However, the 5 aa analogues had lower binding affinities than the full decamer analogues with some unable to bind the receptor (data not shown).
Figure 1.
Peptide 234 is a potent inhibitor of kisspeptin-10 stimulation of IP. Substitution of Leu8 with d-Trp in combination with Ser5 substitution with Gly created potent antagonists (see supplemental Table S1, available at www.jneurosci.org as supplemental material). Additional substitution of Tyr1 with d-Ala (234 shown here) enhanced this (*p < 0.05, **p < 0.01, ***p < 0.001). Peptide 234 alone had no intrinsic IP stimulation. Bars show mean ± SEM of five experiments.
Peptide 234 inhibits kisspeptin-10 stimulation of GnRH neuron firing
As has been demonstrated previously (Han et al., 2005; Pielecka-Fortuna et al., 2008), 1 nm kisspeptin-10 markedly increased GnRH neuron firing activity (Fig. 2A,C). Under these experimental conditions, there was no effect on GnRH neuron firing activity of peptide 234 alone (1 nm before: 0.2 ± 0.1 Hz; after 0.3 ± 0.1 Hz, n = 5, p > 0.05; 10 nm before: 0.3 ± 0.2 Hz, after 0.3 ± 0.2 Hz, n = 6, p > 0.05; 100 nm before: 0.5 ± 0.1 Hz, 0.6 ± 0.1 Hz, n = 7, p > 0.05, paired t test). In contrast to the lack of effect of peptide 234 on basal firing, pretreatment with this peptide blocked the response to 1 nm kisspeptin-10 (p < 0.001 for all doses) compared with cells treated with kisspeptin-10 alone (Fig. 2B,C).
Figure 2.
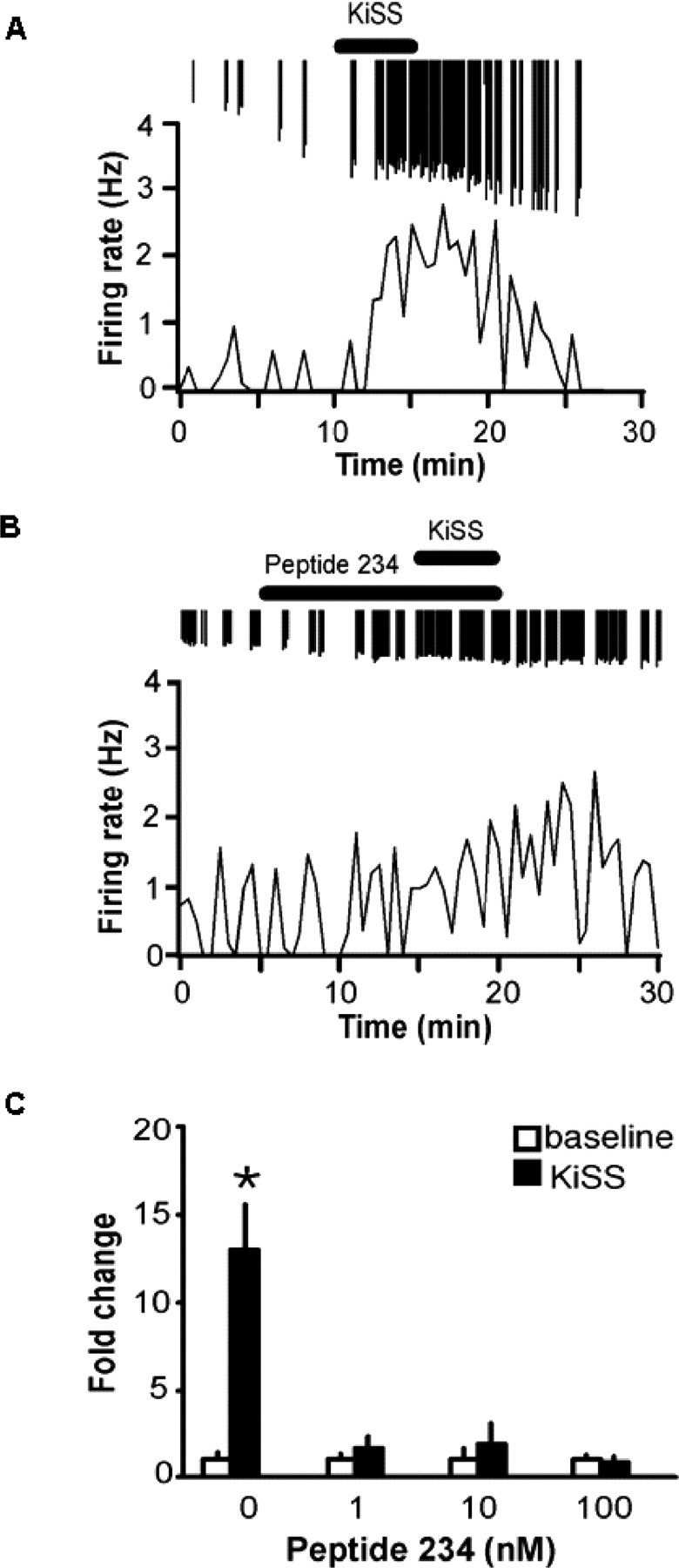
Peptide 234 antagonizes kisspeptin-10 excitation of GnRH neurons. Representative traces of GnRH neuronal firing rate over time. A, Increased GnRH firing rate after 1 nm kisspeptin-10 (bar). Downward spikes are individual action currents. B, Inhibition of kisspeptin-10 (1 nm) stimulation by peptide 234 (1 nm, bar). C, Summary bar graph showing mean ± SEM fold change in firing rate during baseline (white bars) and kisspeptin-10 (black bars); kisspeptin-10 significantly increased firing activity of GnRH neurons (n = 7; *p < 0.002). Response to kisspeptin-10 was significantly reduced with the presence of 1, 10, 100 nm peptide 234 (1 nm, n = 5; 10 nm, n = 6; 100 nm, n = 7; p < 0.001, all groups).
Peptide 234 inhibits pulsatile GnRH release in pubertal female rhesus monkeys
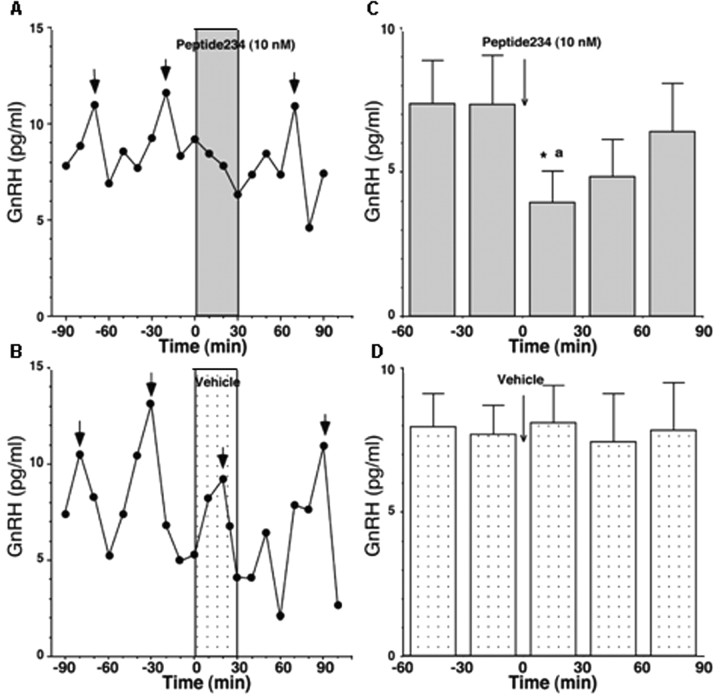
Because peptide 234 inhibited GnRH neuronal firing, we examined whether it also inhibited GnRH release in pubertal female rhesus monkeys using methods described previously (Frost et al., 2008; Keen et al., 2008). Infusion of 10 nm peptide 234 over 30 min through a microdialysis probe located in the stalk-median eminence region promptly and consistently suppressed GnRH pulses as well as mean GnRH levels but did not affect basal levels (Fig. 3A,C). In contrast, vehicle infusion through the probe did not cause any significant changes in GnRH release (Fig. 3B,D). The peptide 234-induced GnRH suppression was significantly different from values before peptide 234 infusion as well as those from the vehicle control (for both, p < 0.05). Based on our previous assessment that the dialysis membrane passes ∼10% of peptides with a similar size to 234 (Frost et al., 2008), it is estimated that the concentration of peptide 234 in the stalk-median eminence region was 1 nm.
Figure 3.
Peptide 234 suppresses GnRH release in vivo. Representative cases from the effects of peptide 234 on GnRH release and group mean (±SEM; n = 6) are shown. A, Pulsatile GnRH release in the hypothalamus was suppressed by 10 nm peptide 234 infusion to the stalk-median eminence regions (dark shaded bar). Short arrows indicate GnRH peaks identified by PULSAR. B, In contrast, vehicle infusion as a control did not cause any significant changes in GnRH release (light shaded bar). C, Data analysis indicated that peptide 234 significantly (p < 0.05) suppressed GnRH release as compared with levels before peptide 234 as well as to the vehicle control. D, Vehicle infusion did not cause any significant changes. The estimated concentration of peptide 234 in the stalk-median eminence region was 1 nm, based on our previous assessment that the dialysis membrane passes ∼10% of peptides with a similar size. *p < 0.05 versus before peptide 234; ap < 0.05 versus control at corresponding time period.
Peptide 234 inhibits kisspeptin-10 stimulated LH in intact male rats and the increase in LH after castration
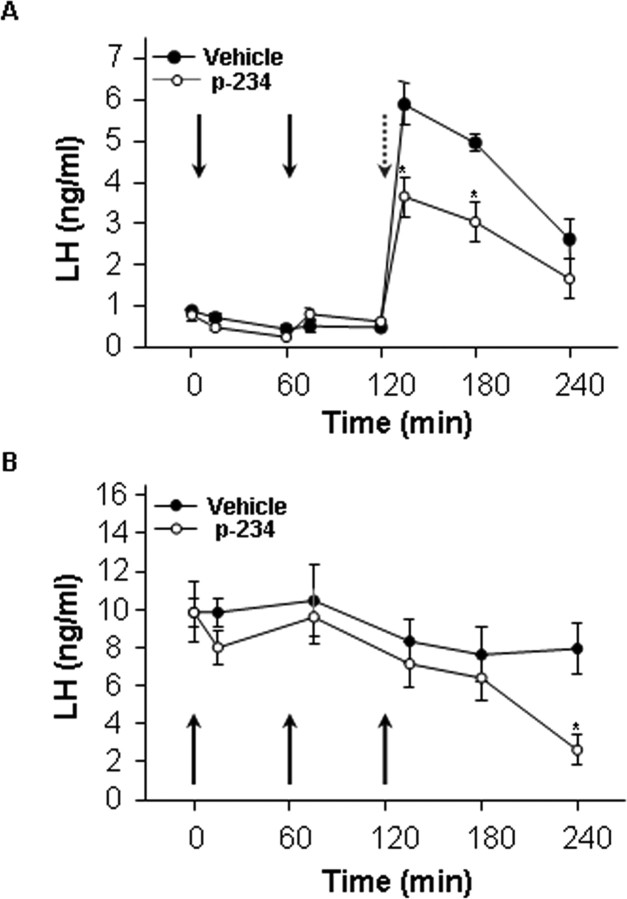
The effects of peptide 234 were tested in vivo using adult male rats. Pharmacological tests involved repeated (×3) intracerebroventricular injections of 1 nmol peptide 234 and serial blood sampling, to assess potential effects of antagonists on basal LH levels. The third injection was accompanied by coadministration of a submaximal dose (100 pmol) of kisspeptin-10 and a 1 nmol dose of peptide 234, to monitor the ability to inhibit kisspeptin-10 stimulation of LH and testosterone via activation of GnRH neurons. Administration of 1 nmol peptide 234 intracerebroventricularly did not significantly modify basal LH levels at any time tested after injection. Central injection of 100 pmol kisspeptin-10 to vehicle-treated animals (at 120 min) evoked the expected rise in serum LH levels with a net LH secretory mass (AUC) of 527 ± 43. Despite its lack of effect on basal LH levels, coadministration of peptide 234 blunted the LH secretory responses induced by kisspeptin-10, with a significant (p < 0.01) reduction in the net LH secretory mass (AUC) to 328 ± 56 during the 120-min period after coinjection of peptide 234 and kisspeptin-10. In good agreement, testosterone levels at 60 min after combined injection of peptide 234 and kisspeptin-10 (1.58 ± 0.1) were significantly (p < 0.01) lower than in animals injected with kisspeptin-10 alone (2.17 ± 0.2). LH levels were elevated in castrated rats over the 240-min monitoring period. Administration of 1 nmol peptide 234 at 0, 60, and 120 min tended to reduce serum LH levels in castrated males; a reduction that reached statistical significance at 240 min (Fig. 4B).
Figure 4.
Peptide 234 effects on basal and kisspeptin-10-stimulated plasma LH in intact and castrated male rats. A, Peptide 234 inhibits kisspeptin-10-induced LH secretion in intact male rats. The animals were infused with 1 nmol peptide 234 at 0 and 60 min, followed by infusion of 100 pmol kisspeptin-10 with 1 nmol peptide 234 at 120 min. The peptide significantly inhibited LH production over the following 2 h (n = 10; *p < 0.05). B, Castrated male rats were given three infusions of 1 nmol peptide 234 at 0, 60, and 120 min, which significantly inhibited LH secretion after 240 min with 1 nmol peptide 234 (n = 10; p < 0.05). Bars show mean ± SEM.
Peptide 234 inhibits LH in castrated male mice and kisspeptin-10 stimulated LH in intact male mice
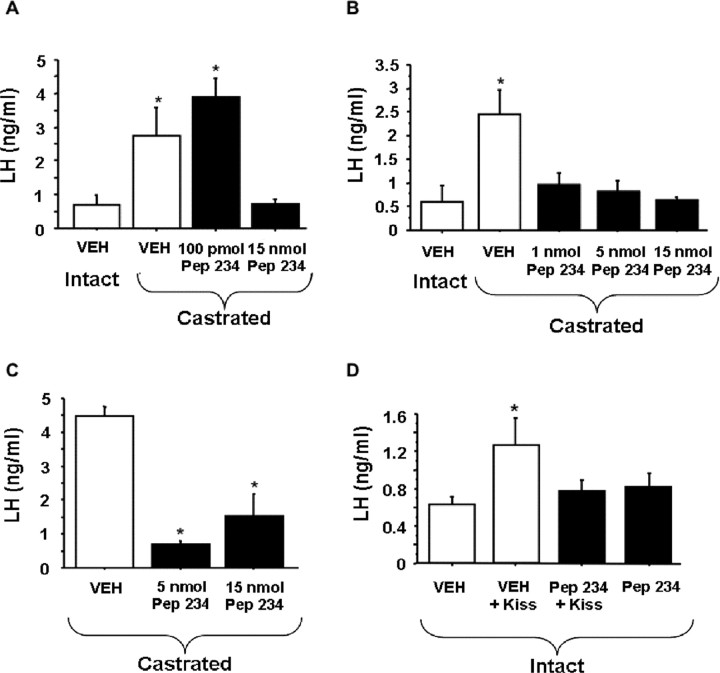
Peptide 234 showed strong antagonistic actions on LH levels in mice. As with the studies in male rats, peptide 234 did not significantly alter plasma LH levels in intact male mice (p > 0.30 vs vehicle controls; data not shown). However, two infusions of peptide 234 at 15 nmol inhibited LH levels in castrated males compared with the elevated LH levels of vehicle-treated castrated males (p < 0.05) (Fig. 5A). The low dose (15 pmol) was ineffective. The subsequent dose–response study confirmed this finding, with all three doses of peptide 234 (1, 5, 15 nmol) reducing LH levels of castrated males to levels observed in intact males (p < 0.01) for all three doses relative to vehicle-treated castrates (Fig. 5B). Similar to two infusions of peptide 234, a single infusion of peptide 234 (5 or 15 nmol) eliminated the postcastration rise in LH (p < 0.05) relative to vehicle-treated castrates (Fig. 5C). The inhibition of LH levels in castrated mice after treatment with peptide 234 appears to be attributable to specific inhibition of kisspeptin signaling (rather than nonspecific effects or effects on other neuroendocrine systems) because pretreatment of intact mice with peptide 234 completely blocked a kisspeptin-induced rise in LH (p < 0.05) (Fig. 5D).
Figure 5.
Peptide 234 inhibition of plasma LH in male mice. A, Two infusions of 15 nmol of peptide 234 inhibit the elevated LH levels of castrated male mice. B, Dose–response graph for castrated male mice given two infusions of peptide 234 indicates that all three doses tested are able to inhibit the postcastration rise in LH. C, Likewise, a single infusion of 5 or 15 nmol of peptide 234 potently inhibits LH levels of castrated mice. D, Exogenously administered kisspeptin (100 fmol) is unable to stimulate LH levels in intact male mice when 100 pmol peptide 234 is infused 5 min beforehand (n = 6–8). Bars show mean ± SEM (*p < 0.05).
Peptide 234 inhibits LH pulses in ovariectomized ewes
Major secretory episodes of LH were clearly distinguishable in control ovariectomized ewes and in treated ewes before peptide 234 administration (Fig. 6). LH pulse amplitude was reduced after intracerebroventricular administration of peptide 234 (p < 0.05); this reduction in amplitude was so marked in some ewes that it made pulse detection difficult and precluded determining if there was an additional effect on pulse frequency. Mean LH levels were similar before and during the infusion but reduced after peptide 234 infusion (p < 0.05) (see supplemental Table S2, available at www.jneurosci.org as supplemental material). Peptide 234 had no effect on the concentrations of prolactin or cortisol before, during, or after infusion (see supplemental Figs. S2, S3, available at www.jneurosci.org as supplemental material).
Figure 6.
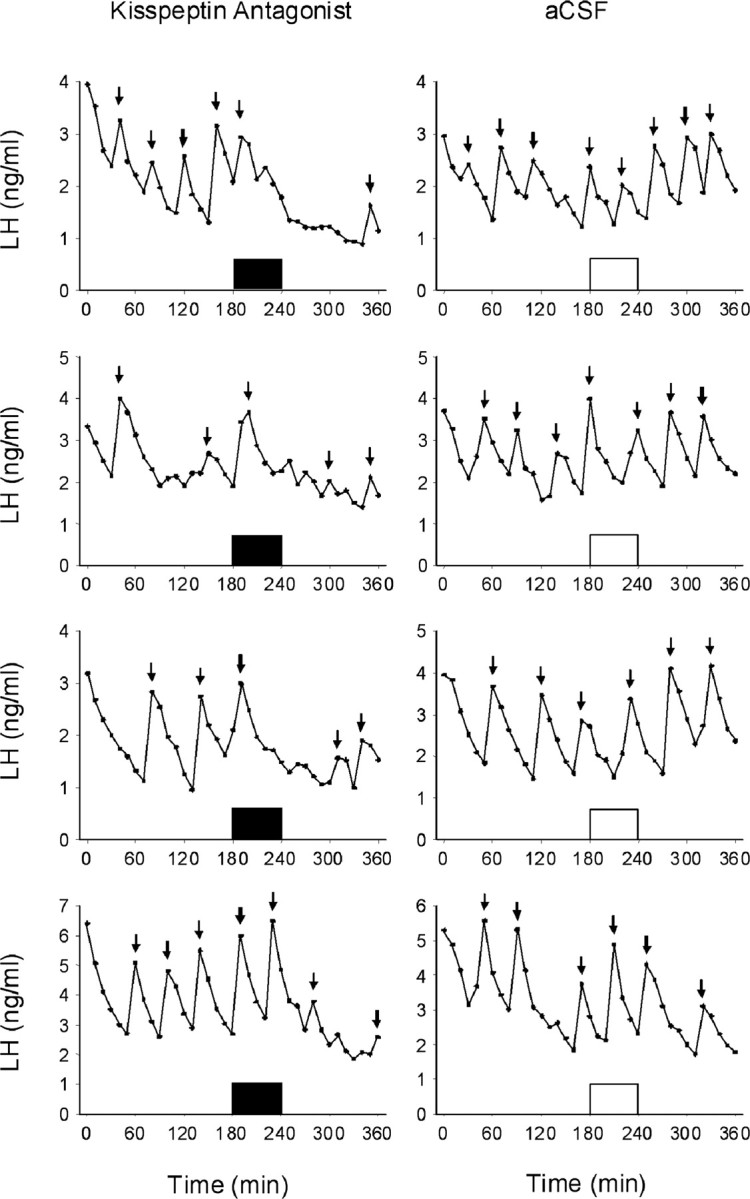
Central infusion of peptide 234 inhibits the secretory pulses of LH in OVX ewes. Concentrations of LH are shown in ewes treated with peptide 234 (closed bars) or control (opened bars). Arrows indicate LH pulses as defined in Materials and Methods. Analysis revealed a significant reduction in the mean LH concentration and pulse amplitude after peptide 234 infusion (see supplemental Table S2, available at www.jneurosci.org as supplemental material).
Discussion
The pleiotropic effects of kisspeptin make the gpr-54 receptor an attractive therapeutic target. Until now, the emphasis has been on developing kisspeptin agonists as antimetastatic agents (Clements et al., 2001; Tomita et al., 2006; Orsini et al., 2007). However, the development of kisspeptin antagonists would be of greater utility for investigating the role of kisspeptin in the neuroendocrine regulation of gonadotropins. To realize this objective, we have systematically substituted amino acids in the minimal kisspeptin structure required for biological activity (kisspeptin-10) and developed antagonists which have high binding affinity, specificity, and efficacy. Previous studies in pursuit of kisspeptin agonists have established that Phe6, Arg9, and Phe10.NH2 constitute a binding pharmacophore (Orsini et al., 2007). The evolutionary conservation of the RF.NH2 moiety among this large and ancient super family of peptides with this motif predicts that these C-terminal residues are essential for receptor engagement, in agreement with their identification in the pharmacophore. Our pilot studies on kisspeptin amino acid substitutions confirmed that changes in the RF.NH2 moiety resulted in a loss of binding. Therefore, we focused on other residues in seeking antagonist structures. First, we truncated the N-terminal 5 aa in the rodent and human kisspeptin-10 sequences and found that this diminished agonist activity and created incomplete antagonistic properties. Substitution of Phe6 or Leu8 in these truncated peptides revealed that d-Trp substitution of Leu8 produced the most promising antagonist. Incorporation of this substitution in the full decapeptide sequences produced better antagonists especially when accompanied by the substitution of Ser5 with the achiral amino acid, glycine, which permits greater flexibility of peptides and can obviate some of the steric hindrances of the other amino acid substitutions that have bulky side chains (e.g., d-Trp).
Further exploration revealed that substitution of the N-terminal Tyr1 with d-amino acids was also tolerable for antagonistic activity but not if d-Tyr1 or d-Trp1 accompanied d-Trp8 substitution. Substitution of the pharmacophore residue Phe6 resulted in a decrease in agonist activity and generation of some antagonist activity (e.g., 211 and 212). However, substitution of Phe6 with d-Trp6 combined with Leu8 substitution by d-Trp8 reduced antagonism compared with d-Trp8 alone (compare 213, 245, and 246 with 210). Certain substitutions of Asn2 (e.g., in 232 and 236) and Trp3 (e.g., in 231 and 235) were also acceptable for antagonism when combined with d-Trp8 substitution of Leu8. Overall, a consensus sequence for antagonism was X1-X2-X3-N-G-F-G-X8-R-F.NH2, where X1 is d-Ala, X2 is Asn or d-Ala or d-Trp, X3 is Trp or d-Trp or d-Ala, and X8 is d-Leu, d-Phe or d-Trp.
Our studies have also identified amino acids that appear to be involved in receptor activation as substitution of Ser5, Leu8, and to some extent, Tyr1, Asn2, and Trp3 contributed to antagonism. This is interesting because research to date suggests that the N terminus contains the activation domain and the C terminus the binding domain. Our findings suggest, however, that the two sites overlap, as it has been shown that Phe6, Arg9, and Phe10 form a pharmacophore for binding (Orsini et al., 2007), thus placing the Leu8 activating residue within the binding pharmacophore.
We selected peptide 234 for ex vivo and in vivo studies based on its in vitro structure-activity profile. Because the action of kisspeptin in the HPG axis is thought to be predominantly mediated through stimulating GnRH secretion (Irwig et al., 2004; Gottsch et al., 2006), we first investigated the ability of peptide 234 to inhibit kisspeptin-10 stimulation of firing in GnRH neurons recorded in acutely prepared brain slices from the mouse. Peptide 234 was found to block kisspeptin-10 stimulation of GnRH neuron firing at 100 nm, 10 nm, and even at 1 nm, which is the same concentration as that of kisspeptin-10 used for stimulation of the GnRH neuron.
The inhibition of kisspeptin stimulation of GnRH neuronal firing by peptide 234 suggests that the antagonist would reduce hypothalamic GnRH secretion in vivo. Thus, we evaluated whether peptide 234 administered directly to the stalk-median eminence region would influence pulsatile GnRH secretion in pubertal female rhesus monkeys. The suppression of GnRH secretion during peptide 234 infusion provides the first direct evidence that signaling from kisspeptin neurons in the hypothalamus is a prerequisite for GnRH pulsatile secretion. The role of kisspeptin in generating GnRH pulses was previously suggested by the observations that kisspeptin-54 pulses coincide with GnRH pulses 75% of the time (Keen et al., 2008). However, because clinical studies suggest that patients with gpr-54 mutations exhibited attenuated LH pulses with an approximately normal pulse frequency (de Roux et al., 2003; Seminara et al., 2003; Tenenbaum-Rakover et al., 2007), it remains equivocal whether kisspeptin neuron input is critical for the generation of pulsatile GnRH secretion or simply reflects modulation of GnRH pulse amplitude. The inhibition of spontaneous GnRH pulses in the monkey model and reduction of LH pulses in the ovariectomized sheep by peptide 234 suggests that kisspeptin is required for both frequency and amplitude of GnRH pulsatile secretion.
Peptide 234 also blocked kisspeptin-10 stimulation of LH in adult male rats and mice presumably by blocking kisspeptin-10 effects on GnRH secretion. In rats, the inhibition was incomplete at 1 nmol 234, suggesting that the dose was insufficient. This dose completely inhibited the kisspeptin-10 stimulation of LH in mice (possibly attributable to their smaller size) as did 5 and 15 nmol.
An unexpected finding that emerged from the studies was the absence of any inhibition of basal LH in intact male rats and mice. This suggests that kisspeptin input is minimal under these conditions and that other factors regulate basal GnRH and LH secretion. This interpretation is supported by the failure of peptide 234 to affect basal firing of the GnRH neuron (Fig. 2), basal GnRH secretion in rhesus monkeys (Fig. 3), and basal LH in ovariectomized ewes (Fig. 6). Further research and confirmation that peptide 234 does not affect basal LH in intact females is merited as the ability to inhibit the ovulatory LH surge but not basal LH in females might presage a long-sought contraceptive that does not ablate endogenous estrogen yet blocks ovulation. These observations are in accordance with the observation of variable residual gonadotropin function in KiSS-1 knock-out mice as reflected in follicular development and sporadic ovulations (Lapatto et al., 2007).
In male mice, peptide 234 blocked the increase in LH that ensues after castration and the removal of negative feedback. A similar result was observed in castrated rats, but the inhibition was less pronounced and delayed probably because of the lower dosage used and the consequent requirement for a build up in peptide 234 in the brain over the three injections. The findings indicate that removal of gonadal steroids leads to a rise in kisspeptin secretion that is translated into increased GnRH and LH secretion. Previous demonstrations of increased KiSS-1 gene expression in the arcuate nucleus in gonadectomized male and female rats (Irwig et al., 2004; Gottsch et al., 2006) is consistent with the presumption that kisspeptin-producing neurons are targets for the feedback actions of sex steroids (Smith et al., 2005). Levels of mRNA may not, however, reflect the biosynthesis and secretion of kisspeptin to engage gpr-54 expressed by GnRH neurons. Our studies with a novel kisspeptin antagonist now provide direct evidence for kisspeptin modulation of the GnRH neuron in negative feedback by the gonads and emphasize the value of a kisspeptin antagonist for elucidating physiological mechanisms.
In ovariectomized sheep, LH pulses (and by inference GnRH pulses) were markedly reduced by administration of peptide 234. This is consistent with the demonstration that peptide 234 inhibited GnRH neuron firing rate in mice and reduced GnRH pulses in rhesus monkeys. These findings imply that the endogenous secretion of kisspeptin is pulsatile in nature and drives the ultradian secretion of GnRH. The effects of peptide 234 appear to be specific to the regulation of gonadotropins because the antagonist had no effect on either prolactin or cortisol secretion in ovariectomized ewes (see supplemental Figs. S2, S3, available at www.jneurosci.org as supplemental material). Moreover, peptide 234 did not bind to GnRH or LH receptors (see supplemental Fig. S4, available at www.jneurosci.org as supplemental material).
The ability of peptide 234 to block kisspeptin-10-induced firing of GnRH neurons suggests therapeutic potential of kisspeptin analogues in reproductive disorders associated with abnormal LH pulse frequency characteristic of polycystic ovarian syndrome (Blank et al., 2006), hypothalamic amenorrhea (Armeanu et al., 1992), stress (Li et al., 2007), and in under nutrition (e.g., anorexia nervosa) (Armeanu et al., 1992).
The inhibition of GnRH pulses in pubertal female rhesus monkeys by peptide 234 provides support for a role for kisspeptin in the initiation of puberty. The demonstration that kisspeptin-10 administration advanced the age of vaginal opening in female rats implicated kisspeptin in the onset of puberty (Navarro et al., 2004). Our preliminary observation that peptide 234 delayed vaginal opening in juvenile female rats (MS in preparation) adds further evidence for the involvement of kisspeptin in the initiation of puberty.
A wide spectrum of pathologies is associated with dysfunction of the HPG axis, and many conditions are exacerbated by sex steroid stimulation. These include infertility, polycystic ovarian syndrome, endometriosis, uterine fibroids, excessive menstrual bleeding, delayed and precocious puberty, and breast, prostatic, and ovarian cancers. Potent and specific kisspeptin antagonists offer potential novel treatments for these conditions.
Footnotes
This work was supported by the Medical Research Council (UK and South Africa); National Institutes of Health Grants R01HD15433, R01HD11355, R01HD41469, R01HD27142, and U54HD12629; National Institute of Child Health and Human Development Grant K99 056157; Grant BFI 2005-07446 from Ministerio de Educación y Ciencia (Spain); funds from Instituto de Salud Carlos III (Red de Centros RCMN C03/08 and Project PI042082; Ministerio de Sanidad, Spain); and the National Health and Medical Research Council, Australia.
References
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Suzuki E, Kessler M, Civelli O, Nothacker HP. Cancer metastasis-suppressing peptide metastin upregulates excitatory synaptic transmission in hippocampal dentate granule cells. J Neurophysiol. 2005;94:3648–3652. doi: 10.1152/jn.00590.2005. [DOI] [PubMed] [Google Scholar]
- Armeanu MC, Berkhout GM, Schoemaker J. Pulsatile luteinizing hormone secretion in hypothalamic amenorrhea, anorexia nervosa, and polycystic ovarian disease during naltrexone treatment. Fertil Steril. 1992;57:762–770. doi: 10.1016/s0015-0282(16)54956-5. [DOI] [PubMed] [Google Scholar]
- Barker-Gibb ML, Scott CJ, Boublik JH, Clarke IJ. The role of neuropeptide Y (NPY) in the control of LH secretion in the ewe with respect to season, NPY receptor subtype and the site of action in the hypothalamus. J Endocrinol. 1995;147:565–579. doi: 10.1677/joe.0.1470565. [DOI] [PubMed] [Google Scholar]
- Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knöfler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic gonadotrophin-releasing hormone expression in female monkeys with different sensitivity to stress. J Neuroendocrinol. 2007;19:594–604. doi: 10.1111/j.1365-2826.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102:15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology. 1993;133:1624–1632. doi: 10.1210/endo.133.4.8404603. [DOI] [PubMed] [Google Scholar]
- Clements MK, McDonald TP, Wang R, Xie G, O'Dowd BF, George SR, Austin CP, Liu Q. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochem Biophys Res Commun. 2001;284:1189–1193. doi: 10.1006/bbrc.2001.5098. [DOI] [PubMed] [Google Scholar]
- Coetsee M, Millar RP, Flanagan CA, Lu ZL. Identification of Tyr(290(6.58)) of the human gonadotropin-releasing hormone (GnRH) receptor as a contact residue for both GnRH I and GnRH II: importance for high-affinity binding and receptor activation. Biochemistry. 2008;47:10305–10313. doi: 10.1021/bi800911z. [DOI] [PubMed] [Google Scholar]
- Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol. 2006;18:806–809. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- Frost SI, Keen KL, Levine JE, Terasawa E. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the Rhesus monkey. J Neurosci Methods. 2008;168:26–34. doi: 10.1016/j.jneumeth.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull. 1988;21:117–121. doi: 10.1016/0361-9230(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol. 2006;254–255:91–96. doi: 10.1016/j.mce.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49:2131–2135. doi: 10.1007/s00125-006-0343-z. [DOI] [PubMed] [Google Scholar]
- Hohmann JG, Teal TH, Clifton DK, Davis J, Hruby VJ, Han G, Steiner RA. Differential role of melanocortins in mediating leptin's central effects on feeding and reproduction. Am J Physiol Regul Integr Comp Physiol. 2000;278:R50–R59. doi: 10.1152/ajpregu.2000.278.1.R50. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Janneau JL, Maldonado-Estrada J, Tachdjian G, Miran I, Motté N, Saulnier P, Sabourin JC, Coté JF, Simon B, Frydman R, Chaouat G, Bellet D. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab. 2002;87:5336–5339. doi: 10.1210/jc.2002-021093. [DOI] [PubMed] [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in LHRH-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 2003;144:813–822. doi: 10.1210/en.2002-220982. [DOI] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- Li XF, Kinsey-Jones JS, Knox AM, Wu XQ, Tahsinsoy D, Brain SD, Lightman SL, O'Byrne KT. Neonatal lipopolysaccharide exposure exacerbates stress-induced suppression of LH pulse frequency in adulthood. Endocrinology. 2007;148:5984–5990. doi: 10.1210/en.2007-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Coetsee M, White CD, Millar RP. Structural determinants for ligand-receptor conformational selection in a peptide G protein-coupled receptor. J Biol Chem. 2007;282:17921–17929. doi: 10.1074/jbc.M610413200. [DOI] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor GPR54, to atherosclerosis prone vessels. Endocrinology. 2007;148:140–147. doi: 10.1210/en.2006-0818. [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernández-Fernández R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143:2284–2292. doi: 10.1210/endo.143.6.8869. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Klein MA, Beavers MP, Connolly PJ, Middleton SA, Mayo KH. Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem. 2007;50:462–471. doi: 10.1021/jm0609824. [DOI] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74:931–937. doi: 10.1095/biolreprod.105.049619. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Khoshaba N, Scarbrough K, Urban JH, Vitaterna MH, Levine JE, Turek FW, Horton TH. Rapid photoperiod-induced increase in detectable GnRH mRNA-containing cells in Siberian hamster. Am J Physiol. 1997;273:R2032–R2039. doi: 10.1152/ajpregu.1997.273.6.R2032. [DOI] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Masson-Pévet M, Pévet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Nelson AL, Ahmed EI, Parfitt DB, Romeo RD, Sisk CL. Female pheromones stimulate release of luteinizing hormone and testosterone without altering GnRH mRNA in adult male Syrian hamsters (Mesocricetus auratus) Gen Comp Endocrinol. 2004;138:211–217. doi: 10.1016/j.ygcen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–1144. doi: 10.1210/jc.2006-2147. [DOI] [PubMed] [Google Scholar]
- Thomas GB, Mercer JE, Karalis T, Rao A, Cummins JT, Clarke IJ. Effect of restricted feeding on the concentrations of growth hormone (GH), gonadotropins, and prolactin (PRL) in plasma, and on the amounts of messenger ribonucleic acid for GH, gonadotropin subunits, and PRL in the pituitary glands of adult ovariectomized ewes. Endocrinology. 1990;126:1361–1367. doi: 10.1210/endo-126-3-1361. [DOI] [PubMed] [Google Scholar]
- Tomita K, Niida A, Oishi S, Ohno H, Cluzeau J, Navenot JM, Wang ZX, Peiper SC, Fujii N. Structure-activity relationship study on small peptidic GPR54 agonists. Bioorg Med Chem. 2006;14:7595–7603. doi: 10.1016/j.bmc.2006.07.009. [DOI] [PubMed] [Google Scholar]