Abstract
Background
Activation of the NOP receptor by its endogenous ligand nociceptin/orphanin FQ reduces ethanol intake in genetically selected alcohol preferring Marchigian Sardinian alcohol preferring (msP) rats. Here we evaluated whether buprenorphine, a partial agonist at μ-opioid and NOP receptors, would reduce ethanol consumption in msP rats via activation of NOP receptors.
Methods
Marchigian Sardinian alcohol preferring rats trained to drink 10% alcohol 2 hours/day were injected with buprenorphine (.03, .3, 3.0, or 6.0 mg/kg intraperitoneally [IP]) 90 min before access to ethanol.
Results
Similar to prototypical μ-agonists, the two lowest doses of buprenorphine significantly increased ethanol consumption (p < .01); in contrast, the two highest doses reduced it (p < .05). Pretreatment with naltrexone (.25 mg/kg IP) prevented the increase of ethanol intake induced by .03 mg/kg of buprenorphine (p < .001) but did not affect the inhibition of ethanol drinking induced by 3.0 mg/kg of buprenorphine. Conversely, pretreatment with the selective NOP receptor antagonist UFP-101 (10.0 or 20.0 μg/rat) abolished the suppression of ethanol drinking by 3.0 mg/kg of buprenorphine.
Conclusions
Buprenorphine has dualistic effects on ethanol drinking; low doses increase alcohol intake via stimulation of classic opioid receptors, whereas higher doses reduce it via activation of NOP receptors. We suggest that NOP agonistic properties of buprenorphine might be useful in the treatment of alcoholism.
Keywords: Buprenorphine, nociceptin/orphanin FQ, alcohol abuse, addiction
Buprenorphine has long been in clinical use for treatment of moderate-to-severe pain (Finco et al 1995; Gundersen et al 1986; Hayes et al 1979; Maunuksela et al 1998; Murphy and MacEvilly 1984; Picard et al 1997; Vanacker et al 1986). More recently, evidence has accumulated in support of its efficacy for maintenance treatment of heroin dependence (Johnson and McCagh 2000; Kakko et al 2003; Ling et al 1996, 1998; Litten and Allen 1999; Mello et al 1993), and the drug has been approved for this indication in numerous countries, including the United States, Australia, Sweden, and France. Observational data from France, where it has been in widespread use, suggest an advantageous safety profile of buprenorphine compared with methadone (Auriacombe et al 2001), whereas its efficacy seems to be comparable when used in an optimal manner (Mattick et al 2003).
An attractive safety profile of buprenorphine, including reduced risk for overdose death due to respiratory suppression and lower street value leading to diminished risk for diversion, is predicted by its complex preclinical pharmacology. Thus buprenorphine has long been known to be a partial agonist at μ-opioid receptors (Cowan et al 1977; Lattanzi et al 2001; Magnan et al 1982; Martin et al 1976; Rosenbaum et al 1985; Sadee et al 1982) but has also antagonistic or agonistic properties at κ- and δ-opioid receptors (Cowan et al 1977; Leader 1987; Negus et al 2002; Pick et al 1997; Rovati et al 1987; Sadee et al 1982; Tyers 1980). In an unexpected development, it has recently been realized that buprenorphine is also agonist/partial agonist at the NOP nociceptin/orphanin FQ (N/OFQ) receptors (Bloms-Funke et al 2000; Lutfy et al 2003; Wnendt et al 1999, Huang et al 2001).
As a result of the aforementioned agonist/antagonist opioidergic properties, and principally owing to the partial stimulation of the μ-opioid receptor, buprenorphine induces most of the known opioid effects like pain relief, feelings of wellbeing and pleasure, respiratory depression, and so forth, but with less intensity than heroin, morphine, methadone, and other opiates that fully stimulate the receptor (Johnson and Strain 1999). Consequently, unlike other opiates, buprenorphine produces modest physical dependence (Fudala et al 1990; Kosten et al 1990; San et al 1992) or respiratory depression (Walsh et al 1994), has a lower addiction potential (Kawamoto et al 1999), and gives only mild withdrawal symptoms even after prolonged treatment and abrupt withdrawal (Fudala et al 1990; Jasinski et al 1978). The lack of pronounced withdrawal symptoms is also likely related to the very slow kinetics of the drug. In addition to a long half-life, buprenorphine penetrates rapidly into the brain and binds to μ-receptors but dissociates from these only at a slow rate (Lewis 1985).
The role of opioids in modulating the reinforcing properties of ethanol has been well documented. First of all, an altered opioidergic system has been described in animals genetically selected for high ethanol preference (De Waele et al 1995; Fadda et al 1999; Gianoulakis et al 1992; Jamensky and Gianoulakis 1997; Marinelli et al 2000; Weiss et al, 1990). In addition, low doses of opioid agonists (e.g., morphine, methadone) increase ethanol consumption (Hubbell et al 1986, 1993; Zhang and Kelley 2002), whereas higher doses decrease it (Sinclair 1974; Sinclair et al 1973; Vacca et al 2002). This latter effect is not specific, and it is associated with sedation, hypomotility, and simultaneous suppression of food intake (Vacca et al 2002).
Nonselective opioid antagonists such as naloxone and naltrexone, instead, dose dependently decrease alcohol intake (Samson and Doyle 1985; Volpicelli et al 1992, 1995; Weiss et al 1990). Most importantly, despite some negative results (Krystal et al 2001), a meta-analysis of available studies unequivocally supports an efficacy of naltrexone for treatment of alcohol dependence (Bouza et al 2004).
An important drawback in the use of full opioid agonists such as methadone in the treatment of opioid dependence is the increase in ethanol intake often reported during maintenance treatment programs with these compounds (Backmund et al 2003; Bickel et al 1987; Ottomanelli 1999). This effect is attributed to the agonistic activity of these drugs at the μ-opioid receptors. Buprenorphine, in contrast, only partially activates the μ-opioid receptor. Furthermore, during our studies of buprenorphine for heroin dependence (Kakko et al 2003), we repeatedly encountered patient reports that motivation to consume ethanol was reduced, as were consumption frequencies and quantities. A review of the preclinical literature did not provide a clear basis for evaluating these observations. Buprenorphine has been found to reduce intravenous and oral ethanol self-administration in rats under some conditions, but reported effects have been complex and not easy to interpret (June et al 1998; Martin et al 1983).
Recent data might shed new light on the complex actions of buprenorphine in relation to alcohol intake. It has been recently found that, in addition to its activity at classical opiate receptors, buprenorphine also acts as agonist/partial agonist at N/OFQ NOP receptors (Bloms-Funke et al 2000; Huang et al 2001; Lutfy et al 2003; Wnendt et al 1999). Interestingly, activation of the NOP receptor system results in a marked functional anti-opioid action (Ciccocioppo et al 2000b; Mogil and Pasternak 2001; Mogil et al 1996) and, as shown in previous studies by our group, administration of N/OFQ reduces ethanol consumption in the genetically selected Marchigian Sardinian alcohol-preferring (msP) rats (Ciccocioppo et al 1999). This effect is mimicked by other NOP receptor agonists and is abolished by pretreatment with the selective NOP antagonists (Ciccocioppo et al 2002).
Taken together, these data led us to hypothesize that NOP agonism might provide a component of buprenorphine’s actions on ethanol motivational properties that confers suppression of alcohol drinking and thus counteracts classical opioid actions of this drug, normally expected to increase drinking. Furthermore, because we had observed clinically that suppression of alcohol drinking by buprenorphine was most pronounced at high doses of the drug (16–32 mg daily), we postulated that this component might be preferentially expressed at the higher end of the dose–response range. Here, we therefore investigated the effect of buprenorphine on voluntary 10% w/v ethanol intake in genetically selected alcohol-preferring msP rats across a wide range of doses. We then used the nonselective opioid receptor antagonist naltrexone and the selective NOP receptor agonist UFP-101 (Calò et al 2005; McDonald et al 2003) to pharmacologically dissect the complex actions of buprenorphine and reveal the respective postulated component of its actions. Finally, to evaluate whether the effect of buprenorphine at the higher doses used in our experiments is selective to ethanol consumption and not due to unspecific actions (i.e., suppression of locomotor activity), the effect of this drug alone or in combination with UFP-101 was analyzed in the open-field test.
Methods and Materials
Animals
Male, genetically selected, alcohol-preferring rats were used. They were bred at the Department of Pharmacological Sciences and Experimental Medicine of the University of Camerino (Marche, Italy) for 53 generations from Sardinian alcohol-preferring rats of the 13th generation, provided by the Department of Neurosciences of the University of Cagliari, Italy (Agabio et al 1996; Lobina et al 1997). These animals are referred to as Marchigian Sardinian alcohol-preferring (msP) rats. At the time of the experiments their body weight ranged between 350 and 400 g. They were housed in a room on a reverse 12-hour light/dark cycle (lights off at 9:00 AM), temperature of 20°–22°C, and humidity of 45%–55%. Rats were offered free access to tap water and food pellets (4RF18, Mucedola, Settimo Milanese, Italy). Experiments took place at 9:30 AM, at the beginning of the dark phase of the light/dark cycle. Separate groups of animals were used in each experiment. All the procedures were conducted in adherence with the European Community Council Directive for Care and Use of Laboratory Animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Intracranial Surgery
For intracranial surgery, msP rats were anesthetized by intramuscular injection of 100–150 μL/rat of a solution containing tiletamine hydrocloridrate (58.17 mg/mL) and zolazepam cloridrate (57.5 mg/mL). A guide cannula for intracerebroventricular (ICV) injections into the lateral cerebroventricle was stereotaxically implanted and cemented to the skull. The following coordinates, taken from the atlas of Paxinos and Watson (1986), were used: AP = 1 mm behind the bregma, L = 1.8 mm from the sagittal suture, and V = 2 mm from the surface of the skull.
Drug Injections
Buprenorphine and naltrexone were purchased from Tocris (Ellisville, Missouri), and UFP-101 ([Nphe(1), Arg(14), Lys(15)]N/OFQ NH[2]) was a generous gift of Dr. R. Guerrini, Department of Pharmaceutical Sciences of the University of Ferrara, Italy. Naltrexone and UFP-101 were dissolved in sterile isotonic saline. Naltrexone was given by intraperitoneal (IP) injection, whereas UFP-101 was injected ICV in a volume of 1 μL/rat by means of a stainless-steel injector 2.5 mm longer than the guide cannula, so that its tip protruded into the ventricle. Buprenorphine was diluted with distilled water and given by IP injection.
Histology
At completion of the experiments, to evaluate the correct cannula placement, immediately before the rat was killed, 1 μl of black India ink was injected ICV and ink diffusion into the ventricles was evaluated.
Experimental Procedures
Ethanol Intake
At the age of 3 months msP rats were selected for their preference for 10% ethanol solution (w/v), offering them free choice between water and 10% ethanol 24 hours/day for 10 days. Water and 10% ethanol were offered in graduated drinking tubes equipped with metallic drinking spouts. The rats used in the following experiments had a 24-hour ethanol intake of 6–7 g/kg with a percent of ethanol preference [mL of ethanol solution/mL of total fluids (water + 10% ethanol) ingested in 24 × 100] higher than 90.
Starting on day 11, while maintained with food and water available during the entire day, rats received 10% ethanol for only 2 hours/day, at the beginning of the dark phase (9:30 AM). Before experiments, rats were acclimated to the limited 2-hour ethanol access for 7 days.
All the experiments were carried out according to a within-subject design, in which each animal received, in a counterbalanced order, all doses and compound tested in that specific experiment (see following experiment descriptions). Before the experiments rats received at least three mock IP and/or ICV injections to familiarize them with the injection procedure.
Water and ethanol intakes were measured by reading the volume consumed from the graduated burettes and were always recorded 30, 60, 90, and 120 min after ethanol was offered to the animals. Food intake was measured by weighing the food containers and taking into account spillage and was measured only at 30, 60, and 120 min. Ethanol, water, and food intakes are expressed as g/kg to reduce the influence of differences in body weight.
Open-Field Behavior
The open-field (66 cm × 66 cm × 20 cm) arena was used to analyze the locomotor effects of buprenorphine (3.0 mg/kg) and UFP-101 (20.0 μg/rat given twice) or their combination. Rats were gently placed in the center of the open-field apparatus and left to explore the arena for 30 min for two consecutive days (habituation trials). Immediately after arena exploration, the animals were taken back to their home cages. On day 3 animals received the respective treatments (see also Experiment 7) and were subjected again to an open-field session (test trial). The numbers of beam brakes at the center and at the periphery of the arena were automatically recorded for a total testing time of 30 min. The floor of the open field was completely cleaned and dried after each trial.
Experiment 1: Effect of Acute IP Injections of Buprenorphine on Voluntary Alcohol Intake
To evaluate the effect of buprenorphine on voluntary 10% ethanol intake, msP rats (n = 10) were injected IP with different doses of buprenorphine (.03, .3, 3.0, and 6.0 mg/kg) or its vehicle (control subjects) at intervals of 3–4 days, 90 min before access to alcohol. Baseline ethanol drinking was re-established between different dose-treatments.
Experiment 2: Effect of Acute IP Injections of Naltrexone on Voluntary Alcohol Intake
To evaluate the effect of naltrexone on voluntary 10% ethanol intake, according to a within-subject design, msP rats (n = 10) received naltrexone (.25, 1.0, and 2.5 mg/kg, IP) or vehicle (control subjects) at intervals of 3–4 days, 95 min before access to ethanol. Baseline ethanol drinking was re-established between different dose-treatments.
Experiment 3: Effect of Acute ICV Injections of UFP-101 on Voluntary Alcohol Intake
To evaluate the effect of the selective N/OFQ receptor antagonist UFP-101 on voluntary 10% ethanol intake, msP rats (n = 7) were injected ICV with 5.0, 10.0, and 20.0 μg/rat or its vehicle, given twice at 95 and 15 min before access to ethanol (10% w/v). In a Latin square design rats received all drug doses or its vehicle. An interval of 3–4 days was imposed between drug treatments, and baseline ethanol drinking was re-established during these periods.
Experiment 4: Effect of IP Injections of Naltrexone on Buprenorphine-Induced Increase of Ethanol Intake
To evaluate the effect of naltrexone on buprenorphine-induced increased ethanol intake, a group of msP rats (n = 10) was treated IP with naltrexone (.25 mg/kg) or its vehicle. Five minutes later, animals received an IP injection of .03 mg/kg of buprenorphine or its vehicle. Drug doses were chosen on the basis of the results obtained in Experiment 1 and Experiment 2. Specifically, the dose of buprenorphine was chosen that selectively increased ethanol intake, and the dose of naltrexone was chosen that was ineffective per se.
Ethanol was given to the animals 90 min after buprenorphine injection, and alcohol, water, and food intake were measured for 2 hours. Tests were carried out at intervals of 3–4 days, and in a Latin square design rats received all drug treatments. Baseline ethanol drinking was re-established between different dose-treatments.
Experiment 5: Effect of IP Injections of Naltrexone on Buprenorphine-Induced Decrease of Ethanol Intake
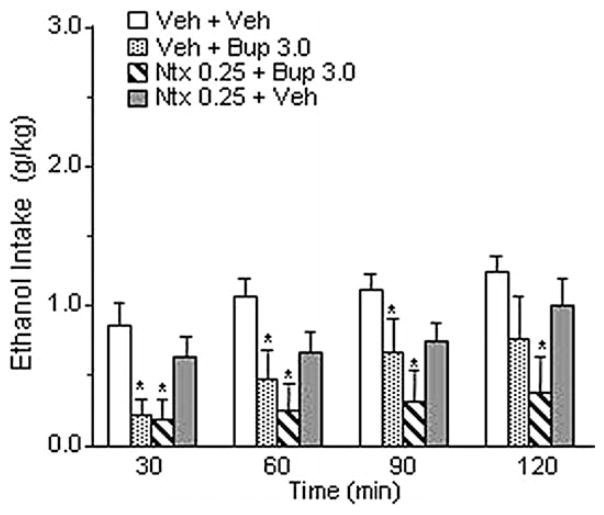
To evaluate the effect of naltrexone on the reduction of ethanol drinking induced by high doses of buprenorphine, msP rats (n = 8) were injected IP with .25 mg/kg of naltrexone or its vehicle. After 5 min, rats received an IP injection of 3.0 mg/kg of buprenorphine or its vehicle. Drug doses were chosen on the basis of the results obtained in Experiment 1 and Experiment 2. Specifically, the dose of buprenorphine that selectively decreased ethanol intake and the dose of naltrexone that was ineffective per se were chosen.
Rats were offered access to 10% ethanol 90 min after buprenorphine injection. Tests were carried out at intervals of 3–4 days, and in a Latin square design rats received all drug treatments. Baseline ethanol drinking was re-established between different dose-treatments.
Experiment 6: Effect of ICV Injections of UFP-101 on Buprenorphine-Induced Decrease of Ethanol Intake
To evaluate the effect of UFP-101 on the reduction of ethanol drinking induced by high doses of buprenorphine, according to a within-subject design, a group of msP rats (n = 10) was injected ICV with UFP-101 or its vehicle at the doses of 10.0 and 20.0 μg/rat, 95 and 15 min before access to 10% ethanol. Buprenorphine (3.0 mg/kg) or its vehicle was given 90 min before access to ethanol. Two injections of UFP-101, a peptidergic NOP receptor antagonist, were given in an attempt to better antagonize the effects of buprenorphine, a non-peptidic, long lasting opioidergic agent. Drug doses were chosen on the basis of the results obtained in Experiment 1 and Experiment 3. Specifically, the dose of buprenorphine that selectively decreased ethanol intake and the doses of UFP-101 that were ineffective per se were chosen.
Tests were carried out at intervals of 3–4 days, and in a Latin square design rats received all drug treatments. Baseline ethanol drinking was re-established between different dose-treatments.
Experiment 7: Effect of Buprenorphine and UFP-101 on Open-Field Behavior
In this experiment, we evaluated the locomotor effects of 3.0 mg/kg of buprenorphine (IP), the selective NOP receptor antagonist UFP-101 (20.0 μg/rat, ICV), or their combination. According to a between-subject design, four groups (n = 6/group) of msP rats were injected ICV with UFP-101 (20.0 μg/rat or its vehicle) 95 and 15 min before exposure to the open field. Buprenorphine (3.0 mg/kg) or its vehicle was given 90 min before exposure to the open field. Drug doses were chosen on the basis of the results obtained in Experiment 6.
Statistical Analysis
Statistical analysis of data for ethanol, food, and water intake was performed by means of two-way analysis of variance (ANOVA) with repeated measures, one factor for treatment and one factor for time. Statistical analysis for the open-field experiment was performed by means of two-way ANOVA with between-subject comparisons for drug treatment and within-subject comparisons for time (habituation vs. test trial). The distance travelled and the time spent resting were analyzed separately. Post-hoc comparisons were carried out by Newman–Keuls Test. Statistical significance was set at p < .05.
Results
Experiment 1: Effect of Acute IP Injections of Buprenorphine on Voluntary Alcohol Intake
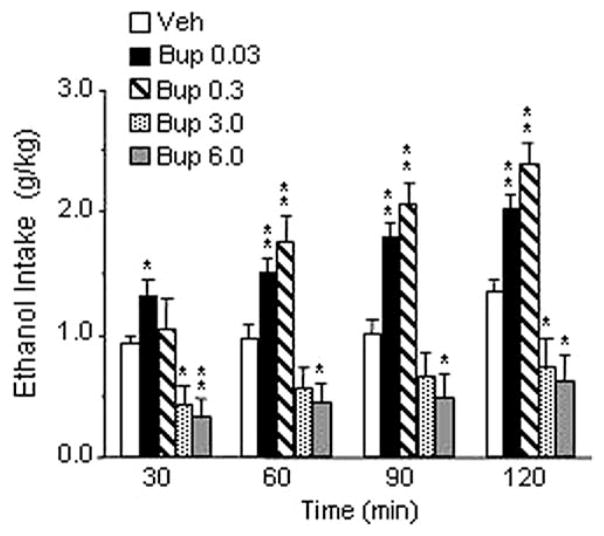
The overall ANOVA revealed a highly significant treatment effect [F(4,9) = 22,31, p < .001]. Post-hoc analysis showed a significant dualistic effect with an increase of ethanol consumption after administration of .03 and .3 mg/kg of buprenorphine (p < .01) and a decrease of drinking after treatment with 3.0 and 6.0 mg/kg of the drug (p < .05). As shown in Figure 1, at the two lowest doses, buprenorphine significantly increased ethanol drinking throughout the 2-hour observation, whereas injection of 6.0 mg/kg of buprenorphine resulted in a significant decrease of drinking at all time points recorded. Similarly, administration of 3.0 mg/kg of the drug induced a significant inhibition of drinking at 30, 60, and 120 min. Difference from control subjects was barely above statistical significance at 60 min. Buprenorphine treatment elicited a significant decrease of food intake [F(4,9) = 5.15, p < .001]. Post-hoc test revealed a significant decrease only at the highest dose (6.0 mg/kg) tested (Table 1). Water intake was not modified by drug treatment (Table 1).
Figure 1.
Effect of intraperitoneal injection of buprenorphine (Bup) (0, .03, .3, 3.0, and 6.0 mg/kg) on ethanol intake in Marchigian Sardinian alcohol-preferring rats. The drug was given 90 min before ethanol access, and alcohol consumption was monitored at 30, 60, 90, and 120 min. Values represent the mean (±SEM) of 10 subjects. Difference from control subjects: *p < .05; **p < .01. Veh, vehicle.
Table 1.
Effects of Buprenorphine, Naltrexone, UFP-101, or Their Combinations on Food and Water Intake in Marchigian Sardinian Alcohol-Preferring Rats
| Treatment | Food Intake (min) |
Water Intake (min) |
|||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | 30 | 60 | 90 | 120 | |
| Buprenorphine (mg/kg, IP) | |||||||
| Veh | 2.9 ± .5 | 4 ± .6 | 5.9 ± .9 | .1 ± 0 | .1 ± 0 | .1 ± .4 | .1 ± .4 |
| Bup .03 | 2.5 ± .6 | 2.9 ± .6 | 8.6 ± .9 | — | — | — | — |
| Bup .3 | 1.3 ± .5 | 3.8 ± 1.1 | 6.6 ± 1.7 | — | — | — | .1 ± 0 |
| Bup 3 | 1 ± .3 | 3 ± .6 | 7.5 ± 1.1 | — | — | — | — |
| Bup 6 | .5 ± .2a | 1.1 ± .4a | 2.1 ± .7a | — | — | — | .1 ± 0 |
| Naltrexone (mg/kg, IP) | |||||||
| Veh | 1.1 ± .5 | 1.6 ± .5 | 4.3 ± 1.1 | .4 ± .3 | .5 ± .3 | .5 ± .3 | .8 ± .3 |
| Nltx .25 | 1.4 ± .8 | 1.9 ± .8 | 3.1 ± .9 | .1 ± 0 | — | .7 ± .3 | 1.3 ± .4 |
| Nltx 1 | .7 ± .5 | .8 ± .5 | 3.3 ± .9 | .4 ± .4 | .5 ± .4 | .5 ± .4 | .6 ± .4 |
| Nltx 2.5 | 1.5 ± 1 | 3.1 ± 1.2 | 4.5 ± 1.2 | .2 ± .2 | .4 ± .4 | .7 ± .6 | .8 ± .7 |
| UFP-101 (μg/rat, ICV) | |||||||
| Veh | 2.7 ± .9 | 3.8 ± 1 | 8.2 ± .9 | .9 ± .9 | .9 ± .9 | 1.4 ± 1.4 | 1.4 ± 1.4 |
| UFP-101 5 | 5 ± 1.2 | 5.5 ± .7 | 7.3 ± 1.1 | 2.3 ± 1.5 | 2.3 ± 1.4 | 2.4 ± 1.4 | 3.2 ± 1.9 |
| UFP-101 10 | 2.2 ± .8 | 3 ± .7 | 5.6 ± 1.5 | 1.1 ± .8 | 1.3 ± .8 | 2.4 ± 1.7 | 2.4 ± 1.7 |
| UFP-101 20 | 4.3 ± 1.2 | 5 ± .9 | 6.8 ± 1.5 | — | .4 ± .4 | .4 ± .4 | 1.5 ± 1.5 |
| Naltrexone (mg/kg, IP) + Buprenorphine .03 (mg/kg, IP) | |||||||
| Veh + Veh | 1.4 ± .8 | 2.8 ± 1.3 | 1.7 ± .8 | — | — | — | — |
| Veh + Bup .03 | 2.4 ± 1 | 5 ± 1.1 | 2.7 ± 1 | — | — | — | 0 ± .2 |
| Nltx .25 + Bup .03 | 2.7 ± 1.1 | 4.8 ± 1.3 | 2.8 ± 1.1 | — | — | — | .1 ± 0 |
| Nltx .25 + Veh | 2.1 ± 1.7 | 2.3 ± .7 | 2 ± .7 | — | — | .1 ± .0 | .3 ± .1 |
| Naltrexone (mg/kg, IP) + Buprenorphine 3 (mg/kg, IP) | |||||||
| Veh + Veh | 3.5 ± 1.3 | 3.8 ± 1.3 | 4.7 ± 1.1 | .2 ± .2 | .4 ± .4 | .6 ± .4 | .6 ± .4 |
| Veh + Bup 3 | .2 ± .2 | .7 ± .6 | 1.9 ± 1.1 | .1 ± 0 | .2 ± .1 | .4 ± .2 | .5 ± .3 |
| Nltx .25 + Bup 3 | .7 ± .5 | 1.2 ± .9 | 2.8 ± 1.7 | .2 ± .1 | .3 ± .1 | .7 ± .2 | .8 ± .2 |
| Nltx .25 + Veh | 2.4 ± 1.1 | 2.6 ± 1.1 | 4.5 ± 1.4 | — | — | .1 ± 0 | .1 ± 0 |
| UFP-101 (μg/rat, ICV) + Buprenorphine 3 (mg/kg, IP) | |||||||
| Veh + Veh | 2.3 ± .9 | 3.3 ± .8 | 5.50 ± 1.5 | .1 ± 0 | .1 ± 0 | .1 ± 0 | .1 ± .1 |
| Veh + Bup 3 | .8 ± .4 | 1.5 ± .9 | 2.86 ± 1.5 | .2 ± 0 | .3 ± .3 | .3 ± .3 | .4 ± .4 |
| UFP 10 + Bup 3 | 3.4 ± .7 | 5.3 ± 1.2 | 7.34 ± 1.6 | .8 ± .7 | .9 ± .8 | 1 ± .9 | 1.1 ± 1 |
| UFP 20 + Bup 3 | 2.6 ± .5 | 4.2 ± 1.1 | 6.84 ± 1.6 | .1 ± 0 | .2 ± .2 | .2 ± .2 | .3 ± .3 |
IP, intraperitoneal; Veh, vehicle; Bup, buprenorphine; Nltx, naltrexone; UFP, UFP-101; ICV, intracerebroventricular. Treatments were conducted as described above for the respective experiments. Each value represents the mean (± SEM) of intake corrected for body weight (g/kg).
p < .01, difference from vehicle.
Experiment 2: Effect of Acute IP Injections of Naltrexone on Voluntary Alcohol Intake
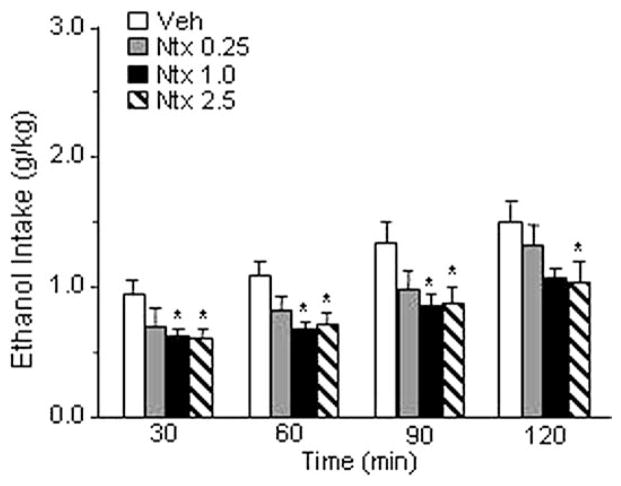
The overall ANOVA demonstrated a significant effect of naltrexone [F(3,9) = 4.257, p < .05]. As shown in Figure 2, post-hoc comparisons revealed a significant inhibition of ethanol intake after administration of 1.0 or 2.5 mg/kg of naltrexone, whereas injection of .25 mg/kg of the drug did not significantly modify alcohol consumption. Neither food intake nor water intake were modified by naltrexone (Table 1).
Figure 2.
Effect of intraperitoneal injection of naltrexone (Ntx) (0, .25, 1.0, and 2.5 mg/kg) on ethanol intake in Marchigian Sardinian alcohol-preferring rats. Drug injections were given 95 min before ethanol access, and alcohol consumption was monitored at 30, 60, 90, and 120 min. Values represent the mean (±SEM) of 10 subjects. Difference from control subjects: *p <.05. Veh, vehicle.
Experiment 3: Effect of Acute ICV Injections of UFP-101 on Voluntary Alcohol Intake
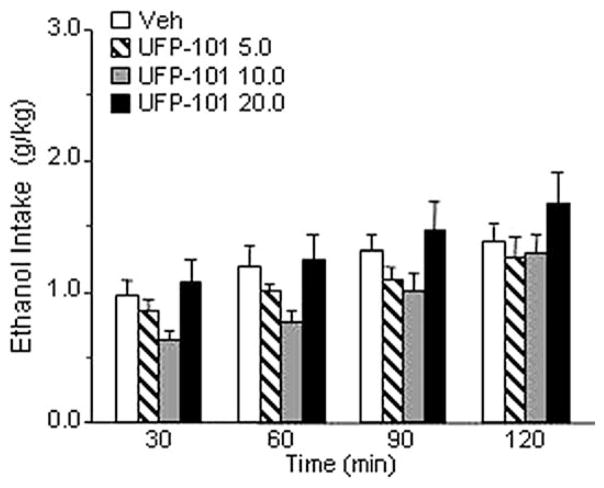
The overall ANOVA showed that treatment with UFP-101 (5.0, 10.0, or 20.0 μg/rat) given twice at 95 and 15 min before access to ethanol (Figure 3) did not modify ethanol drinking in msP rats [F(3,6) = 2.264, p = ns]. Neither food intake nor water intake were modified by UFP-101 treatment (Table 1). This is in line with previous studies in which other selective NOP antagonists were used (Ciccocioppo et al 2002).
Figure 3.
Effect of UFP-101 (0, 5.0, 10.0, and 20.0 μg/rat) on ethanol intake in Marchigian Sardinian alcohol-preferring rats. The drug was given intracerebroventricular twice at 95 and 15 min before ethanol access, and alcohol consumption was monitored at 30, 60, 90, and 120 min. Values represent the mean (± SEM) of seven subjects. Difference from control subjects was not statistically significant. Veh, vehicle.
Experiment 4: Effect of IP Injections of Naltrexone on Buprenorphine-Induced Increased Ethanol Intake
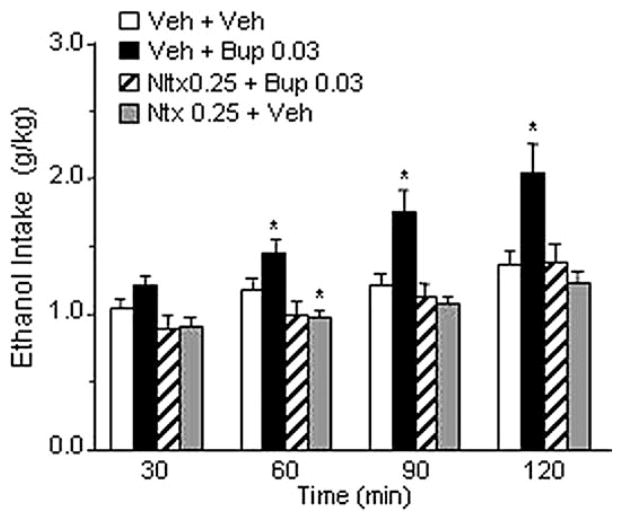
The overall ANOVA revealed a highly significant treatment effect [F(3,9) = 9.50, p < .01]. Confirming the results of Experiment 1, the dose of .03 mg/kg buprenorphine significantly increased ethanol drinking (p < .01). This effect was abolished by pretreatment with .25 mg/kg of naltrexone. Consistent with the data obtained in Experiment 2, administration of .25 mg/kg of naltrexone alone did not significantly affect ethanol drinking (Figure 4). Water and food intake were not influenced by drug treatments (Table 1).
Figure 4.
Effect of intraperitoneal (IP) injection of naltrexone (Ntx), at a dose ineffective per se (.25 mg/kg), on buprenorphine-induced increased ethanol intake (.03 mg/kg) in Marchigian Sardinian alcohol-preferring rats. Buprenorphine (Bup) was injected IP 5 min after Ntx administration, and ethanol was given to the animals 90 min after Bup. Alcohol consumption was monitored at 30, 60, 90, and 120 min. Values represent the mean (±SEM) of 10 subjects. Difference from control subjects: *p < .05. Veh, vehicle.
Experiment 5: Effect of IP Injections of Naltrexone on Buprenorphine-Induced Decreased Ethanol Intake
The overall ANOVA showed a significant effect of treatment [F(3,7) = 5.90, p < .01]. As shown in Figure 5 and consistent with the result of Experiment 1, administration of 3.0 mg/kg of buprenorphine significantly reduced ethanol drinking (p < .05). Pretreatment with naltrexone (0.25 mg/kg, IP) did not block high-dose buprenorphine-induced decrease of ethanol intake, which remained significantly lower compared with control subjects (p < .01). Administration of naltrexone alone reduced ethanol drinking slightly but not significantly. Water and food intake were not modified by drug treatments (Table 1).
Figure 5.
Effect of intraperitoneal (IP) injection of naltrexone (Ntx), at a dose ineffective per se (.25 mg/kg), on buprenorphine-induced decreased ethanol intake (3.0 mg/kg) in Marchigian Sardinian alcohol-preferring rats. Five minutes after Ntx administration animals received an IP injection of buprenorphine (Bup), and ethanol was given to the animals 90 min after Bup. Alcohol consumption was monitored at 30, 60, 90, and 120 min. Values represent the mean (± SEM) of eight subjects. Difference from control subjects: *p < .05. Veh, vehicle.
Experiment 6: Effect of ICV Injections of UFP-101 on Buprenorphine-Induced Decreased Ethanol Intake
The overall ANOVA showed a significant effect of treatment [F(3,9) = 9.16, p < .001]. As in previous experiments, administration of buprenorphine at the dose of 3.0 mg/kg significantly reduced alcohol intake (p < .05). Pretreatment with UFP-101 at the dose of 10.0 μg/rat (given twice) completely blocked this effect of buprenorphine. Administration of 20.0 μg/rat of the N/OFQ antagonist (given twice) not only blocked buprenorphine-induced reduction of ethanol drinking but resulted in a significant increase of drinking, compared with control subjects (p < .05). As shown in Figure 6, significant differences from control subjects were observed throughout the observation period. Water and food intake were never modified by drug treatments (Table 1).
Figure 6.
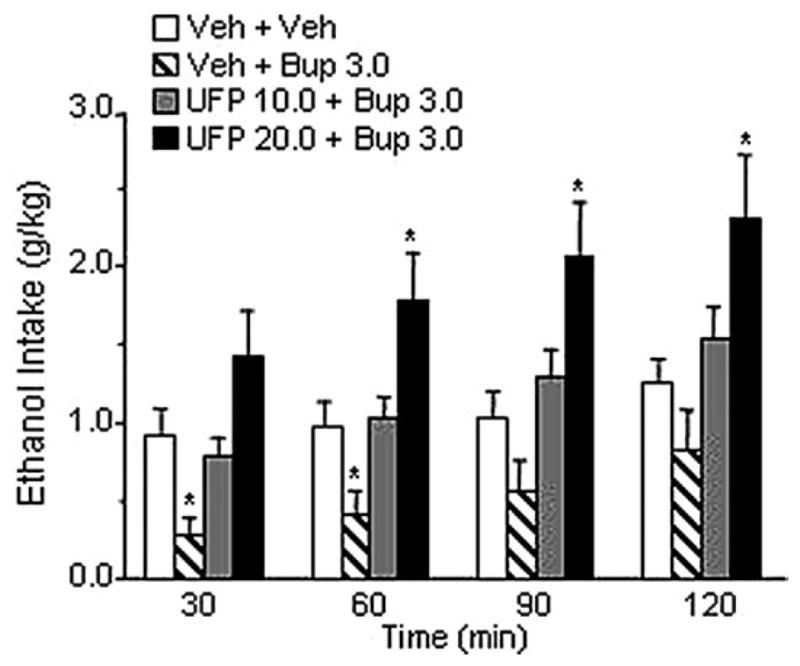
Effect of intracerebroventricular (ICV) injection of UFP-101, at doses ineffective per se (10.0, 20.0 μg/rat), on buprenorphine-induced decreased ethanol intake (3.0 mg/kg) in Marchigian Sardinian alcohol-preferring rats. The UFP-101 was ICV injected 95 and 15 min before access to ethanol. Buprenorphine (Bup) was given 90 min before access to ethanol. Alcohol consumption was monitored at 30, 60, 90, and 120 min. Values represent the mean (±SEM) of 10 subjects. Difference from control subjects: *p < .05. Veh, vehicle.
Experiment 7: Effect of Buprenorphine and UFP-101 on Open-Field Behavior
The general locomotor activity of the animals was not influenced by drug treatments. The overall ANOVA showed nonsignificant differences on time spent resting [F(3,20) = .11, p = ns] and distance traveled [F(3,120) = .14, p = ns] (Table 2). Comparisons between pretreatment and treatment provided further within-subject evidence of the absence of locomotor effects due to drug injections.
Table 2.
Locomotor Activity in Naïve Marchigian Sardinian Alcohol-Preferring Rats Treated With Buprenorphine, UFP-101, or Their Combination
| Treatment | Time Resting (min) |
Distance Traveled (cm) |
||
|---|---|---|---|---|
| Pretreatment | Treatment | Pretreatment | Treatment | |
| Veh + Veh | 23.6 ± 1.4 | 23 ± 1.4 | 3717.6 ± 1232.5 | 3859.6 ± 972 |
| UFP + Veh | 22.9 ± 1.2 | 23.7 ± 1.3 | 3618.6 ± 615.5 | 3050.3 ± 715 |
| Veh + Bup | 23.6 ± 1 | 24.2 ± .7 | 3575 ± 709.1 | 2997.6 ± 393.2 |
| UFP + Bup | 24.6 ± 1.5 | 21.9 ± .2 | 3415 ± 1008.3 | 3961 ± 706.3 |
UFP-101 (20.0 μg/rat, intracerebroventricular was injected twice, 95 and 15 min before placing the animal into the open-field chambers. Buprenorphine (3.0 mg/kg, intraperitoneal) was given 90 min before placing the animal into the open-field chambers. Each value represents the mean (±SEM) of six subjects. Veh, vehicle; UFP, UFP-101; Bup, Buprenorphine.
Discussion
We report a dualistic action of buprenorphine on ethanol consumption. At low doses (.03 and .3 mg/kg), this drug increased ethanol consumption, whereas at the dose of 3.0 mg/kg, it markedly and selectively decreased it. Food and water consumption as well as motor behavior as assessed in the open field were not influenced in these animals after treatment with 3.0 mg/kg of buprenorphine. If the drug is given at higher doses (6.0 mg/kg), it further reduces ethanol consumption but food intake is also decreased concomitantly, indicating that nonspecific inhibition of ingestive behavior might occur at this highest dose.
The increase of ethanol drinking observed after administration of low doses of buprenorphine can be explained on the basis of the ability of this drug to activate the μ-opioid receptor subtype. In fact, previously published studies have demonstrated that treatment with low doses of morphine or the selective μ–agonist DAMGO increases ethanol consumption in rats (Hubbell et al 1986, 1993, Zhang and Kelley 2002). Consistent with this notion, in the present study we have shown that pretreatment with naltrexone, at a dose that does not modify ethanol drinking per se, completely abolishes the increase of alcohol consumption evoked by low doses of buprenorphine. Surprisingly, however, naltrexone was unable to block the reduction of ethanol consumption induced by higher doses of buprenorphine. This demonstrates that the inhibition of ethanol drinking observed after administration of higher doses of buprenorphine is not mediated by classical opioidergic mechanisms.
Searching for a mechanism that might mediate the high-dose suppressive effects of buprenorphine on alcohol intake, we tested the hypothesis that these could be mediated by its ability to activate the NOP receptors (Bloms-Funke et al 2000; Wnendt et al 1999). Therefore, we tested the effect of the highly selective NOP receptor antagonist UFP-101 on high-dose buprenorphine-induced reduction of ethanol drinking. The results of this experiment seem to confirm our hypothesis. Central administration of the NOP antagonist at doses that did not influence ethanol drinking per se fully blocked the inhibitory effect of buprenorphine. In addition, at the highest dose of UFP-101, the NOP antagonist inverted the high-dose action of buprenorphine (i.e., whereas animals receiving 3.0 mg buprenorphine alone drank less than vehicle-treated control subjects, animals receiving the same dose of buprenorphine after UFP-101 pretreatment paradoxically drank more than control subjects receiving neither of the drugs). This seemingly paradoxical effect likely reflects that a complete blockade by UFP-101 of NOP receptors unmasks opposite buprenorphine effects mediated through activation of μ-opioid receptors. Under these circumstances, the activation of μ-opioid receptors and the subsequent increase of ethanol consumption are not longer counterbalanced by the concomitant stimulation of the NOP receptors.
In a number of other studies, biphasic or even triphasic dose-related effects have been described for buprenorphine (Dum and Herz 1981; Pick et al 1997; Rance et al 1979; Tyers 1980). In a detailed investigation by Huang et al (2001), it was shown that buprenorphine binds at nanomolar concentration to μ-, δ-, and κ-receptors and at micromolar concentration to the NOP receptors. At functional level it acts as a partial agonist at μ-, κ-, or NOP receptors and as an antagonist at δ-receptors. In contrast, its major metabolite, norbuprenorphine, is a full agonist at δ- and NOP receptors, and partial agonist at μ- and κ-receptors. This complex pharmacology might account for the fact that at low doses (1.0 mg/kg, IP) buprenorphine is an effective analgesic but at higher doses its antinociceptive effects are diminished (Dum and Herz 1981; Lizasoain et al 1991; Lutfy et al 2003). In general, researchers attributed both the safety and the biphasic actions of buprenorphine to its partial agonist activity at μ-opioid receptors and to its agonistic/antagonistic properties at κ-opioid receptors (Kamei et al 1995; Lattanzi et al 2001).
Our present data, however, prompt a reanalysis of buprenorphine’s pharmacological profile and an examination of whether activation of NOP receptors plays a major role in shaping it (Lutfy et al 2003; Wnendt et al 1999). That this might be the case is suggested by the fact that co-administration of UFP-101 (see present results) and J-113397 (Lutfy et al 2003), two selective NOP receptor antagonists (Kawamoto et al 1999; McDonald et al 2003), completely eliminates the biphasic effect of buprenorphine on ethanol intake (present data) and on analgesia (Lutfy et al 2003), respectively.
It is known that activation of brain NOP receptors by the endogenous ligand N/OFQ results in an anti-opioid action (Ciccocioppo et al 2000a, 2000b). For example, N/OFQ injected intracranially blocks the analgesic effects of morphine (King et al 1998; Mogil and Pasternak 2001; Mogil et al 1996), prevents the development of morphine-induced conditioned place preference (Ciccocioppo et al 2000b; Murphy et al 1999), and inhibits morphine-induced dopamine release in the nucleus accumbens (Di Giannuario and Pieretti 2000). In addition, it has been demonstrated that activation of NOP receptors by N/OFQ results in a marked inhibition of ethanol self-administration and ethanol-seeking in rodents (Ciccocioppo et al 1999, 2004). On the basis of these data it is conceivable to hypothesize (see also Wnendt et al 1999) that the low abuse liability, the relatively safe profile of buprenorphine, and its efficacy in reducing alcohol drinking might be due to the activation of NOP receptors induced by this drug.
The results of the present study suggest a potential new application of buprenorphine in pharmacological treatment of alcohol dependence. We propose that a combination of buprenorphine with naltrexone could be particularly beneficial in this regard. First, on the basis of the documented efficacy of naltrexone for this indication (Bouza et al 2004), simultaneous blockade of the μ-opioid and activation of the NOP receptors should result in a synergistic inhibition of alcohol drinking. Second, co-administration of naltrexone and buprenorphine would eliminate concerns of giving an opioid agonist to subjects without opioid dependence. In particular, co-administration of buprenorphine with a novel depot naltrexone preparation (Kranzler et al 2004) seems attractive in this regard, because it would ensure compliance with naltrexone treatment before administration of buprenorphine.
A second potential application is prompted by the observation that concomitant use of different drugs of abuse is on the rise and increases the likelihood of overdose and suicide (Risser and Schneider 1994; Roy et al 1990; Ruttenber and Luke 1984) and participation in HIV risk behaviors (Petry 1999) and reduces the treatment outcomes (Rounsaville et al 1987; Schuckit 1985). Alcohol is the drug most frequently co-abused with illicit substances (Helzer and Pryzbeck 1988; Hesselbrock et al 1985). In the United States, approximately 50% of heroin addicts applying to methadone programs are also regular users of alcohol (Ball and Ross 1991). Alcohol consumption further increases under methadone-maintenance therapy, and this represents a serious limitation in the long term clinical use of this compound (Backmund et al 2003; Hunt et al 1986; Stastny and Potter 1991). The agonistic activity of methadone at μ-opioid receptors could be at the origin of this effect. In this respect, buprenorphine might offer important advantages over methadone, because owing to its ability to simultaneously activate the NOP receptors, it should reduce rather than increase alcohol consumption. Clinical studies are urgently needed to evaluate the efficacy of buprenorphine to control ethanol abuse in alcoholic patients, possibly in association with naltrexone, and to systematically evaluate its efficacy in the treatment of concomitant opiate and alcohol dependence.
Finally, the present observations raise an intriguing possibility in relation to the therapeutic efficacy of buprenorphine in heroin dependence. As reviewed by Mattick et al (2004), clinical studies of buprenorphine that have used high doses of buprenorphine have consistently shown superior outcomes to those where low doses have been used. Clinical experience indicates further improvements beyond the doses studied systematically. Yet a recent study with positron emission tomography and carfentanyl displacement indicates that there is virtually no increase in μ-opioid occupancy by buprenorphine between the maximal clinically used dose, 32 mg daily, and one-half of that dose. This prompts the question of whether some of the additional efficacy of high buprenorphine doses might also be mediated through an activation of NOP receptors. If this proves to be the case, it would demonstrate a novel treatment principle for this disorder that lacks addictive properties (Lewis and Walter 1992).
Acknowledgments
This study was supported by the European Union’s Fifth Framework Program, grant QLRT-2001-01048 (to MH and RC); National Institute on Alcohol Abuse and Alcoholism, grant AA01435 (to FW subcontract to RC); and by a Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) grant 2004 to (MM). We wish to thank Mr. Marino Cucculelli for his skilful technical assistance.
References
- Agabio R, Cortis G, Fadda F, Gessa GL, Lobina C, Reali R, Colombo G. Circadian drinking pattern of Sardinian alcohol-preferring rats. Alcohol Alcohol. 1996;31:385–388. doi: 10.1093/oxfordjournals.alcalc.a008166. [DOI] [PubMed] [Google Scholar]
- Auriacombe M, Franques P, Tignol J. Deaths attributable to methadone vs buprenorphine in France. JAMA. 2001;285:45. doi: 10.1001/jama.285.1.45. [DOI] [PubMed] [Google Scholar]
- Backmund M, Schutz CG, Meyer K, Eichenlaub D, Soyka M. Alcohol consumption in heroin users, methadone-substituted and codeine-substituted patients--frequency and correlates of use. Eur Addict Res. 2003;9:45–50. doi: 10.1159/000067733. [DOI] [PubMed] [Google Scholar]
- Ball J, Ross A. The Effectiveness of Methadone Maintenance Treatment. New York: Springer-Verlag; 1991. [Google Scholar]
- Bickel WK, Marion I, Lowinson JH. The treatment of alcoholic methadone patients: A review. J Subst Abuse Treat. 1987;4:15–19. doi: 10.1016/0740-5472(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–1146. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Calo G, Guerrini R, Rizzi A, Salvadori S, Burmeister M, Kapusta DR, et al. UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drug Rev. 2005;11:97–112. doi: 10.1111/j.1527-3458.2005.tb00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Panocka I, Massi M. Nociceptin/orphanin FQ and drugs of abuse. Peptides. 2000a;21:1071–1780. doi: 10.1016/s0196-9781(00)00245-x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000b;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol seeking-behaviour by the anti-opioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Polidori C, Antonelli L, Salvatori S, Guerrini R, Massi M. Pharmacological characterization of the nociceptin receptor which mediates reduction of alcohol drinking in rats. Peptides. 2002;23:117–125. doi: 10.1016/s0196-9781(01)00587-3. [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waele J-P, Kiianmaa K, Gianoulakis C. Distribution of the μ and δ opioid sites in the brain of the alcohol-preferring AA and alcohol-avoiding AANA lines of rats. J Pharmacol Exp Ther. 1995;275:518–527. [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release in the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda P, Tronci S, Colombo G, Fratta W. Differences in the opioid system in selected brain regions of alcohol-preferring and alcohol-non-preferring rats. Alcohol Clin Exp Res. 1999;23:1296–1305. [PubMed] [Google Scholar]
- Finco G, Polati E, Gottin L, Bartoloni A, Milan B, Zanoni L, Valle L. Intravenous patient-controlled analgesia (PGA) in the treatment of postoperative pain: Rationale and clinical application. Chir Ital. 1995;47:20–25. [PubMed] [Google Scholar]
- Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction. II. Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin Pharmacol Ther. 1990;47:525–534. doi: 10.1038/clpt.1990.67. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, de Waele J-P, Kiianmaa K. Differences in the brain and pituitary β-endorphin system between the alcohol-preferring AA and alcohol avoiding ANA rats. Alcohol Clin Exp Res. 1992;16:453–459. doi: 10.1111/j.1530-0277.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Gundersen RY, Andersen R, Narverud G. Postoperative pain relief with high-dose epidural buprenorphine: A double-blind study. Acta Anaesthesiol Scand. 1986;30:664–667. doi: 10.1111/j.1399-6576.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Fraser AR, Hampton JR. Randomized trial comparing buprenorphine and diamorphine for chest pain in suspected myocardial infarction. Br Med J. 1979;2:300–302. doi: 10.1136/bmj.2.6185.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol. 1988;49:219–224. doi: 10.15288/jsa.1988.49.219. [DOI] [PubMed] [Google Scholar]
- Hesselbrock MN, Meyer RE, Keener JJ. Psychopathology in hospitalized alcoholics. Arch Gen Psychiatry. 1985;42:1050–1055. doi: 10.1001/archpsyc.1985.01790340028004. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Hubbell CL, Czirr SA, Hunter GA, Beaman CM, Lecann NC, Reid LD. Consumption of ethanol solution is potentiated by morphine and attenuated by naloxone persistently across repeated daily administrations. Alcohol. 1986;3:39–54. doi: 10.1016/0741-8329(86)90070-4. [DOI] [PubMed] [Google Scholar]
- Hubbell CL, Mankes RF, Reid LD. A small dose of morphine leads rats to drink more alcohol and achieve higher blood alcohol concentrations. Alcohol Clin Exp Res. 1993;17:1040–1043. doi: 10.1111/j.1530-0277.1993.tb05661.x. [DOI] [PubMed] [Google Scholar]
- Hunt DE, Strug DL, Goldsmith DS, Lipton DS, Robertson K, Truitt L. Alcohol use and abuse: Heavy drinkers among methadone clients. Am J Drug Alcohol Abuse. 1986;12:147–164. doi: 10.3109/00952998609083749. [DOI] [PubMed] [Google Scholar]
- Jamensky NT, Gianoulakis C. Content of dynorphins and κ-opioid receptors in distinct rain regions of C57BL/6 and DBA/2 mice. Alcohol Clin Exp Res. 1997;21:1455–1464. [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: A potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Johnson RE, McCagh JC. Buprenorphine and naloxone for heroin dependence. Curr Psychiatry Rep. 2000;2:519–526. doi: 10.1007/s11920-000-0012-8. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain CS. Other medications for the treatment of opioid dependence. In: Strain EC, Stitzer M, editors. Methadone Treatment for Opioid Dependence. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- June HL, Cason CR, Chen SA, Lewis MJ. Buprenorphine alters ethanol self-administration in rats: Dose-response and time-dependent effects. Psychopharmacology. 1998;140:29–37. doi: 10.1007/s002130050735. [DOI] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: A randomised, placebo-controlled trial. Lancet. 2003;361:662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Suzuki T, Misawa M, Nagase H, Kasuya Y. Buprenorphine exerts its antinociceptive activity via μ1 opioid receptors. Life Sci. 1995;56:285–290. doi: 10.1016/0024-3205(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, et al. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist:1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397) J Med Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- King M, Chang A, Pasternak GW. Functional blockade of opioid analgesia by orphanin FQ/nociceptin. Biochem Pharmacol. 1998;55:1537–1540. doi: 10.1016/s0006-2952(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Krystal JH, Chamey DS, Price LH, Morgan CH, Kleber HD. Opioid antagonist challenges in buprenorphine maintained patients. Drug Alcohol Depend. 1990;25:73–78. doi: 10.1016/0376-8716(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L Drug Abuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: A multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Lattanzi R, Negri L, Giannini E, Schmidhammer H, Schutz J, Improta G. HS-599: A novel long acting opioid analgesic does not induce place-preference in rats. Br J Pharmacol. 2001;134:441–447. doi: 10.1038/sj.bjp.0704280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader JD. Buprenorphine has potent κ opioid receptor antagonistic activity. Neuropsychopharmacology. 1987;26:1445–1447. doi: 10.1016/0028-3908(87)90112-2. [DOI] [PubMed] [Google Scholar]
- Lewis JW. Buprenorphine. Drug Alcohol Depend. 1985;14:363–372. doi: 10.1016/0376-8716(85)90067-5. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Walter D. Buprenorphine-background to its development as a treatment for opiate dependence. NIDA Res Monogr. 1992;121:5–11. [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P, et al. Buprenorphine maintenance treatment of opioid dependence: A multi-center, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Allen JP. Medications for alcohol, illicit drug, and tobacco dependence. An update of research findings. J Subst Abuse Treat. 1999;16:105–112. doi: 10.1016/s0740-5472(98)00028-2. [DOI] [PubMed] [Google Scholar]
- Lizasoain I, Lez JC, Lorenzo P. Buprenorphine: bell-shaped dose-response curve for its antagonistic effects. Gen Pharmacol. 1991;22:297–300. doi: 10.1016/0306-3623(91)90452-c. [DOI] [PubMed] [Google Scholar]
- Lobina C, Agabio R, Diaz G, Fa M, Fadda F, Gessa GL, et al. Constant absolute ethanol intake by Sardinian alcohol preferring rats independent of ethanol concentrations. Alcohol Alcohol. 1997;32:19–22. doi: 10.1093/oxfordjournals.alcalc.a008229. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, et al. Buprenoprhine-induced antinociception is mediated by μ-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23:10331–10337. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan J, Paterson SJ, Tafani A, Kosterlitz HW. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol. 1982;319:197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sci. 2000;66:1915–1927. doi: 10.1016/s0024-3205(00)00517-8. [DOI] [PubMed] [Google Scholar]
- Martin A, Pilotto R, Singer G, Oei TPS. The suppression of ethanol self injection by buprenorphine. Pharmacol Biochem Behav. 1983;19:985–986. doi: 10.1016/0091-3057(83)90403-3. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Mattick RP, Ali R, White JM, O’Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: A randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003;98:441–452. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2004;3:CD002207. doi: 10.1002/14651858.CD002207.pub2. [DOI] [PubMed] [Google Scholar]
- Maunuksela EL, Korpela R, Olkkola T. Double-blind, multiple-dose comparison of buprenorphine and morphine in postoperative pain of children. Br J Anaesth. 1998;60:48–55. doi: 10.1093/bja/60.1.48. [DOI] [PubMed] [Google Scholar]
- McDonald J, Calò G, Guerrini R, Lambert DG. UFP-101, a high affinity antagonist for the nociceptin/orphanin FQ receptor: Radioligand and TPgamma(35)S binding studies. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:183–187. doi: 10.1007/s00210-002-0661-8. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Lukas SE, Gastfriend DR, Teoh SK, Holman BL. Buprenorphine treatment of opiate and cocaine abuse: Clinical and preclinical studies. Harv Rev Psychiatr. 1993;1:168–183. doi: 10.3109/10673229309017075. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Murphy DF, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Murphy DF, MacEvilly M. Pain relief with epidural buprenorphine after spinal infusion: A comparison with intramuscular (i.m.) morphine. Acta Anaesthesiol Scand. 1984;28:144–146. doi: 10.1111/j.1399-6576.1984.tb02030.x. [DOI] [PubMed] [Google Scholar]
- Negus SS, Bidlack JM, Mello NK, Furness MS, Rice KC, Brandt MR. Delta opioid antagonist effects of buprenorphine in rhesus monkeys. Behav Pharmacol. 2002;13:557–570. doi: 10.1097/00008877-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Ottomanelli G. Methadone patients and alcohol abuse. J Subst Abuse Treat. 1999;16:113–121. doi: 10.1016/s0740-5472(98)00030-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. North Ryde, N.S.W., Australia: Academic Press; 1986. [Google Scholar]
- Petry NM. Alcohol use in HIV patients: What we don’t know may hurt us. Int J STD AIDS. 1999;10:561–570. doi: 10.1258/0956462991914654. [DOI] [PubMed] [Google Scholar]
- Picard PR, Tramer MR, McQuay HJ, Moore RA. Analgesic efficacy of peripheral opioids (all except intra-articular): A qualitative systematic review of randomised controlled trials. Pain. 1997;72:309–318. doi: 10.1016/s0304-3959(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Pick CG, Peter Y, Schreiber S, Weizman R. Pharmacological characterization of buprenorphine, a mixed agonist-antagonist with k3 analgesia. Brain Res. 1997;744:41–46. doi: 10.1016/s0006-8993(96)01069-4. [DOI] [PubMed] [Google Scholar]
- Rance MI, Lord JAH, Robinson T. Biphasic dose response to buprenorphine in the rat tail flick assay: Effect of naloxone pretreatment. In: Way EL, editor. Endogenous and Exogenous Opiate Agonists and Antagonists. New York: Pergamon Press; 1979. pp. 387–390. [Google Scholar]
- Risser D, Schneider B. Drug related deaths between 1985 and 1992 examined at the Institute of Forensic Medicine in Vienna. Addiction. 1994;89:851–857. doi: 10.1111/j.1360-0443.1994.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JS, Holford NH, Sadee W. In vivo receptor binding of opioid drugs at the mu site. J Pharmacol Exp Ther. 1985;233:735–740. [PubMed] [Google Scholar]
- Rounsaville BJ, Dolisky ZS, Babor TF, Meyer RE. Psychopathology as a predictor of treatment outcome in alcoholics. Arch Gen Psychiatry. 1987;44:505–513. doi: 10.1001/archpsyc.1987.01800180015002. [DOI] [PubMed] [Google Scholar]
- Rovati L, Pazzucconi F, Panerai AE. Involvement of μ and κ receptors in the analgesic effect of buprenorphine. Med Sci Res. 1987;15:659–660. [Google Scholar]
- Roy A, Lamparski D, Dejong J, Moore V, Linnola M. Characteristics of alcoholics who attempt suicide. Am J Psychiatry. 1990;147:761–765. doi: 10.1176/ajp.147.6.761. [DOI] [PubMed] [Google Scholar]
- Ruttenber AJ, Luke JL. Heroin-related deaths: New epidemiological insights. Science. 1984;226:14–16. doi: 10.1126/science.6474188. [DOI] [PubMed] [Google Scholar]
- Sadee W, Rosenbaum LS, Herz A. Buprenorphine: Differential interaction with opiate receptor subtypes in vivo. J Pharmacol Exp Ther. 1982;223:157–162. [PubMed] [Google Scholar]
- Samson HH, Doyle TF. Oral ethanol self-administration in the rat: Effect of naloxone. Pharmacol Biochem Behav. 1985;2:92–99. doi: 10.1016/0091-3057(85)90491-5. [DOI] [PubMed] [Google Scholar]
- San L, Cami J, Femandez T, Olle JM, Peri JM, Torrens M. Assessment and management of opioid withdrawal symptoms in buprenorphine-dependent subjects. Br J Addict. 1992;87:55–62. doi: 10.1111/j.1360-0443.1992.tb01900.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. The clinical implications of primary diagnostic groups among alcoholics. Arch Gen Psychiatry. 1985;42:1043–1049. doi: 10.1001/archpsyc.1985.01790340021003. [DOI] [PubMed] [Google Scholar]
- Sinclair JD. Morphine suppresses alcohol drinking regardless of prior alcohol access duration. Pharmacol Biochem Behav. 1974;2:409–412. doi: 10.1016/0091-3057(74)90088-4. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Adkins J, Walker S. Morphine-induced suppression of voluntary alcohol drinking in rats. Nature. 1973;246:425–427. doi: 10.1038/246425a0. [DOI] [PubMed] [Google Scholar]
- Stastny D, Potter M. Alcohol abuse by patients undergoing methadone treatment programmes. Br J Addict. 1991;86:307–310. doi: 10.1111/j.1360-0443.1991.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Tyers MB. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980;69:503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL, Colombo G. Boosting effect of morphine on alcohol drinking is suppressed not only by naloxone but also by the cannabinoid CB1 receptor antagonist, SR 141716. Eur J Pharmacol. 2002;445:55–59. doi: 10.1016/s0014-2999(02)01712-0. [DOI] [PubMed] [Google Scholar]
- Vanacker B, Vandermeersch E, Tomassen J. Comparison of i.m. buprenorphine and buprenorphine/naloxone combination in the treatment of post-operative pain. Curr Med Res Opin. 1986;10:139–144. doi: 10.1185/03007998609110432. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Volpicelli LA, O’Brien CP. Medical management of alcohol dependence: clinical use and limitations of naltrexone treatment. Alcohol Alcohol. 1995;30:789–798. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Weiss F, Mitchner M, Bloom BE, Koob GF. Free choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology. 1990;101:178–186. doi: 10.1007/BF02244123. [DOI] [PubMed] [Google Scholar]
- Wnendt S, Kruger T, Janocha E, Hildebrandt D, Englberger W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol. 1999;56:334–338. doi: 10.1124/mol.56.2.334. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology. 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]