Abstract
BACKGROUND
Rodents with deficiency of or resistance to the proopiomelanocortin-derived peptide γ-melanocyte stimulating hormone (γ-MSH) develop marked salt-sensitive hypertension. We asked whether this hypertension was accompanied by abnormal glucose metabolism.
METHODS
γ-MSH-deficient Pc2−/− mice, and resistant Mc3r−/− mice were studied acutely for measurement of blood pressure and glucose and insulin concentrations after ≥ 1 week of a high-sodium diet (HSD; 8% NaCl) compared to a normal-sodium diet (NSD; 0.4% NaCl). Mc3r−/− also underwent glucose tolerance test (GTT) and insulin tolerance test.
RESULTS
Both knockout strains were hypertensive and also exhibited fasting hyperglycemia and hyperinsulinemia on the HSD. Mc3r−/− mice on the HSD had impaired glucose tolerance and insulin-mediated glucose disposal compared to wild-type mice on either the HSD or the NSD, or to Mc3r−/− mice on the NSD.
CONCLUSIONS
These results indicate an interaction of interrupted γ-MSH signaling with the HSD to cause hypertension on the one hand and abnormal glucose metabolism, with the characteristics of insulin resistance, on the other. Further study of the nature of this interaction should provide new insight into the mechanisms by which salt-sensitive hypertension and insulin resistance are linked.
A relationship between hypertension and insulin resistance was first recognized more than 20 years ago,1,2 but despite keen interest in the subject, there is no widely accepted mechanism by which these two independent risk factors of cardiovascular disease are linked.
We reported that mice with genetic interruption of normal signaling of the pituitary proopiomelanocortin-derived peptide γ-melanocyte stimulating hormone (γ-MSH), whether from impaired secretion from the pituitary or from absence of its receptor Mc3r, develop marked salt-sensitive hypertension.3 The hypertension is rapidly corrected by infusion of exogenous γ-MSH,3,4 and prevented by continuous administration of a stable γ-MSH analog, in rodents with deficiency of this hormone.3,5 We asked whether these models of genetic salt-sensitive hypertension due to interruption of normal γ-MSH signaling were accompanied by altered glucose metabolism.
METHODS
We studied male proconvertase 2 wild-type and knockout mice (Pc2+/+,−/−)6 obtained from The Jackson Laboratory, and male Mc3r+/+ and −/− mice7 obtained from Dr Roger Cone, University of Oregon for Health Sciences. The Pc2−/− mice and their littermate wild-type controls are on a B6; 129 background, whereas the Mc3r−/− mice and their littermate wild-type controls are on a C57B6Jx129 background. The mice were maintained in the transgenic breeding core of the Association for Assessment of Laboratory Animal Care–approved University of California San Francisco Animal Care Facility and were studied at 3–4 months of age, at which time they weighed 18–26 g. Mice were genotyped using DNA from tail biopsies as described by us.3 They were kept in the temperature-controlled vivarium with a 12-h light–dark cycle; the study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of University of California San Francisco. The mice ingested either a normal-sodium diet (NSD, 0.4% NaCl, Purina Mills Purified Diet 5001; Purina Mills, Richmond, IN) or the high-sodium diet (HSD, 8% NaCl, cat. no. 32892; Purina Mills) for ≥7 days. Mice were brought to the laboratory in the fasted state and anesthetized with ketamine 100 mg/kg and xylazine 15 mg/kg, both given IP, and placed on a heated operating table. A catheter was inserted into the femoral artery and attached to a Statham P23id blood pressure transducer to record mean arterial pressure (MAP) and heart rate on a direct writing recorder. A blood sample was taken for measurement of whole blood glucose; the rest of the sample was centrifuged and the plasma frozen for subsequent measurement of plasma insulin concentration. This procedure was followed in Pc2+/+, Pc2−/−, Mc3r+/+, and Mc3r−/− mice on the NSD or HSD (n = 5/group).
Separate Mc3r+/+ and Mc3r−/− mice on the NSD or HSD underwent further study to evaluate glucose metabolism more fully. Fasted mice (n = 5/group) were prepared as above and control measurements of MAP and heart rate, as well as a control blood sample, obtained. They then received an IP injection of a 20% glucose solution in normal saline, 1 mg (5 μl)/g body weight for a glucose tolerance test (GTT). Blood samples (25 μl) for determination of blood glucose and plasma insulin concentrations were obtained 20, 40, 60, 90, and 120 min later; MAP and heart rate were monitored throughout. Other, non-fasted, Mc3r+/+ and Mc3r−/− mice (n = 5/group) on the NSD or HSD underwent an insulin tolerance test. After surgical preparation as above and collection of control measurements, they received an IP injection of bovine insulin, 1 United States Pharmacopeia (USP) unit/kg body weight. Blood samples (15 μl) were obtained 20, 40, 60, 90, 120, and 150 min later for measurement of blood glucose concentration. In longer experiments, supplemental doses of ketamine and xylazine, 50–70% of the initial dose, were administered to maintain a stable plane of surgical anesthesia.
Because Mc3r−/− mice exhibit a metabolic phenotype,7,8 and because the two-test diets differed in the percent of calories derived from fat and the sucrose content as well as in other ways, we carried out the GTT in two additional groups of fasted Mc3r−/− mice (5/group). One group was fed the control NSD throughout and given tap water to drink, thus resembling the Mc3r−/−/NSD group described above; the other was fed the same NSD but given 0.9% saline as drinking water for ≥12 days to achieve sodium loading.
Blood glucose was measured using glucometer (Accensia Elite XL, Bayer, Mishowaka, IN). Plasma insulin was determined in 5 μl of plasma using an ultrasensitive rat insulin ELISA kit (Crystal Chem, Downers Grove, IL) according to the manufacturer’s instructions. Data are presented as means ± s.e.m. Differences within groups were determined by repeated measures analysis of variance, whereas differences between group means were assessed by the unpaired t-test or by one-way analysis of variance with the Bonferroni post hoc test using GraphPad Instat version 3.05 (GraphPad Software, San Diego, CA). In some analyses, two-way analysis of variance was used (Analyze-it Software, Leeds, UK). When groups being compared exhibited unequal variances, the data were log transformed. A P value <0.05 was used to indicate the presence of a significant difference.
RESULTS
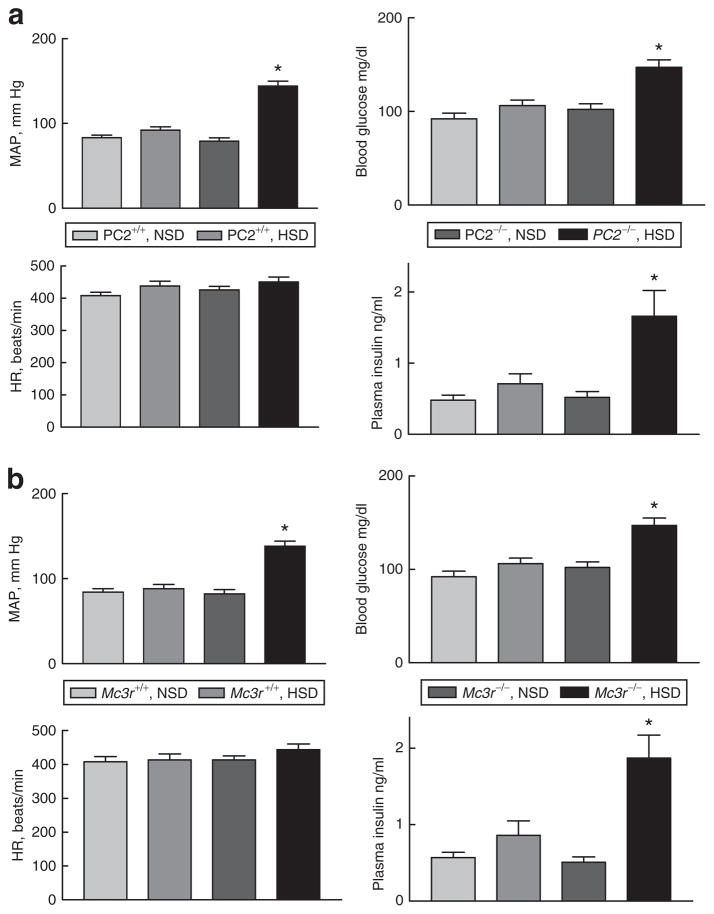
Both Pc2−/− and Mc3r−/− mice developed hypertension when ingesting the HSD, as we had reported earlier3 (Figure 1). They also exhibited fasting hyperglycemia and hyperinsulinemia compared to each wild-type strain on either diet, or to the knockout mice ingesting the NSD. Two-way analysis of variance indicated significant effects of genotype and dietary sodium for both strains for fasting glucose and insulin concentrations and MAP (P <0.003), as well as a significant genotype × diet interaction (P <0.05).
Figure 1.
Mean arterial pressure (MAP), heart rate, fasting blood glucose concentration, and plasma insulin in (a) PC2+/+ and −/− mice on the normal-(NSD) or high-sodium diet (HSD) and in (b) Mc3r+/+ and −/− mice. While ingesting the HSD, both knockout strains developed fasting hyperglycemia and hyperinsulinemia in association with hypertension. N = 5 mice/group; *P < 0.003 or greater vs. the other groups by two-way analysis of variance.
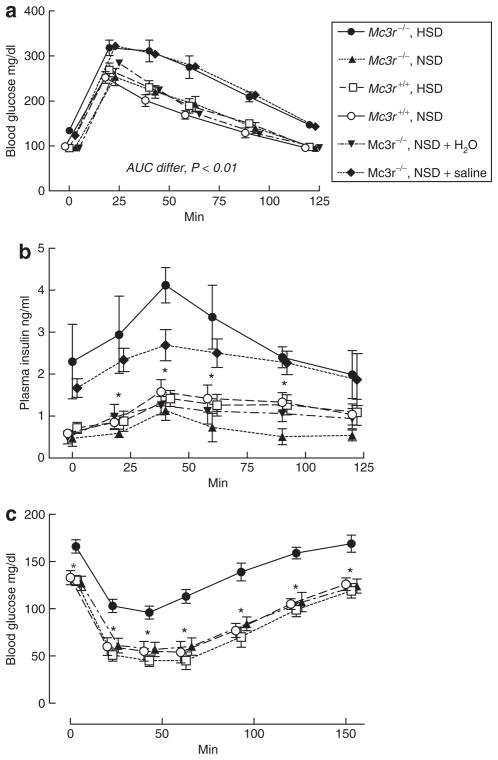
We characterized this abnormal glucose metabolism further in Mc3r−/− mice ingesting the NSD vs. the HSD (Figure 2). In the GTT, Mc3r−/− mice fed the HSD had a significantly greater area under the curve of the blood glucose/time relationship compared to Mc3r−/− mice on the NSD or Mc3r+/+ mice on either diet (Figure 2a); these latter three results did not differ from each other. Plasma insulin concentration exhibited a similar pattern; the control level in Mc3r−/− mice fed the HSD was elevated over the other three groups, although this was not significant (P <0.15) as it had been in the mice shown in Figure 1. However, plasma insulin was significantly elevated in Mc3r−/− mice on the HSD throughout the 2-h period of the GTT compared to the other three groups (Figure 2b).
Figure 2.
(a) Blood glucose concentration during a 2-h glucose tolerance test in Mc3r+/+ and −/− mice fed the normal- (NSD) or high-sodium diet (HSD), and in Mc3r−/− mice fed the NSD but given either tap water or normal saline as drinking water. Mc3r−/− on the HSD and on the NSD with saline drinking water had a significantly greater area under the glucose-time curve than wild-type mice fed either diet or Mc3r−/− mice ingesting the NSD and drinking tap water. (b) Plasma insulin concentrations in the experiments shown in a. Mc3r−/− mice on the HSD or on the NSD plus saline drinking water were hyperinsulinemic throughout the glucose tolerance test compared to wild-type mice on either diet or knockouts on the NSD. (c) Blood glucose concentration during a 150 min insulin tolerance test. Blood glucose fell significantly less in Mc3r−/− mice fed the HSD compared to the other groups, suggesting blunted insulin-mediated glucose uptake. N = five mice per group; *values in Mc3r−/− mice on the HSD and on the NSD plus saline drinking water significantly different from the other three groups, P < 0.05 or greater by one-way analysis of variance.
To control more fully for differences in dietary composition, we studied two additional groups of fasted Mc3r−/− mice, both of which ate the control, NSD diet throughout but one of which was given 0.9% saline as drinking water. MAP in the water-drinking group was 84 ± 3 mm Hg, whereas it was 127 ± 3 mm Hg in the group given normal saline to drink (P <0.001). The saline-drinking mice exhibited fasting hyperglycemia (123 ± 2 vs. 95 ± 2 mg/dl) and hyperinsulinemia (1.67 ± 0.22 vs. 0.49 ± 0.15 ng/ml) compared to the water-drinking group (P < 0.003 for both). These results are similar to those shown in Figure 1. Results of the GTT in these two groups are shown in Figure 2a,b. The glucose-time relationship of the knockout mice fed the NSD plus saline drinking water was virtually identical to that in Mc3r−/− mice fed the HSD, and the area under the curve of these two groups was significantly greater than all the other groups (P < 0.01, Figure 2a). The plasma insulin response was also similar in the salt-loaded knockout mice (Figure 2b), although at the 40 min time point the value in the knockout mice fed the HSD was significantly higher than that in the knockouts fed the NSD plus normal saline drinking water (P < 0.05). These data indicate that the impaired glucose metabolism induced by the HSD in Mc3r−/− mice can be largely attributed to the high-sodium intake rather than to some other difference between the HSD and the NSD.
Results of the insulin tolerance test showed a pattern consistent with the GTT (Figure 2c): nonfasting Mc3r−/− mice on the HSD had a higher basal blood glucose concentration and, although insulin caused a decrease in glucose in all groups, knockout mice on the HSD maintained a higher level throughout the 150 min study compared to Mc3r−/− mice on the NSD or to wild-type mice on either diet. The impaired glucose tolerance and insulin-mediated glucose disposal of the Mc3r−/− mice fed the HSD are suggestive of insulin resistance.
Body weights in the different groups did not differ markedly. Pc2+/+ mice fed the NSD weighted 24.2 ± 0/7 g, Pc2+/+ fed the HSD 22.6 ± 0.5 g, Pc2−/− on the NSD 22.4 ± 0.5 g, and Pc2−/− on the HSD 21.0 ± 0.4 g. The last value was significantly less than the weight of Pc2+/+ mice on the NSD (P < 0.01), but no other differences were detected. The corresponding weights for Mc3r+/+ NSD, Mc3r+/+ HSD, Mc3r−/− NSD, and Mc3r−/− HSD were 23.2 ± 0.8, 23.3 ± 0.6, 23.1 ± 0.5, and 22.9 ± 0.4 g (P = not significant).
DISCUSSION
These experimental results demonstrate that the elevation in blood pressure in two models of salt-sensitive hypertension resulting from impaired signaling of the POMC-derived peptide γ-MSH is accompanied by abnormal glucose metabolism. Mice with γ-MSH deficiency due to genetic absence of the POMC processing enzyme PC2 (Pc2−/−)3 exhibit fasting hyperglycemia and hyperinsulinemia when ingesting the HSD, as do mice lacking the cellular receptor for γ-MSH (Mc3r−/−) which exhibit the features of hormone resistance.3 The abnormal glucose metabolism in this latter strain is further characterized by impaired glucose tolerance and blunted insulin-mediated glucose uptake.
The phenotypes of the Pc2−/− and Mc3r−/− mice could have some impact on carbohydrate metabolism. The initial report of the Pc2−/− knockout described a mild impairment in growth and a tendency to fasting hypoglycemia and a flat glucose tolerance curve attributed to reduced circulating glucagons,6,9 although abnormalities in insulin processing have also been reported in these mice.10 The development of fasting hyperglycemia during ingestion of the HSD is therefore all the more noteworthy. Mc3r−/− mice have a phenotype characterized by a decrease in skeletal muscle mass and increase in fat mass with an increase in resting energy expenditure;7,8 one study has reported basal hyperinsulinemia in these mice,8 whereas another has documented the development of hyperglycemia and hyperinsulinemia with ingestion of a high-fat diet.11 We could not confirm hyperinsulinemia in our mice when ingesting the NSD, but were able to unmask it with ingestion of the HSD. The elevation in plasma insulin concentration during the GTT (Figure 2b) suggests no major defect in insulin secretory capacity. Our results do not support a role of the HSD itself in contributing to altered glucose metabolism and insulin resistance as has been observed in some rodent studies:12,13 in wild-type mice, the HSD resulted in trivial differences in fasting blood glucose and insulin concentrations compared with values after ingesting the NSD (Figure 1).
The mechanism(s) by which a HSD induces both hypertension and impaired glucose tolerance in mice with interrupted γ-MSH signaling is not known. Our earlier study3 showed that a small dose of the peptide, which had no effect on MAP when given intravenously, rapidly lowered MAP to normal when given into the cerebroventricular system of hypertensive Pc2−/− mice fed the HSD. This indicated a central site of action of the administered γ-MSH, and led us to speculate that the peptide acts centrally as a tonic brake on sympathetic nervous out-flow.14 Loss of this brake as in γ-MSH deficient Pc2−/− or resistant Mc3r−/− mice could cause increased sympathetic outflow and hypertension during ingestion of the HSD. Much evidence suggests that activation of the sympathetic nervous system can also lead to insulin resistance,15 perhaps providing an explanation for the effects of the HSD on both blood pressure and glucose metabolism in rodents with impaired γ-MSH signaling. This will be an important possibility to test in future studies.
Acknowledgments
Portions of this work have appeared in abstract form in J Am Soc Nephrol 2006; 17:213A. This work was supported by grant HL68871 from the National Institutes of Health.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities—the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 3.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111:1251–1258. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayan H, Ni XP, Almog S, Humphreys MH. Suppression of gamma-melanocyte-stimulating hormone secretion is accompanied by salt-sensitive hypertension in the rat. Hypertension. 2003;42:962–967. doi: 10.1161/01.HYP.0000097601.83235.F8. [DOI] [PubMed] [Google Scholar]
- 5.Ni XP, Humphreys MH. Prevention of salt-induced hypertension by an analog of γ-melanocyte stimulating hormone in the rat. Am J Hypertens. 2007;20:362–365. doi: 10.1016/j.amjhyper.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Furuta M, Yano H, Zhou A, Rouillé Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 8.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 9.Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, Steiner DF. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J Biol Chem. 2001;276:27197–27202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- 10.Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem. 1998;273:3431–3437. doi: 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- 11.Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogihara T, Asano T, Ando K, Chiba Y, Sekine N, Sakoda H, Anai M, Onishi Y, Fujishiro M, Ono H, Shojima N, Inukai K, Fukushima Y, Kikuchi M, Fujita T. Insulin resistance with enhanced insulin signaling in high-salt diet-fed rats. Diabetes. 2001;50:573–583. doi: 10.2337/diabetes.50.3.573. [DOI] [PubMed] [Google Scholar]
- 13.Boini KM, Hennige AM, Huang DY, Friedrich B, Palmada M, Boehmer C, Grahammer F, Artunc F, Ullrich S, Avram D, Osswald H, Wulff P, Kuhl D, Vallon V, Häring HU, Lang F. Serum- and glucocorticoid-inducible kinase 1 mediates salt sensitivity of glucose tolerance. Diabetes. 2006;55:2059–2066. doi: 10.2337/db05-1038. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys MH. Cardiovascular and renal actions of melanocyte-stimulating hormone peptides. Curr Opin Nephrol Hypertens. 2007;16:32–38. doi: 10.1097/MNH.0b013e3280117fb5. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, Reid J, Van Zwieten PA. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–920. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]