Abstract
Objectives
Acute and chronic rejection remain unresolved problems after lung transplantation, despite heavy multidrug immunosuppression. In turn, the strong immunosuppression has been responsible for mortality and pervasive morbidity. It also has been postulated to interdict potential mechanisms of alloengraftment.
Methods
In 48 lung recipients we applied 2 therapeutic principles: (1) recipient pretreatment with antilymphoid antibody preparations (Thymoglobulin [SangStat, Fremont, Calif] or Campath [alemtuzumab; manufactured by ILEX Pharmaceuticals, LP, San Antonio, Tex; distributed by Berlex Laboratories, Richmond, Calif]) and (2) minimal posttransplant immunosuppression with tacrolimus monotherapy or near-monotherapy. Our principal analysis was of the events during the critical first 6 posttransplant months of highest immunologic and infectious disease risk. Results were compared with those of 28 historical lung recipients treated with daclizumab induction and triple immunosuppression (tacrolimus-prednisone-azathioprine).
Results
Recipient pretreatment with both antilymphoid preparations allowed the use of postoperative tacrolimus monotherapy with prevention or control of acute rejection. Freedom from rejection was significantly greater with Campath than with Thymoglobulin (P = .03) or daclizumab (P = .05). After lymphoid depletion with Thymoglobulin or Campath, patient and graft survival at 6 months was 90% or greater. Patient and graft survival after 9 to 24 months is 84.2% in the Thymoglobulin cohort, and after 10 to 12 months, it is 90% in the Campath cohort. There has been a subjective improvement in quality of life relative to our historical experience.
Conclusion
Our results suggest that improvements in lung transplantation can be accomplished by altering the timing, dosage, and approach to immunosuppression in ways that might allow natural mechanisms of alloengraftment and diminish the magnitude of required maintenance immunosuppression.
Traditional immunosuppressive strategies for organ transplantation have involved, from the time of operation, the use of potent multidrug regimens, including a calcineurin inhibitor, high doses of prednisone, and an antimetabolite with or without a short course of antilymphoid antibody (induction). Despite the strong prophylactic immunosuppression, the incidence of acute rejection in the first 6 to 12 months after lung transplantation has remained high.1 Moreover, complications from the chronic immune depression and from drug-specific toxicities have been the rule rather than the exception, with infection-related mortality the most common cause of death in the first 3 years after transplantation.2 As a result, the 1- and 5-year patient survivals reported from national and international lung transplantation registries are 73% to 77% and 42% to 45%, respectively.2,3
It has also been suggested that excessive early postoperative immunosuppression can potentially subvert postulated mechanisms of alloengraftment.4 Two therapeutic principles were applied in 48 lung recipients to avoid this self-defeating consequence of treatment, beginning in June 2002. First, the lung recipients were infused with a single large dose of a potent antilymphoid preparation during the few hours preceding the operation or intraoperatively but before allograft reperfusion. The pretreatment was either with a rabbit antithymocyte globulin (Thymoglobulin; SangStat, Fremont, Calif; n = 38) or with the broadly reacting humanized anti-CD52 monoclonal antibody alemtuzumab (Campath-1H [alemtuzumab], hereafter referred to as Campath; manufactured by ILEX Pharmaceuticals, LP, San Antonio, Tex; distributed by Berlex Laboratories, Richmond, Calif; n = 10). Second, the recipients were treated after transplantation with tacrolimus monotherapy alone or in combination with very low doses of prednisone (usually ≤5 mg/d).
Methods
Recipient and Donor Demographics
All adult patients undergoing single- or double-lung transplantation or heart-lung transplantation at the University of Pittsburgh between June 2002 and September 2003 were managed with the protocol described below, except for 5 recipients in whom there were logistical difficulties. From June 2002 through June 2003, 37 recipients were pretreated with 4 to 7 mg/kg intravenous Thymoglobulin; a 38th patient was added in September 2003. Between June 2003 and August 2003, the second cohort of 10 patients was pretreated with 30 mg of intravenous Campath instead of Thymoglobulin. Results are compared with those of 28 unselected patients who underwent single- or double-lung transplantation or heart-lung transplantation at the University of Pittsburgh between December 2001 and June 2002 who were managed with daclizumab (Zenapax) induction, followed by triple-drug immunosuppressive therapy.
The characteristics of the patients and donors in the 3 groups are shown in Table 1. There was no significant difference between groups for any recipient or donor variable. The indications for transplantation were broader in the Thymoglobulin-treated and daclizumab-treated cohorts than in the Campath group (Table 1). In the Thymoglobulin-treated cohort, other risk factors included 1 (3%) patient with a preexisting, donor-specific, anti-class II antibody; 3 (8%) patients with scleroderma; 1 (3%) patient with cystic fibrosis with Burkholderia gladioli colonization; 2 (5%) patients with sarcoidosis (odds ratio for death at 1 year of 2.03 by International Society for Heart and Ling Transplantation [ISHLT] Registry2); and one recipient of a simultaneous liver.
TABLE 1.
Demographics of lung transplant recipients in the Thymoglobulin, Campath, and daclizumab groups
| Thymoglobulin | Campath | Daclizumab | P value | |
|---|---|---|---|---|
| Number | 38 | 10 | 28 | |
| Average age (y) | 47 ± 13 (25–68) | 55 ± 11 (37–70) | 49 ± 14 (22–65) | NS |
| Sex (M/F) | 17/21 | 7/3 | 15/13 | NS |
| Underlying disease | NS | |||
| COPD | 12 (37%) | 2 (20%) | 8 (29%) | |
| CF | 8 (21%) | 5 (18%) | ||
| IPF | 5 (13%) | 4 (40%) | 8 (29%) | |
| A1A deficiency | 5 (13%) | 1 (3%) | ||
| Scleroderma/crest | 3 (8%) | 1 (10%) | 2 (7%) | |
| Sarcoidosis | 2 (5%) | |||
| PPH | 1 (3%) | 1 (3%) | ||
| Pulmonary fibrosis | 1 (3%) | |||
| EG | 1 (3%) | |||
| OB/Retx | 3 (30%) | 1 (3%) | ||
| Other | 2 (7%) | |||
| Type of transplantation | NS | |||
| Single | 17 (45%) | 5 (50%) | 12 (43%) | |
| Double | 20 (53%) | 4 (40%) | 15 (54%) | |
| Heart, double lung | 1 (10%) | |||
| Double lung + liver | 1 (2%) | |||
| Living lung donor lobar | ||||
| CMV (D+/R−) | 13/38 (34%) | 2/10 (20%) | 7/28 (25%) | NS |
| EBV (D+/R−) | 2/38 (5%) | 0/10 | 1/28 (3%) | NS |
| HLA mismatch | 4.5 ± 1.3 | 5.2 ± 0.8 | 5.2 ± 0.9 | NS |
| PRA > 10% | 1/38 (3%) | 1/10 (10%) | 2/28 (7%) | NS |
| Ischemia time (min) | 345 ± 101 | 354 ± 85 | 329 ± 82 | NS |
| Donor age (y) | 35 ± 15 (14–63) | 39 ± 16 (11–62) | 35 ± 15 | NS |
COPD, Chronic obstructive pulmonary disease; CF, cystic fibrosis; IPF, idiopathic pulmonary fibrosis; A1A deficiency, α1-antitrypsin deficiency; PPH, primary pulmonary hypertension; EG, eosinophilic granuloma; OB/Retx, obliterative bronchiolitis retransplantation; CMV, cytomegalovirus; EBV, Epstein-Barr virus; PRA, panel reactive antibody.
Immunosuppressive Protocol
The antibody infusions (Thymoglobulin, Campath, or daclizumab) were initiated as soon as possible after confirmation of the acceptability of the donor organs. Thymoglobulin was begun slowly, with rate escalation every 30 minutes. Campath was infused at a steady rate over 2 hours. Patients who received Thymoglobulin or Campath were coadministered 1 g of methylprednisolone to suppress cytokine reactions. In all patients 250 mg of methylprednisolone was administered immediately before lung allograft reperfusion (1 dose for single-lung or heart-lung recipients and 2 doses for double-lung recipients).
Thymoglobulin-treated and Campath-treated patients were given twice-daily oral tacrolimus (Prograf; Fujisawa Healthcare, Inc, Deerfield, Ill) beginning on postoperative day 1. Tacrolimus doses were adjusted to achieve a 12-hour trough level of 12 to 15 ng/mL. All Thymoglobulin-treated patients received 5 mg/d prednisone beginning on postoperative day 1. Five of the 10 Campath-treated patients were given 5 mg/d (n = 4) or 7.5 mg/d (n = 1) prednisone from postoperative day 1 because they were receiving corticosteroids preoperatively. The other 5 were treated with tacrolimus only. No patient received an antimetabolite.
For patients who were given daclizumab, the protocol called for 5 doses (1 mg/kg each on day 0, posttransplant day 7, and posttransplant weeks 2, 4, and 6). These patients also received a methylprednisolone taper beginning the day of transplantation and extending over 5 days from 240 mg/d to 40 mg/d, followed by 20 mg of prednisone daily. Twice-daily tacrolimus (target 12-hour trough level of 15–20 ng/mL) and azathioprine (2–3 mg/kg) were begun the day of transplantation.
Diagnosis and Treatment of Rejection
Acute rejection was diagnosed by means of histologic examination of transbronchial or open lung biopsy specimens according to the revised working formulation for the histologic classification of pulmonary allograft rejection.5 The specimens were examined and graded by clinical pathologists according to our standard routine. Subsequent review of biopsy specimens was carried out at weekly conferences. Rejection was treated with corticosteroids (ie, 10-day oral prednisone taper [100 mg to previous baseline] or 1–2 doses of 500–1000 mg of methylprednisolone). In the Thymoglobulin- and Campath-conditioned patients, grade 2 acute rejection often was not treated in the absence of deterioration of allograft dysfunction unless there were histopathologic findings of issue injury. In contrast, all grade 2 acute rejection episodes were treated in the daclizumab cohort.
Higher-grade rejections were treated with 2 to 3 doses of 1000 mg of methylprednisolone, with occasional augmentation of maintenance steroids or the addition of other baseline agents. After Campath became available in the autumn of 2002, it was used to treat steroid-resistant rejection. A few patients were switched from tacrolimus to cyclosporine because of drug-specific toxicity.
Assays of Lymphoid Depletion
Patients receiving Thymoglobulin or Campath pretreatment were assessed for leukocyte subsets by means of flow cytometry at baseline (immediately before transplantation) and monitored for recovery at posttransplant days 1, 7, 14, and 30 and months 3 and 6.
Monitoring of Allograft Function
In all cohorts surveillance spirometry and bronchoscopy with bronchoalveolar lavage and transbronchial biopsy were performed at 2 and 8 weeks after transplantation and then every 2 to 3 months, as well as when clinically indicated.
Infection Monitoring and Prophylaxis
Infection prophylaxis against cytomegalovirus (CMV) in Thymoglobulin- and Campath-treated patients consisted of 450 to 900 mg/d valganciclovir (Valcyte; Roche Laboratories, Nutley, NJ) for 6 months. For prophylaxis against fungus and yeast, 6 mg/kg voriconazole (Vfend; Pfizer, New York, NY) was administered intravenously every 12 hours for 2 doses, followed by 200 mg twice daily for 4 months. Pneumocystis carinii prophylaxis was with one trimethoprim/sulfamethoxazole (TMP/sulfa) single strength tablet 3 times per week.
Daclizumab-treated patients received valganciclovir and TMP/ sulfa as described but received 3 months of fluconazole (Diflucan, Pfizer) prophylaxis in lieu of voriconazole.
Spaced Reduction of Tacrolimus
In 17 Thymoglobulin-pretreated recipients who were 6 months or more after transplantation, an effort was made to reduce tacrolimus dosing to 4 times a week or 3 times per week. The decisions were made either as a search for the lowest level of treatment consistent with stable graft function in response to low-level nephrotoxicity or because of concern about smoldering infection or incipient posttransplant lymphoproliferative disease.
Informed Consent and Data Compilation
Because the efficacy and safety of the immunosuppressive strategy had been demonstrated with other kinds of organ transplantation,6,7 the treatment regimen was deemed with the agreement of the University of Pittsburgh Institutional Review Board to be within the boundaries of standard therapy. The protocol was reviewed and authorized by the Presbyterian University Hospital Innovative Practices Committee and by the Pharmacy and Therapeutics Committee. Patients provided standard informed consent. In addition, all patients provided informed consent for enrollment under an institutional review board-approved protocol for studies not routinely performed in our conventional practice and for reporting of outcomes. Data were collected in a prospective manner. Data integrity and safety and efficacy monitoring were ensured by means of establishment of a formal weekly review of cases. Because Campath cases were compiled recently, the comparisons between the therapeutic antibodies are limited to 6 months.
Statistical Analysis
The χ2 statistic was used to determine the significance of nominal variables. Continuous variables were compared by either a Student t test or a Kruskal-Wallis test. All P values reported are 2 tailed. Survival and freedom-from-rejection analysis was determined by Kaplan-Meier actuarial analysis, and differences were compared with the log-rank test. Values are reported as means ± SD.
Results
Tolerability of Pretreatment Protocol
The antibody infusions were begun as soon as possible after determination of the acceptability of donor organs. Frequently, this preceded initiation of general anesthesia. All infusions were completed before graft reperfusion. Thymoglobulin was infused over an average of 225 ± 68 minutes (range, 120–433 minutes), whereas Campath was infused over 2 hours. Both agents were generally well tolerated. In one patient with obstructive lung disease, significant bronchospasm began before starting Thymoglobulin and continued afterward. Thymoglobulin was transiently stopped and later resumed at a slower rate, allowing almost a full dose (4 mg/kg) to be administered before allograft reperfusion. Another patient who received Thymoglobulin had significant hyperthermia, resulting in slowing of the rate of infusion.
Immune Cell Reconstitution
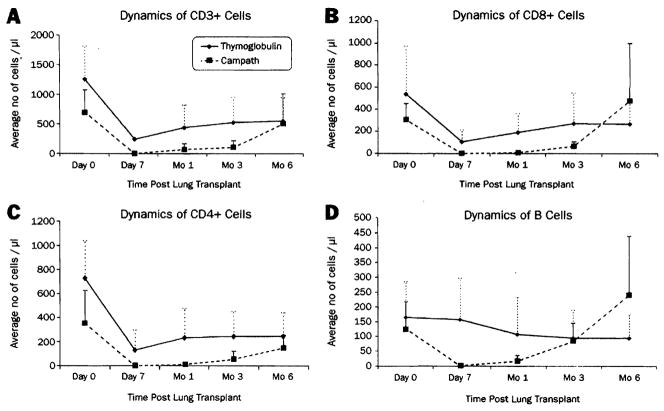
Pretreatment with 4 to 7 mg/kg Thymoglobulin resulted in profound depletion of T cells (CD3+) by postoperative day 1. T-cell counts remained less than baseline values for 3 months, with a gradual return toward normal levels by 3 to 6 months after transplantation (Figure 1, A). The CD8 population recovered more rapidly than the CD4 population. Although CD8 cell counts returned to baseline by 1 to 3 months after transplantation, CD4 cell counts remained at less than baseline value for up to 6 months (Figure 1, B–C). This pattern resulted in an inverted CD4/CD8 ratio that persisted up to 6 months after transplantation. B cells were not depleted by Thymoglobulin (Figure 1, D).
Figure 1.
The effect of Thymoglobulin and Campath on leukocyte subsets and their recovery after transplantation. The dashed line represents the value of 100 cells/μL (see text).16 Values are expressed as means ± SD.
The level of T-cell depletion achieved was significantly greater and more sustained with Campath than with Thymoglobulin (Figure 1, A) and included CD8 and CD4 phenotypes (Figure 1, B–C). CD4 (helper) cells were the most profoundly affected. The mean value for CD3+ cell counts depicted in Figure 1, A, at 6 months was due to high cell counts in 2 patients. The remainder of patients had cell counts that were 290 cells/μL or less.
Unlike Thymoglobulin, B cells were significantly depleted with Campath but returned to normal levels by 3 months (Figure 1, D).
Rejection Episodes
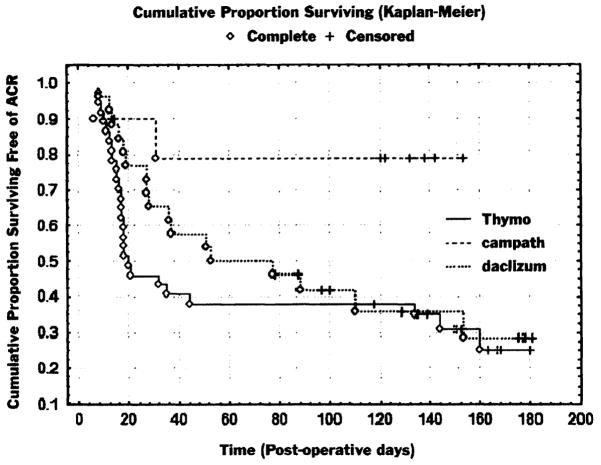
The incidence and overall burden of acute rejection was compared in patients who survived at least 2 weeks after transplantation (37/38 Thymoglobulin-treated patients, 10/10 Campath-treated patients, and 26/28 daclizumab-treated patients). The percentage of patients with at least one episode of grade 2 or greater acute rejection in the first 6 posttransplant months was no different between the Thymoglobulin- and daclizumab-treated cohorts (25/37 [68%] vs 17/26 [65%], P = not significant [NS]). In contrast, the Campath cohort had significantly fewer patients with rejection (2/10 [20%]; P = .008 vs Thymoglobulin and P = .02 vs daclizumab). Similarly, there was no difference in the percentage of patients with high-grade rejection (≥grade 3) between the Thymoglobulin- and daclizumab-treated cohorts (16/37 [43%] vs 12/26 [46%], P = NS), whereas no Campath-conditioned patients had high-grade rejection (P = .01 vs Thymoglobulin and P = .01 vs daclizumab). Figure 2 shows the Kaplan-Meier plot of freedom from greater than or equal to grade 2 rejection, demonstrating the lower incidence of rejection in the Campath cohort.
Figure 2.
Kaplan-Meier plot of freedom from grade 2 or greater rejection. ACR, Acute cellular rejection.
In addition, in those patients who experienced rejection, the number of rejection episodes was fewer in the Campath cohort compared with the Thymoglobulin and daclizumab cohorts. The average number of grade 2 or greater episodes of rejection per patient was 1.45 in the Thymoglobulin cohort and 1.15 in the daclizumab cohort (P = NS) but was significantly less in Campath-treated patients (0.2 episodes per patient [ie, a single episode of grade 2 rejection in 2 patients], P = .007 vs Thymoglobulin and P = .03 vs daclizumab). Neither Campath -preconditioned patient was treated with augmented immunosuppression for the grade 2 rejection. The frequency of high-grade rejection episodes was similarly no different between Thymoglobulin- and daclizumab-treated patients (0.81 vs 0.57 episodes per patient, respectively; P = NS) but was significantly less in Campath-treated patients (0 episodes, P = .02 vs Thymoglobulin and P = .032 vs daclizumab). No patients in any group had histopathologic evidence of obliterative bronchiolitis during the first 6 months.
Survival
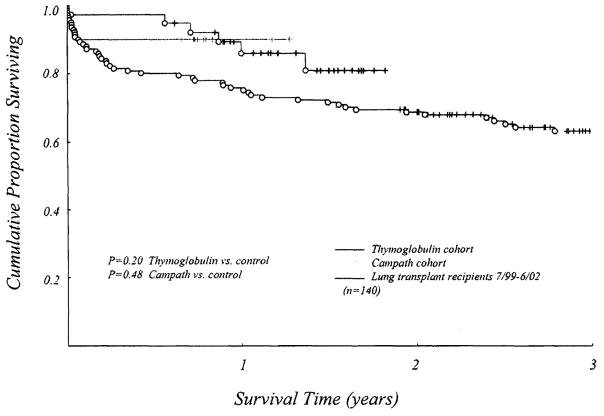
Six-month survival was not statistically different between the groups. Although survival in the Thymoglobulin and Campath groups was greater than our previous 3-year experience, the difference did not reach statistical significance (Figure 3). Six-month survival in the Thymoglobulin-treated cohort was 97% (37/38). The only death during this period occurred on postoperative day 9 because of the sequelae of ischemia-reperfusion injury. One patient required early retransplantation (postoperative day 54) for allograft dysfunction after transplantation for sarcoidosis. The patient survived. Thus, graft survival at 6 months was 95% (36/38).
Figure 3.
Kaplein-Meier actuarial survival curves for the Thymoglobulin and Campath cohorts, as well as for the group of patients receiving lung transplants at the University of Pittsburgh Medical Center from July 1999 to June 2002. Although pretreatment with either Thymoglobulin or Campath with resultant management as described in the text resulted in a greater than 10% difference in 1-year survival between the Thymoglobulin and Campath groups and the historical group, the difference did not reach statistical significance. This is likely because of the small sample size.
Six-month survival in the Campath-treated cohort was 90% (9/10). The only death occurred on postoperative day 23 in a 70-year-old recipient of a double-lung transplant (for emphysema) after an apneic episode on the ward.
Six-month survival in the daclizumab group was 89% (25/28).
Pulmonary Function
The average forced expiratory volume in 1 second (FEV1) in the Thymoglobulin-treated cohort at 6 months was 70% ± 19%, whereas that in the Campath-treated cohort was 80% ± 27% (P = NS). One single-lung recipient in the Thymoglobulin-pretreated group reached a peak FEV1 at 2 months after transplantation, with a subsequent decrease in values. Although transbronchial and open lung biopsies have not revealed any evidence of pathologic obliterative bronchiolitis, the FEV1 values meet bronchiolitis obliterans syndrome 2 criteria.8 This patient had preexisting donor-specific anti-HLA class II antibody (Table 1). No Campath-treated patients met bronchiolitis obliterans syndrome criteria.
Infection
There was no significant difference in infection rates among the 3 groups, although there was a trend toward less CMV-induced disease in the Campath cohort. Eighteen percent (5/28) of patients in the daclizumab group, compared with 8% (3/38) in the Thymoglobulin group and 0% in the Campath group, had histologically proved CMV-induced disease (P = .23). There were 3 cases of Nocardia species (one each of Nocardia Nova, Nocardia farcinica, and Nocardia asteroides complex) recorded in patients receiving Thymoglobulin pretreatment, whereas none occurred in the other groups (P = .19).
Survival After 6 Months
The potential follow-up for the 38 Thymoglobulin-pretreated recipients is now 9 to 23 months. Although 37 patients were alive at 6 months, 3 more deaths occurred by 1 year (2 caused by sepsis and 1 caused by posttransplant lymphoproliferative disease), and 2 additional deaths occurred after 13 and 16 months (viral influenza and chronic rejection, respectively). Thus, the 1-year actuarial survival is 86%. Thirty-two (84.2%) of the original 38 patients are alive, with a mean follow-up of 17.1 ± 4.0 months. Except for the death at 3 weeks of a 70-year-old emphysematous recipient, all of the other Campath-pretreated patients are alive, with a mean follow-up of 10.5 ± 4.4 months (range, 9–17 months).
Spaced Reduction of Tacrolimus
In 16 of the 32 surviving patients pretreated with Thymoglobulin, an attempt was made to reduce tacrolimus doses to 3 or 4 times a week. Five of the 16 patients remain stable on spaced dosing after a mean period of 8.5 ± 2.8 months (range, 6–12 months). Weaning was discontinued in the other 11 patients because of decreases in pulmonary function or because of histopathologic evidence of destructive immunity on biopsy. Despite augmentation of immunosuppression, full restoration of baseline function was achieved in only 3 of these patients. In contrast, the other 16 surviving recipients of the Thymoglobulin cohort who have been maintained on daily (twice a day or once a day dosing) tacrolimus monotherapy or near-monotherapy have had no deterioration in pulmonary function after a mean follow-up of 14.6 ± 3.7 months.
Discussion
There are obvious limitations to this study. Because it was a phase I-II study rather than a prospective randomized trial, definitive comparisons could not be made with contemporaneous (or, in our report, historical) control patients treated with conventional multiple drug immunosuppression. In addition, follow-ups are still short, particularly for the cohort of Campath-pretreated recipients. Nevertheless, it is noteworthy that our 6-month patient survival (97% and 90% in the Thymoglobulin and Campath cohorts, respectively) is competitive with the 89% 6-month survival with triple-drug immunosuppression in our immediately preceding experience and the benchmark 79% of the ISHLT registry.2 Moreover, current survival is 84.2% of our Thymoglobulin-pretreated recipients with follow-ups of 9 to 23 months and has remained at 90% since postoperative week 3 in the Campath-pretreated patients who are now 9 to 17 months after transplantation. In addition, no further deaths have occurred as additional patients (>40) have received transplants with Campath preconditioning. These results were achieved without an increased risk of infection and, in fact, with a trend toward less CMV-induced disease in an unselected series of patients who had a large representation of high-risk factors.
Both Thymoglobulin and Campath are potent lymphoid-depleting agents. Thymoglobulin9,10 has properties similar to the original polyclonal antilymphoid globulins that were introduced clinically nearly 40 years ago, and the more T-cell specific muromonab (OKT3). Campath was developed by Hale and Waldmann11 and first used for kidney transplantation by Calne and colleagues.12 In accord with reports by Knechtle,13 Kirk,14 and their associates, our data demonstrate that Campath has a more profound and prolonged lymphoid depletion than Thymoglobulin, including depression of the B-lymphocyte and natural killer lineages without causing major decreases in platelet counts. The Campath effect lasted for 6 months compared with the few weeks of Thymoglobulin. Because the extent and duration of T-cell depletion is thought to correlate with the avoidance of rejection,15 including that of thoracic organs,16 the lower rate of rejection in the Campath cohort relative to the antilymphoid globulin group was not surprising.
Moreover, the acute rejections were less severe in the patients pretreated with Campath. The incidence of grade 2 or higher acute rejection in the Thymoglobulin-treated patients was 66%, although the treated acute rejection rate was 47%. The incidence of rejection was similar to that seen with standard triple immunosuppression without use of antibody induction,17 as well as in some series in which antilymphoid globulins were administered postoperatively.1 In contrast, only 2 of the 10 Campath-pretreated patients had 1 episode each of grade 2 acute rejection (neither treated), and no patients had grade 3 rejection or higher. These patients were treated with steroid boluses or a short course of another agent but otherwise managed throughout with low maintenance immunosuppression. Five of the 10 recipients treated with Campath never were administered anything but tacrolimus, whereas the other 5 also were given 5 mg/d or less prednisone, usually because the patients previously had received chronic steroid therapy.
The timing of the lymphoid depletion in our patient deserves particular emphasis. The theory underlying our treatment strategy is that the seminal mechanism of organ alloengraftment is passenger leukocyte-driven clonal exhaustion-deletion,18 an immune activation-dependent mechanism that can be variably eroded by heavy prophylactic postoperative immunosuppression.4 Lymphoid depletion before exposure to donor alloantigen was designed to avoid this pitfall, reduce the anticipated donor-specific response into a more deletable range, and allow the safe use of minimum posttransplant immunosuppression. Thus our hypothesis was (and is) that organ engraftment is a state of variable partial tolerance that can be made more complete by modifying the timing and dosage of immunosuppression.
Our view of organ engraftment and its relation to acquired tolerance has been controversial. A large body of historical and recent work has led to alternative hypotheses to explain organ engraftment, acquired tolerance, or both.19,20 These theories emphasize the importance of immunoregulatory T cells, other changes in the host immune response, or both that can downregulate alloimmune or autoimmune responses. In addition to T-cell immunoregulation, subsets of other special cells, changes in the host cytokine profile, or the development of enhancing antibodies might play a role. In an attempt to foster these mechanisms, lymphoid depletion has been done elsewhere at and after the time of transplantation. In recent trials Campath (or Thymoglobulin) was administered in 2 doses: the first intraoperatively and the second a few days later. Preliminary results, especially after renal transplantation, have been encouraging.12–14
Our strategy of lymphoid depletion before, rather than at or after, transplantation also has been shown to be effective for kidney, liver, and other kinds of organ transplantation.6,7,21 Implementing this regimen in a clinical setting of thoracic organ transplantation entailed 2 concerns. The first question was whether the use of minimalist posttransplant immunosuppression might result in an unacceptable loss of allografts to uncontrollable acute rejection. In the experience reported herein, the risk of short-term graft loss to acute rejection was no greater than with conventional immunosuppression in either the Thymoglobulin- or Campath pretreated recipients.
The second question concerned the extent to which daily minimal maintenance immunosuppression could be sustained after recovery from the lymphoid depletion or reduced further, without the penalty of breakthrough acute rejection or indolent chronic rejection. Studies in mice by Wu and colleagues22 have raised the possibility that recovery from lymphoid depletion (homeostatic proliferation) might be associated with heightened long-term immune reactivity. The adverse consequences could include vigorous later rejection that could become evident only with long follow-up. This issue can be tentatively addressed with observations in the Thymoglobulin-pretreated recipients, 32 of whom survive after 17.1 ± 4.0 months (range, 9–23 months).
Fifteen of the 32 patients have been maintained on tacrolimus monotherapy for 14.6 ± 3.7 months (range, 9–22 months), with no deterioration of graft function. An additional patient who had been maintained on twice-daily tacrolimus had a late rejection episode (11 months) and required the addition of mycophenolate mofetil after treatment with steroids. In 16 others in whom spaced reduction of tacrolimus was attempted, this was tolerated by only 5 patients. Therefore we have concluded that attempts at spaced reduction of tacrolimus should not be made unless there is a specific reason to do so (eg, calcineurin inhibitor related renal dysfunction or an ongoing infection). A similar approach is being taken in the Campath-pretreated patients. We emphasize, however, that much longer follow-ups will be needed before the threat posed by chronic rejection can be fully evaluated.
Randomized trials comparing the results with the different timing of antilymphoid antibody treatment, as well as comparisons of the lymphoid-depleting strategies with conventional immunosuppression, could lead to better care of lung recipients. In addition to analyses of clinical outcomes, detailed immune monitoring in such trials might help resolve disputes about the mechanisms of engraftment. Finally, the observations with lung transplantation are expected to be generalizable to other organs, including the heart. We have pretreated 3 heart lung recipients with Campath (one included in the current report) and a recent heart-only recipient. All 4 patients have been free of rejection on tacrolimus monotherapy.
Acknowledgments
We thank Terry L. Mangan for secretarial assistance and all members of the University of Pittsburgh Lung and Heart-Lung Transplantation Program for their dedication to patient care.
Footnotes
Read at the Eighty-fourth Annual Meeting of The American Association for Thoracic Surgery, Toronto, Ontario, Canada, April 25–28,2004.
References
- 1.Brock MV, Borja MC, Ferber L, Orens JB, Anzcek RA, Krishnan J, et al. Induction therapy in lung transplantation: a prospective controlled trial comparing OKT3, anti-thymocyte globulin and daclizumab. J Heart Lung Transplant. 2001;20:1282–90. doi: 10.1016/s1053-2498(01)00356-4. [DOI] [PubMed] [Google Scholar]
- 2.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Mohacsi PJ, Keck BM, et al. The registry of the International Society for Heart and Lung Transplantation: Twentieth official adult lung and heart-lung transplant report—2003. J Heart Lung Transplant. 2003;22:625–35. doi: 10.1016/s1053-2498(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 3.The US Organ Procurement and Transplantation Network and The Scientific Registry of Transplant Recipients: transplant data 1992–2001. Washington (DC): Department of Health and Human Services; 2002. Annual Report. [Google Scholar]
- 4.Starzl TE, Zinkernagel RM. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;1:233–9. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 6.Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–10. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro R, Jordan ML, Basu A, Scantlebury V, Potdar S, Tan HP, et al. Kidney transplantation under a tolerogenic regimen of recipient pretreatment and low-dose postoperative immunosuppression with subsequent weaning. Ann Surg. 2003;238:520–7. doi: 10.1097/01.sla.0000089853.11184.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper JD, Billingham M, Egan T, Hertz MI, Higgenbottam T, Lynch J, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12:713–6. [PubMed] [Google Scholar]
- 9.Preville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–8. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 10.Mueller TF. Thymoglobulin: an immunologic overview. Curr Opin Organ Transplant. 2003;8:305–12. [Google Scholar]
- 11.Hale G, Waldmann H, Dyer M. Specificity of monoclonal antibody Campath-1H. Bone Marrow Transplant. 1988;3:237–9. [PubMed] [Google Scholar]
- 12.Calne R, Friend P, Moffatt S, Bradley A, Hale G, Firth J, et al. Prope tolerance, perioperative Campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients [published erratum appears in Lancet. 1998;352:408] Lancet. 1998;351:1701–2. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 13.Knechtle SJ, Pirsch JD, Fechner HJ, Jr, Becker BN, Friedl A, Colvin RB, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–30. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–9. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 15.Cosimi AB, Wortis HH, Delmonico FL, Russell PS. Randomized clinical trial of antithymocyte globulin in cadaver renal allograft recipients: importance of T cell monitoring. Surgery. 1976;80:155–63. [PubMed] [Google Scholar]
- 16.Krasinskas AM, Kreisel D, Acker MA, Bavaria JE, Pochettino A, Kotloff RM, et al. CD3 monitoring of antithymocyte globulin therapy in thoracic organ transplantation. Transplantation. 2002;73:1339–41. doi: 10.1097/00007890-200204270-00026. [DOI] [PubMed] [Google Scholar]
- 17.Bhorade SM, Jordan A, Villanueva J, Yu A, Kramer H, Vigneswaran WT, et al. Comparison of three tacrolimus-based immunosuppressive regimens in lung transplantation. Am J Transplant. 2003;3:1570–5. doi: 10.1046/j.1600-6135.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–13. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker LSK, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–9. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 20.Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003;3:147–58. doi: 10.1038/nri1002. [DOI] [PubMed] [Google Scholar]
- 21.Marcos A, Eghtesad B, Fung JJ, Fontes P, Patel K, deVera M, et al. The use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation. Transplantation. 2004;78:966–71. doi: 10.1097/01.tp.0000142674.78268.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]