Abstract
The new immunosuppressive agent, cyclosporin A, was used with low doses of steroids to treat eight patients undergoing hepatic transplantation and three patients undergoing cadaveric renal transplantation. Seven of the eight liver recipients are well, including one who was given two livers. The three kidney recipients, who had developed cytotoxic antibodies after previously rejecting grafts with conventional immunosuppressive therapy, have had good results despite conditions which usually preclude attempts at transplantation. The ability to control rejection effectively and safely without chronic high-dose steroid therapy may make the described therapeutic regimen valuable for pediatric recipients of whole organs.
Cadaveric organ transplantation in infants and children has been an unreliable and morbidity-laden undertaking. The ability to control rejection has been unpredictable with conventional immunosuppressive therapy including azathioprine and prednisone with or without anti-lymphocyte globulin. Even with “success” the frequent need for high-dose steroid therapy has all too often degraded the quality of post-transplantation life.
We report here an alternative method of immunosuppression with cyclosporin A and low doses of steroids that has been used in eight liver and three cadaveric kidney recipients who have been followed for 3½ months to 1⅓ years. Although the experience is small and the follow-up periods short, the results have made us believe that transplantation of a variety of organs will become a practical reality in larger numbers of pediatric health care centers.
METHODS
Case material
Liver recipients
The eight patients were 2⅙ to 16 years of age (Table I). Four had biliary atresia; of these, three had had one or more previous attempts at porticoenterostomy (Kasai procedure), and the earlier operations made transplantation difficult. Other diagnoses are shown in Table I.
Table I.
Features of eight patients treated with orthotopic liver transplantation using cyclosporin A (CyA) and low doses of steroids
| Patient | Age (yr)/sex | Diagnosis | Transplantation date | Major complications | Initial biliary construction | Bilirubin (mg) | Present daily drug doses |
|
|---|---|---|---|---|---|---|---|---|
| CyA (mg/kg) | Prednisone(mg) | |||||||
| 1 | 16/F | Budd-Chiari syndrome | 8-23-80 | Cholangitis from retained stent | Duel to duct* | 6 | 10 | 10† |
| 2 | 8/F | Byler disease | 9-16-80 | Hepatic artery thrombosis | Duct to duct | Died 19 days | — | — |
| 3 | 10/F | Intrahepatic biliary atresia | 9-28-80 | Disrupted biliary anastomosis | Duct to duct* | < 1 | 7.2 | 10 |
| 4‡ | 23/4/M | Biliary atresia | 5-9-81 | None | Duct to Roux limb | < 1 | 9 | 5 |
| 5‡ | 4/F | Biliary atresia | 5-29-81 | Respiratory distress syndrome; biliary obstruction | Gallbladder to Roux limb* | < 1 | 5.5 | 5 |
| 6‡ | 2 1/6/M | Biliary atresia | 6-3-81 6-24-81 | Liver abscess; Candida peritonitis | Gall bladder to Roux limb*; Duct to Roux limb | < 1 | 9.3 | 5 |
| 7 | 10½/M | Alpha-1-antitrypsin deficiency | 6-12-81 | None | Duct to duct | < 1 | 8 | 3.5 |
| 8 | 10 1/6/F | Congenital biliary cirrhosis | 7-4-81 | None | Duct to duct | < 1 | 9.3 | 5 |
Required secondary revision.
Plus intermittent hydrocortisone boluses.
Previous Kasai procedure(s).
Donor selection was random in respect to the HLA A, B and D loci. Matches of the four antigens of the A and B loci averaged 1.0 ± 0.7 (SD), range 0 to 2. Two DR matches were present in one case, one match existed in two cases, and no matches were present in the other six donor-recipient combinations.
In two instances, grafts were placed into recipients who had pre-existing anti-donor antibodies. In one (No. 8) an A blood type donor provided a liver for a B type recipient. In the other (No. 5), the serum of the recipient contained T warm cytotoxic antibodies that killed all the lymphocytes of the donor.
Kidney recipients
The three girls had undergone one or two previous transplantations (Table II). After the failed initial transplantations, the sera of all three had developed T warm cytotoxic antibodies which reacted against the cells of more than 40, 95, and 95% respectively, of the 48 lymphocyte donors who contributed to an antibody screening panel. The cytotoxic crossmatches of the recipients with their actual donors were negative using current sera, but in Patients 2 and 3 T warm crossmatches were positive with stored sera that had been collected a few weeks or months previously. In most centers the latter finding is considered a positive cross-match and precludes renal transplantation. One to three HLA antigens were matched (Table II). No DR antigen matches were found between any of the donors and recipients.
Table II.
Features of three patients who had cadaveric renal retransplantation using cyclosporin A (CyA) and steroids
| Patient | Age(yr)/sex | Year and fate of previous grafts | Transplantation date | HLA matches (of possible four) | Adverse immunologic factors | Major complications | Present creatinine(mg/dl) | Present daily drug doses |
|
|---|---|---|---|---|---|---|---|---|---|
| CyA(mg/kg) | Prednisone(mg) | ||||||||
| 1 | 8 11/12 F | 1981, rejected 7 days | 6-18-81 | 1 | Widely reacting T warm antibodies; negative cross-match with stored sera | None | 1.3 | 10 | 7.5 |
| 2 | 13 5/6 F | 1978, rejected 1 yr | 7-4-81 | 3 | Widely reacting T warm antibodies; positive cross-match with stored sera | None | 1.7 | 9 | 15 |
| 3 | 13 1/3 F | 1979, rejected 2 wk; 1979 rejected 4 wk | 9-6-81 | 3 | Widely reacting T warm antibodies; positive cross-match with stored sera | None | 1.1 | 10 | 15 |
The operations
Liver transplantation
Two of the nine donor livers were obtained locally, and the other seven from cities 300 to 2,000 miles away. The hepatic grafts were preserved with Collin’s solution1 and transplanted by previously described techniques2,3 after one to 10½ hours of cold ischemia. Antibiotics were given prophylactically and for a few days postoperatively. Subsequent antibiotic therapy was given for specific indications only.
Kidney transplantation
The kidneys were preserved with asanguinous perfusion or with Collin’s solution and transplanted by conventional techniques4 approximately one day after their removal.
Immune suppression
Combination therapy with CyA and steroids has been described previously.5–9
Liver recipients
CyA was started four to six hours before operation with a single oral dose of 17.5 mg/kg. Afterward the same daily dose was continued intramuscularly or orally as soon as diet was resumed (Figs. 1 and 2) but with half the total amount in the morning and the other half in the evening. The daily dose was reduced if nephrotoxicity or other side effects were suspected (Fig. 2). All surviving patients are still receiving CyA.
Fig. 1.
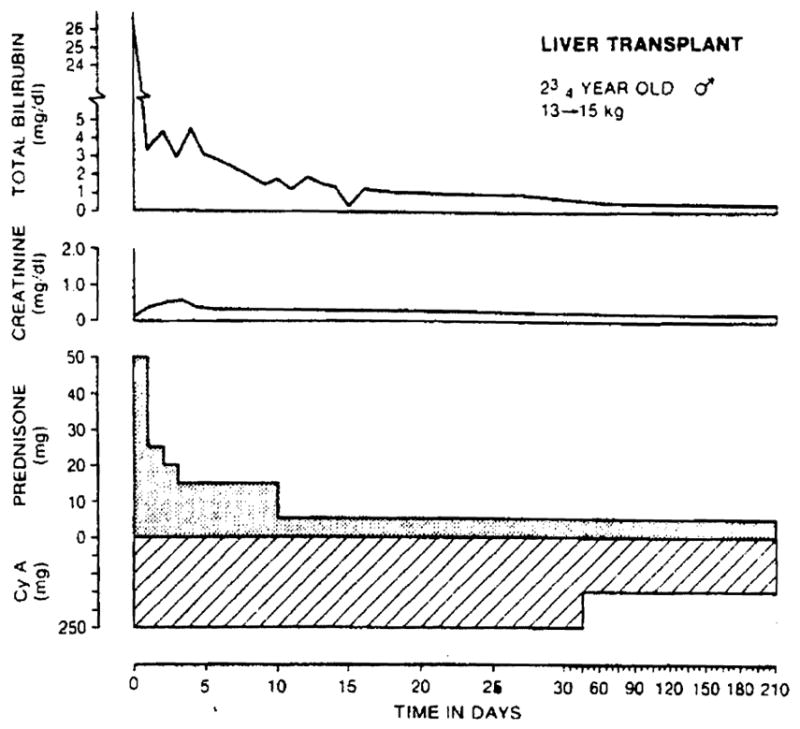
Uncomplicated recovery after liver transplantation. The 2¾-year-oId patient had biliary atresia. Note the low doses of prednisone which were used after an initial period of high intensity treatment.
Fig. 2.
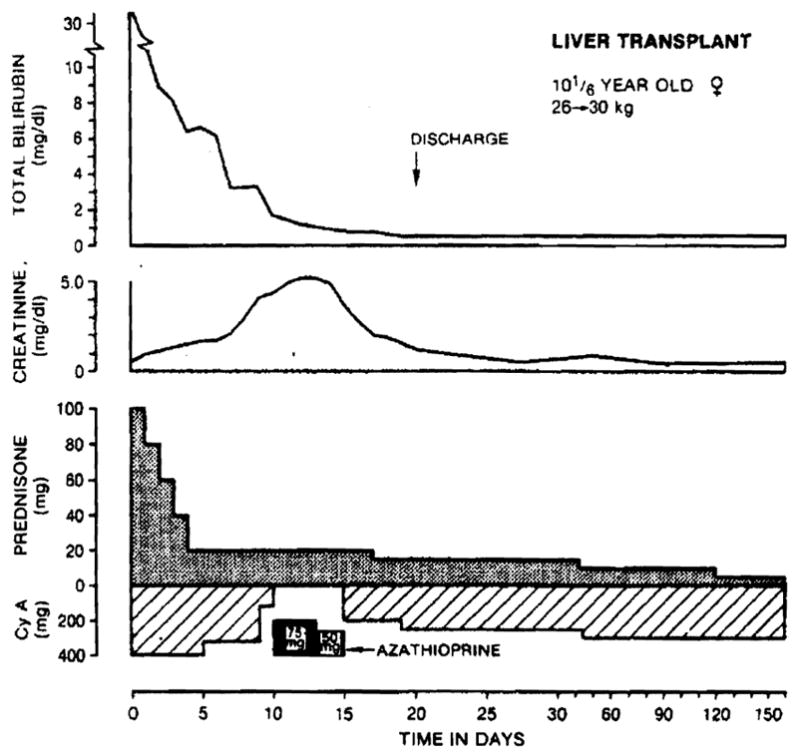
Recovery after liver transplantation of a 10½-year-old patient who developed transient nephrotoxicity from cyclosporin A. Note the five-day switch from cyclosporin A to azathioprine therapy. The original diagnosis was alpha1-antitrypsin deficiency.
Intravenous prednisone or hydrocortisone therapy was begun intraoperatively and converted afterward to the oral route as soon as possible. Postoperatively, patients who were in reasonable physiologic condition were given a five-day burst of prednisone (Figs. 1 and 2) beginning at 60 to 100 mg/day (depending on weight) and ending with maintenance doses of 5 to 20 mg/day. The steroid burst was omitted in those liver recipients who were gravely ill at the time of operation; they were given 10 to 20 mg/day. Further steroid weaning was variable. If rejections supervened, these were treated with boluses of hydrocortisone, 0.3 to 1.0 gm intravenously.
Kidney recipients
Immunosuppressive therapy (Figs. 3 and 4) was similar to that after liver transplantation except that steroids were used more liberally at the outset.5, 6, 8, 9 In two of the kidney recipients, a second five-day burst of high dose steroid therapy was given (Fig. 3).
Fig. 3.
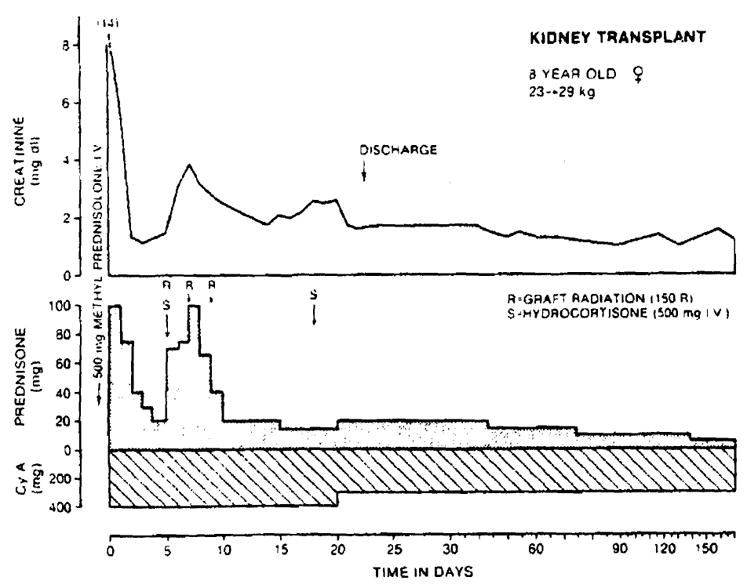
Recovery after renal retransplantation under immunosuppression with cyclosporin A and prednisone. The second burst of prednisone therapy was necessitated by an acute rejection, but within live months the patient’s prednisone dose had been reduced to 7.5 mg/day.
Fig. 4.
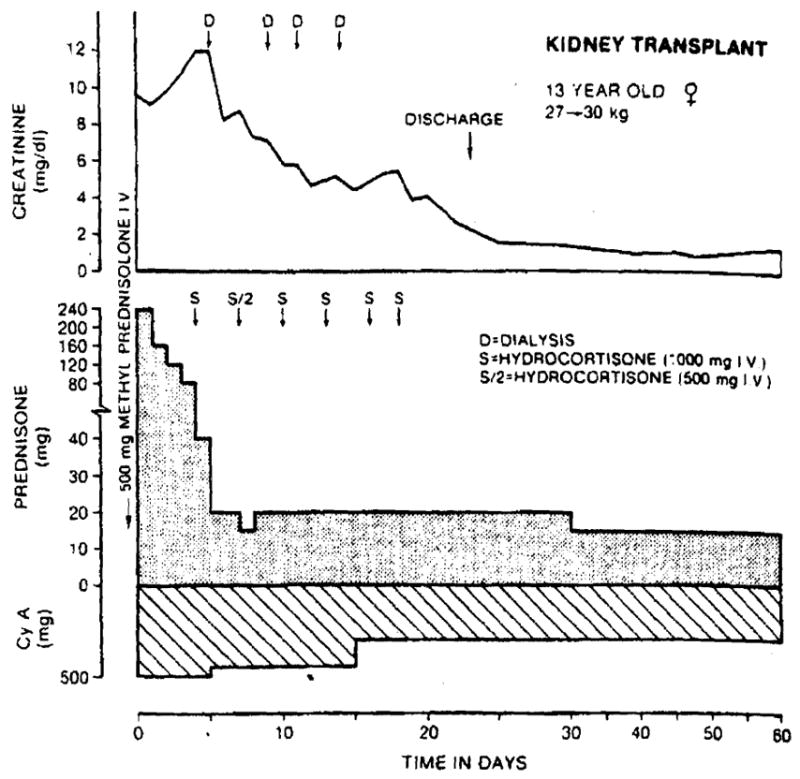
Renal transplantation in a 13-year-old girl who had previously rejected two grafts. Subsequently she developed widely reactive T warm antibodies (to 99% of the population) making her a noncandidate for transplant. Eventually a kidney was obtained from a donor against whose lymphocytes the current recipient serum did not react, although stored serum still gave a positive crossmatch. The transplanted kidney survived the immediate immunologic insult and began to function within two weeks. She has had a good result.
RESULTS
Liver transplantation
Survival
One of the eight liver recipients died after 19 days. The homograft hepatic artery had thrombosed. The death was ascribed to surgical error although no obvious technical mistake could be found at the anastomosis. The other patients have lived for 6 to 16½ months. All are at home.
Liver function
All but one of the surviving patients has a serum bilirubin concentration less than 1.0 mg/dl (Table I). The exceptional patient developed jaundice one year postoperatively with a peak bilirubin concentration of 58 mg/dl. Eventually, a retained stent in the reconstructed common duct was removed at reoperation. In the succeeding four months, the bilirubin concentration has slowly fallen to its present level of 6 mg/dl. Other liver function tests of the survivors have been normal or near normal except for persistent low-grade elevations of serum transaminases or alkaline phosphatase in the exceptional patient described above.
Retransplantation
One of the seven surviving patients (No. 6, Table I) developed a huge abscess of the right hepatic lobe which contained a variety of bacteria as well as Candida albicans. After two weeks the damaged liver was replaced with a fresh homograft. The boy had a stormy postoperative course, but recovered completely.
Biliary tract reconstruction
Five of the nine livers in the eight patients had bile duct reconstruction with duct-to-duct anastomosis (Table I). In one case, an anastomotic disruption necessitated reoperation and conversion to choledochojejunostomy with a Roux-en-Y jejunal limb. Late secondary operation for stent removal was necessary in another recipient.
Biliary reconstruction of two livers was satisfactory by primary Roux-en-Y choledochojejunostomy. The two final livers were reconstructed by anastomosing the graft gallbladder to a Roux limb (cholecystojejunostomy). One required conversion to choledochojejunostomy. The other liver was removed at retransplantation (Patient 6). Defective biliary drainage may have contributed to the destruction of this liver (see previous section).
Other major complications
Early deterioration of renal function occurred in two patients, both of whom had received nephrotoxic antibiotics as well as CyA. The most severe example is shown in Fig. 2. The complication was treated by a reduction of CyA dosage, and in one instance (Fig. 2) by omitting the dose of CyA for several days and substituting azathioprine; dialysis was not required. One patient (No. 5) had severe pulmonary edema and paralysis of the right phrenic nerve, requiring ventilatory support for three weeks. Patient 6 had recurrent laryngeal nerve paralysis.
Maintenance drug therapy (Table I)
The ability to use low maintenance doses of prednisone was noteworthy. In past experience with conventional immunosuppression, the single most important determinant of the quality of life following liver transplantation has been the requirements for steroid therapy.3, 10
Hospitalization
The days in hospital after transplantation of the seven survivors averaged 56 ± 34 (SD) and ranged from 18 to 93. With conventional immunosuppressive therapy, patients with what were then considered to be favorable courses spent an average of nearly five months of the first postoperative year in the hospital, and those with protracted survival despite troubled courses spent more than six months of the first postoperative year in the hospital.10
Renal transplantation
The two patients whose recently stored sera had positive T warm crossmatches with their donors had a short-lived postoperative diuresis followed by oliguria for two and three one-half weeks, respectively, requiring temporary dialysis support (Fig. 4). They recovered completely.
The third patient had a severe but quickly reversible rejection after six days (Fig. 3). Hospitalization was required for 23, 38, and 24 days. The serum creatinine concentration in all three patients is below 2.0 mg/dl (Table II). Follow-up has been 4 to 6½ months.
DISCUSSION
When renal transplantation was made feasible by the combined use of azathioprine and prednisone,4 there were expressions of concern about subjecting pediatric recipients to such drastic therapy.11 However, the first report of consistent success in treating infants and children4, 12 was promptly confirmed,13–16 although acceptable results were obtained only with consanguineous donors. A number of special pediatric transplantation centers were established,17–20 at which there usually has been a major effort to find genetically related donors. Failure to do so and consequent transplantation of cadaveric kidneys has been associated with a higher incidence of graft loss, death, and unacceptable morbidity from chronic high-dose steroid therapy. With organs such as the liver, there has been no option to the use of cadaveric donors. Major progress in cadaveric transplantation has been stalled for almost two decades despite efforts at tissue typing, better understanding of rejection, and modest improvements in immunosuppression made possible by adding antilymphoid globulins2, 18, 21 or thoracic duct drainage to the original azathioprine-prednisone therapy.
Thus the description by Borel et al22, 23 of a fungus extract, cyclosporin A, which was a powerful immunosuppressant, and news of its first encouraging trials by Calne et al24–25 were major and long awaited events. The drug became available for clinical testing in the United States in late 1979. Our consequent experience with CyA, for kidney and liver transplantation, and our recommendations about its use with conservative doses of steroids have been reported and are based principally upon observations in adults.5–9
In spite of the superior results obtained when CyA is used alone24–27 or in combination with low-dose steroids,5–9 the agent has not been available for evaluation in patients under 18 years of age because of the unknown risks from side effects. The initial fear that there might be an exorbitant incidence of de novo lymphomas25 has proved unfounded.7, 8 Nephrotoxicity, hepatotoxicity, hirsutism, motor dyskinesias, and gum hyperplasia have been manageable by dose adjustments.7, 26
Cyclosporin A was released on a compassionate basis to our pediatric liver recipients after it had become apparent that the chance of survival in adults after hepatic replacement was nearly doubled with use of the drug.8 The same benefit has been seen in the children receiving livers, of whom seven of eight are alive after six to 16½ months. The pediatric recipients were by no means free of the complications that have plagued efforts at liver transplantation in the past, but with the good control of rejection achieved with low-dose steroid therapy, these complications (including retransplantation in the face of an otherwise lethal infection in the first graft) could be managed.
Cyclosporin A was released compassionately to the three kidney recipients for equally forceful reasons. These patients were having problems with dialysis. Under the best of circumstances, retransplantation has a lowered graft survival, and a one-year mortality that has been as high as 40%.28 In addition, with the presence of widely reacting T warm antibodies in their sera, our patients had even poorer prospects of successful cadaveric renal retrans-plantation; in most centers, they would have been considered noncandidates for such an heroic effort. Two of the three patients had been waiting for more than three years for a cadaveric kidney. Although severe immunologic reactions ensued postoperatively, necessitating brief continuation of dialysis for two of the three recipients, all three have had a good result and have not required excessive steroid therapy.
Caution is justifiable in restricting the use of new techniques and drugs to adults during their developmental phase. However, pediatric recipients whose growth and development patterns are severely inhibited by conventional immunosuppression with high doses of steroids should benefit most from CyA therapy when it is released for more general use. In our liver and kidney recipients, the plan is to reduce the maintenance prednisone doses even lower than the present level.
Randomized controlled trials comparing cyclosporin A and steroid therapy with conventional immunosuppression for renal transplantation in adults are underway in a number of centers, including our own. The results of these trials should be applicable in making decisions about treatment protocols for infants and children.
Abbreviations used
- CyA
cyclosporin A
- DR
D-related
References
- 1.Benichou J, Halgrimson CG, Weil R, III, Koep LJ, Starzl TE. Canine and human liver preservation for 6–18 hours by cold infusion. Transplantation. 1977;24:407. doi: 10.1097/00007890-197712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE (with the assistance of C W Putnam) Experience in hepatic transplantation. Philadelphia: The WB Saunders Company; 1969. pp. 1–553. [Google Scholar]
- 3.Starzl TE, Koep LJ, Halgrimson CG, Hood J, Schröter GPJ, Porter KA, Weil R., III Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE. Experience in renal transplantation. Philadelphia: The WB Saunders Company; 1964. [Google Scholar]
- 5.Starzl TE, Weil R, III, Iwatsuki S, Klintmalm G, Schröter GPJ, Koep LJ, Iwaki Y, Terasaki PI, Porter KA. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Iwatsuki S, Klintmalm G, Schröter GPJ, Weil R, III, Koep LJ, Porter KA. Liver transplantation, 1980, with particular reference to cyclosporin A. Transplant Proc. 1981;13:281. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Klintmalm GBG, Weil R, III, Porter KA, Iwatsuki S, Schroter GP, Fernandez-Bueno C, MacHugh N. Cyclosporin A and steroid therapy in 66 cadaver kidney recipients. Surg Gynecol Obst. 1981;153:486. [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Klintmalm GBG, Porter KA, Iwatsuki S, Schröter GP. Liver transplantation with use of cyclosporin A and prednisone. N Engl J Med. 1981;305:266. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Hakala TR, Rosenthal JT, Iwatsuki S, Shaw BW. Variable convalescence and therapy after cadaveric renal transplantations under cyclosporin A and steroids. Surg Gynecol Obstet. in press. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Koep LJ, Schröter GPJ, Hood J, Halgrimson CG, Porter KA, Weil R., III The quality of life after liver transplantation. Transplant Proc. 1979;11:252. [PMC free article] [PubMed] [Google Scholar]
- 11.Riley CM. Thoughts about kidney homotransplantation in children. J Pediatr. 1964;65:797. [Google Scholar]
- 12.Starzl TE, Marchioro TL, Porter KA, Faris TD, Carey TA. The role of organ transplantation in pediatrics. Pediatr Clin North Am. 1966;13:381. doi: 10.1016/s0031-3955(16)31843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams GM, Lee HM, Hume DM. Renal transplants in children. Transplant Proc. 1969;1:262. [PubMed] [Google Scholar]
- 14.Gonzalez LL, Martin L, West CD, Spitzer R, McEnery P. Renal homotransplantation in children. Arch Surg. 1970;101:232. doi: 10.1001/archsurg.1970.01340260136022. [DOI] [PubMed] [Google Scholar]
- 15.Fine RN, Korsch BM, Stiles Q, Riddell H, Edelbrock HH, Brennan LP, Grushkin CM, Leiberman E. Renal homotransplantation in children. J Pediatr. 1970;76:347. doi: 10.1016/s0022-3476(70)80473-5. [DOI] [PubMed] [Google Scholar]
- 16.Najarian JS, Simmons RL, Tallent MB, Kjellstrand CM, Buselmeier TJ, Vernier RL, Michael AF. Renal transplantation in infants and children. Ann Surg. 1971;174:583. doi: 10.1097/00000658-197110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilly JR, Giles G, Hurwitz R, Schröter GPJ, Takagi H, Gray S, Penn I, Halgrimson CG, Starzl TE. Renal homotransplantation in pediatric patients. Pediatrics. 1971;47:548. [PMC free article] [PubMed] [Google Scholar]
- 18.Levy RH, Ingelfinger J, Grupe WE, Toper M, Eraklis AJ. Unique surgical and immunological features of renal transplantation in children. J Pediatr Surg. 1978;13:576. doi: 10.1016/s0022-3468(78)80096-7. [DOI] [PubMed] [Google Scholar]
- 19.Martin LW, McEnery PT, Rosenkrantz JG, Cox JA, West CD, Lecoultre C. Renal homotransplantation in children. J Pediatr Surg. 1979;14:571. doi: 10.1016/s0022-3468(79)80142-6. [DOI] [PubMed] [Google Scholar]
- 20.Potter DE, Holliday MA, Piel CF, Federska NJ, Belzer FO, Salvatiera O., Jr Treatment of end-stage renal disease in children: a 15-year experience. Kidney Int. 1980;18:103. doi: 10.1038/ki.1980.115. [DOI] [PubMed] [Google Scholar]
- 21.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation, and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 22.Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 23.Borel JF, Feurer C, Magnee C, Stahelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977;32:1017. [PMC free article] [PubMed] [Google Scholar]
- 24.Calne RY, Thiru S, McMaster P, Craddock GN, White DJG, Evans DB, Dunn DC, Pentlow BD, Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;1:1323. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 25.Calne RY, Rolles K, Thiru S, McMaster P, Craddock GN, Aziz S, White DJG, Evans DB, Dunn DC, Henderson RG, Lewis P. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 26.Calne RY, White DJG, Evans DB, Thiru S, Henderson RG, Hamilton DV, Rolles K, McMaster P, Duffy TJ, MacDougall BRD, Williams R. Cyclosporin A in cadaveric organ transplantation. Br Med J. 1981;282:934. doi: 10.1136/bmj.282.6268.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calne RY, Williams R, Lindop M, Farman JV, Tolley ME, Rolles K, MacDougall B, Neuberger J, Wyke RJ, Raftery AT, Duffy TJ, Wight DGD, White DJG. Improved survival after orthotopic liver grafting. Br Med J. 1981;283:115. doi: 10.1136/bmj.283.6284.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asher NL, Ahrenholz DH, Simmons RL, Najarian JS. 100 Second renal allografts from a single transplantation institution. Transplantation. 1979;27:30. doi: 10.1097/00007890-197901000-00008. [DOI] [PubMed] [Google Scholar]


