Abstract
The pubic symphysis is a unique joint consisting of a fibrocartilaginous disc sandwiched between the articular surfaces of the pubic bones. It resists tensile, shearing and compressive forces and is capable of a small amount of movement under physiological conditions in most adults (up to 2 mm shift and 1° rotation). During pregnancy, circulating hormones such as relaxin induce resorption of the symphyseal margins and structural changes in the fibrocartilaginous disc, increasing symphyseal width and mobility. This systematic review of the English, German and French literature focuses on the normal anatomy of the adult human pubic symphysis. Although scientific studies of the joint have yielded useful descriptive data, comparison of results is hampered by imprecise methodology and/or poorly controlled studies. Several aspects of the anatomy of the pubic symphysis remain unknown or unclear: the precise attachments of surrounding ligaments and muscles; the arrangement of connective tissue fibres within the interpubic disc and the origin, structure and function of its associated interpubic cleft; the biomechanical consequences of sexual dimorphism; potential ethnic variations in morphology; and its precise innervation and blood supply. These deficiencies hinder our understanding of the normal form and function of the joint, which is particularly relevant when attempting to understand the mechanisms underlying pregnancy-related pubic symphyseal pain, a neglected and relatively common cause of pubic pain. A better understanding of the normal anatomy of the human pubic symphysis should improve our understanding of such problems and contribute to better treatments for patients suffering from symphyseal pain and dysfunction.
Keywords: interpubic disc, pregnancy-related pelvic girdle pain, pubic ligaments, pubic symphysis
Introduction
The pubic symphysis forms the strong midline union between the pubic bones of the pelvis. The symphyseal nature of this joint was recognized as long ago as 1543 by Vesalius who challenged the prevailing Hippocratic belief that the pubic bones became widely separated in labour (Eastman, 1948). Hunter (1762) provided one of the first detailed descriptions of the joint, emphasizing the strength of its ligaments and its mobility in the last trimester of pregnancy (Fig. 1). Modern anatomical reference texts classify the pubic symphysis either as a secondary cartilaginous (McMinn, 1994; Standring, 2008) or fibrocartilaginous (Rosse & Gaddum-Rosse, 1997) joint. Functional terms in older texts, such as an amphiarthrodial (Gray, 1858) or diarthro-amphiarthrodial (Testut & Latarjet, 1928) joint, are no longer in common use.
Fig. 1.
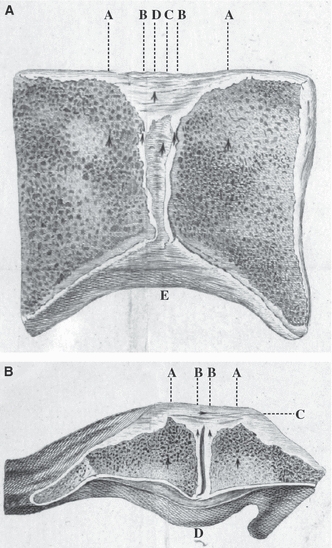
Sections of the pubic symphysis as depicted by William Hunter (1762). (A) Coronal section from a nulliparous female. AA, pubic bone; BB, cartilage; C, interior ‘ligamentous substance’; D, superior pubic ligament; E, inferior pubic ligament. (B) Axial section from a woman with puerperal fever. AA, pubic bone; BB, cartilage; C, interior ‘ligamentous substance’, the anterior pubic ligament that blends with tendinous fibres from adjacent muscle attachments; D, posterior pubic ligament that projects prominently in some subjects. The interpubic cleft is visible in the centre of the joint.
The joint comprises the medial surfaces of the pubic bones and an intervening fibrocartilaginous disc, which may contain a cleft. Functionally, it resists tension, shearing and compression and yet is able to widen during pregnancy. Pain in the region of the pubic symphysis, referred to as symphyseal pain, symphysitis or symphyseal dysfunction, can affect a diverse group of individuals including athletes, patients with traumatic pelvic injuries, and pregnant women (Gibbon & Hession, 1997; Owens et al. 2002; Robinson et al. 2007; Ronchetti et al. 2008; Cheer & Pearce, 2010). During pregnancy, symphyseal pain typically causes difficulty with weight-bearing activities such as walking and climbing stairs, and turning over in bed (Jain et al. 2006); symptoms do not necessarily resolve after childbirth and are very disabling for some women (Owens et al. 2002). The reported incidence and point prevalence of pregnancy-related pelvic girdle pain, which includes symphyseal pain, varies widely. This is in part related to differences in the definition of signs and symptoms, but a generally accepted figure for point prevalence is 20% (Vleeming et al. 2008). One study reported the prevalence of specific symphyseal symptoms to be approximately 3% (Owens et al. 2002). Given the scale and significance of this problem, and the lack of effective management strategies (Jain et al. 2006), it is imperative to understand the normal anatomy of the pubic symphysis. This should enable us to better understand morphological changes that may occur in this joint during pregnancy and the postpartum period.
This systematic review of the anatomy of the adult human pubic symphysis focuses on its constituent parts, blood supply, innervation, biomechanics, and pregnancy-related changes. Early development of the joint is not considered in detail, and only animal studies relevant to human anatomy have been included.
Search strategy
The initial search strategy is summarized in Fig. 2. Primary articles refer to those in which the anatomy of the pubic symphysis was the focus of the article, whereas secondary articles contained relevant data, but the anatomy of the joint was a more peripheral concern. Selected historical texts were also included, particularly if they provided anatomical information based on the author's original observations. Additional searches were conducted to retrieve relevant papers on biomechanics (search terms: pubic symphysis and biomechanics or mobility), pregnancy (pubic symphysis and pregnancy), and laboratory animal studies (pubic symphysis or symphysis pubis and mouse or rat or animal).
Fig. 2.
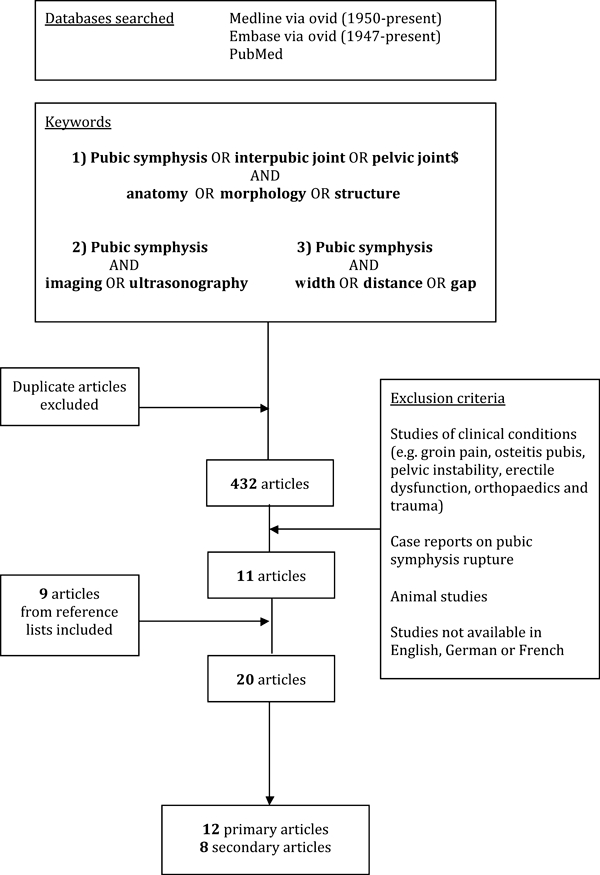
Search strategy for scientific articles included in the systematic review.
Results of systematic review
The most recent gross anatomical study of the pubic symphysis was published more than 20 years ago (Gamble et al. 1986). Many anatomical studies have methodological flaws such as missing information on parity and/or precise age of the specimens, and poorly described dissection and measurement protocols (Table 1). Only the three oldest studies (Aeby, 1858; Zulauf, 1901; Loeschcke, 1912) provide quantitative data from relatively large numbers of specimens, including young and pregnant cadavers but, even in these studies, precise reference points for measurements were poorly documented.
Table 1.
Details of anatomical studies focusing on the adult human pubic symphysis
| Author (year) | Type of study | Specimens/subjects | Anatomical data | Comment |
|---|---|---|---|---|
| Aeby (1858) | Gross | 61 cadavers 61 interpubic discs 36♂ 15–30 years (n = 12), > 30 years (n = 24) 25♀ 15–30 years (n = 12), > 30 years (n = 13) 47 interpubic clefts Sex: 25♂, 22♀ | Descriptive: articular surfaces, hyaline cartilage, interpubic disc and cleft, and ligaments Quantitative: dimensions of interpubic disc and cleft, including relationship to age and sex | Poorly described methods Anatomical reference points for measurements unclear Pregnancy-related changes described |
| Zulauf (1901) | Gross and microscopic | 107 cadavers Age: 10 days–75 years Sex: 57♂, 50♀ | Descriptive: interpubic cleft and disc Quantitative: interpubic cleft in relation to age and sex | Poorly documented methods |
| Loeschcke (1912) | Gross and microscopic | 76 cadavers 19♂ 25–75 years 19♀ 13–69 years 12 pregnant ♀ 20–39 years 26 postpartum ♀ 20–76 years | Descriptive: interpubic disc, ligaments, articular surfaces, interpubic cleft Quantitative: interpubic cleft and retropubic eminence, width of pubic symphysis related to age | Main focus on physiological changes within the pelvis during pregnancy and birth Unclear how width of pubic symphysis measured |
| Barnes (1934) | Radiographic | 27 cadavers 19♂ and ♀ adults, eight newborns 500 patients 20♂and♀ 2–14 years; 30♀ 17–23 years 140 non-pregnant ♀ 24–70 years 130♂ 25–75 years 180 pregnant ♀ (99 multipara, 81 primipara) | Quantitative: width of pubic symphysis in relation to age and sex | Anatomical reference points for width of pubic symphysis unclear No information on assessors of radiographs |
| Sutro (1936) | Microscopic and radiographic | 75 cadavers Age: 0–75 years Sex: 41♂, 34♀ (22 nullipara, 12 multipara) | Descriptive: articular surfaces, ligaments, interpubic cleft and disc, degenerative changes | Mostly descriptive with little quantitative data |
| Schmidt (1956) | Microscopic | 20 cadavers Age: 20–80 years Sex: ♂and♀ | Descriptive: distribution of collagen fibres in interpubic disc | Methods not described in detail (number of specimens sectioned in each plane uncertain) No consideration of age or sex differences |
| Vix & Ryu (1971) | Gross, microscopic and radiographic | 15 cadavers: age and sex unknown 400 adult patients: precise age unknown Sex: 200♂, 200♀ | Descriptive: articular surfaces, interpubic disc and cleft Quantitative: greatest transverse width of pubic symphysis in mid-third | Paucity of data on specimens (age, parity) No reference points for measurements given No information on assessors of radiographs |
| Putschar (1976) | Microscopic | 198 cadavers Age: 0–87 years Sex: 87♂, 111♀ | Descriptive: interpubic cleft, articular surfaces, retropubic eminence, age-related changes, degenerative changes | Summary of results from main study published 1931 Proportion of adult specimens uncertain |
| Gamble et al. (1986) | Gross, microscopic and radiographic | 20 cadavers 12 fetuses, four neonates, four adults Age and sex unknown | Descriptive: articular surfaces, shape and width of pubic symphysis, interpubic disc, ligaments, adjacent muscles, nerve and blood supply | Missing data (precise age, sex, parity) Only general descriptive results provided |
| Sgambati et al. (1996) | Radiographic | 96 patients Age: 14–89 years Sex: 64♂, 32♀ | Quantitative: anterior width of pubic symphysis at three levels (superior, middle, inferior), relationship to age and sex | Retrospective study Methods poorly described No reference points for measurements No consideration of parity in females No information on assessors of radiographs Included mean ± 2 SDs |
| Alicioglu et al. (2008) | Radiographic (CT) | 542 patients Age: 17–94 years (mean 56.7 ± 15.0 years) Sex: 264♂, 278♀ | Quantitative: width of pubic symphysis at three points (anterior, middle, posterior) through mid-section of joint, correlated to age, sex, BMI and parity | Retrospective study Width of symphysis related to gender and age No reliability data provided (one investigator, single measurements) |
| Wurdinger et al. (2002) | MR imaging | 11 healthy volunteers Age: 22–42 years, (mean 30 years) Sex: non-pregnant nulliparous♀ | Quantitative: width of pubic symphysis in coronal section, related to age | Unclear which MR images used for measuring pubic symphysis width and where measurement was taken No data on inter-rater reliability (two investigators) |
BMI, body mass index; CT, computed tomography; MR, magnetic resonance.
Articular surfaces
The articular surfaces of the pubic bones are oval in shape, slightly convex, and orientated obliquely in the sagittal plane, running posteroinferiorly in a craniocaudal direction (Knox, 1831; Luschka, 1864; Fick, 1904; Testut & Latarjet, 1928). The mean length of the articular surfaces is reported to be between 30–35 mm and mean width 10–12 mm (Testut & Latarjet, 1928). Posteriorly, the surfaces are parallel but usually diverge anteriorly, superiorly and inferiorly (Aeby, 1858; Fick, 1904). The upper and lower borders of the pubic symphysis are at the same horizontal level in almost all adult men but, in a random sample of adult women of unknown parity, 16% of upper and 5% of lower margins were uneven (Vix & Ryu, 1971) (Fig. 3).
Fig. 3.
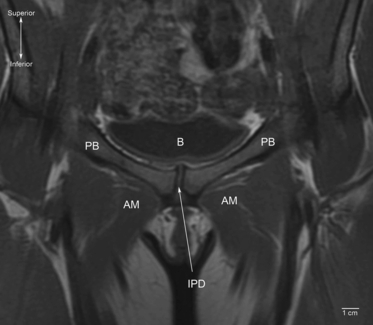
Coronal T1-weighted magnetic resonance image of the pubic symphysis in a supine 33-year-old woman (anterior view). Note that the joint is asymmetric. B, bladder; AM, adductor muscle group; PB, pubic bone; IPD, interpubic disc.
The articular surfaces are covered in hyaline cartilage, which varies between 1 and 3 mm in thickness (Aeby, 1858; Luschka, 1864; Fick, 1904; Frazer, 1920; Sutro, 1936; Frick et al. 1991), although Gamble et al. (1986) stated that the hyaline cartilage was only 200–400 μm thick in adults. Loeschcke (1912) noted that the thickness of the hyaline cartilage decreased with advancing age.
The subchondral bony surfaces are irregular in young adults (Luschka, 1864; Fick, 1904) but, radiographically, become smooth and straighter at around 30 years of age (Todd, 1930) before degenerative changes (joint narrowing, subchondral sclerosis and irregularity) set in from around the sixth decade (Todd, 1930). These features are used by biological anthropologists to assist age and sex determination (Todd, 1920; Gilbert & McKern, 1973; Brooks & Suchey, 1990; White & Folkens, 2005). Putschar (1976) described 8–12 subchondral transverse bony ridges in young individuals and noted that these gradually disappeared by around 25 years of age. A computed tomographic (CT) study indicated that the subchondral bone is more compact anteriorly in males and posteriorly in females (Putz & Mueller-Gerbl, 1992) but the study subjects and methods were poorly documented. In a radiographic study of cadavers, Sutro (1936) noted that the subchondral bone appeared increasingly porotic after the age of 50 years.
Bony fusion of the pubic symphysis is described in some adult primates such as the red leaf monkey (Presbytis rubicunda) (Tague, 1993) but has not been documented in healthy adult humans.
Ligaments
Four ligaments reinforce the pubic symphysis, but only the superior and inferior pubic ligaments are listed in Terminologia Anatomica (Federative Committee on Anatomical Terminology, 1998).
Superior pubic ligament
The superior pubic ligament bridges the superior margins of the joint and is attached to the pubic crest as far laterally as the pubic tubercles (Fick, 1904; Gamble et al. 1986) (Fig. 4). This ligament has been variably described as having connections with the interpubic disc (Testut & Latarjet, 1928; Frick et al. 1991), pectineal ligament (Fick, 1904), linea alba (Luschka, 1864; Testut & Latarjet, 1928), and periosteum of the superior pubic ramus (Frick et al. 1991). Luschka (1864) stated that the ligament was composed of irregular fibrous tissue although early reports mentioned a yellowish colour (Fick, 1904; Testut & Latarjet, 1928), suggesting the possibility of elastic fibres. The strength and significance of this ligament are controversial, with some arguing that it is important in reinforcing the joint (Luschka, 1864) and others that it is functionally inconsequential (Rosse & Gaddum-Rosse, 1997).
Fig. 4.
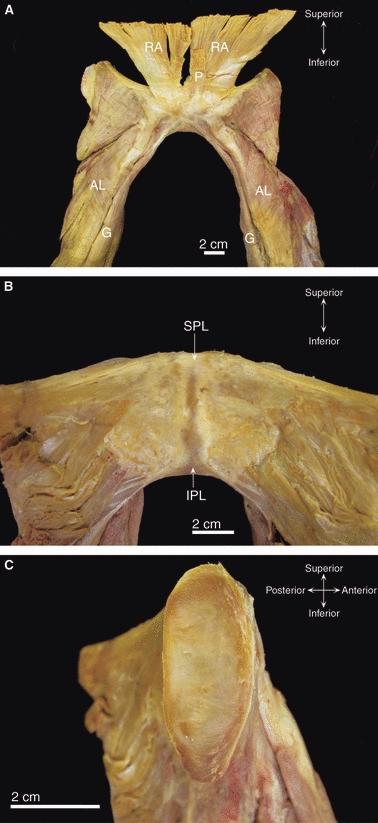
Dissection images of the pubic symphysis from an elderly cadaver of unknown parity. (A) Anterior view of the pubic symphysis showing blending of the tendons of rectus abdominis (RA) and pyramidalis (P) with the anterior pubic ligament. Note the decussation of the gracilis (G) tendons. AL, adductor longus. (B) Posterior view of the superior (SPL) and inferior (IPL) pubic ligaments. (C) The left medial pubic surface after bisection of the fibrocartilaginous interpubic disc.
Inferior pubic ligament
The inferior pubic ligament, also referred to as the subpubic (Gray, 1858; Frazer, 1920) or arcuate (Frick et al. 1991; Standring, 2008) pubic ligament, forms an arch spanning the inferior pubic rami (Fick, 1904; Frazer, 1920; Rosse & Gaddum-Rosse, 1997) (Fig. 4). Luschka (1864) and Testut & Latarjet (1928) emphasized that only its inferior fibres are attached to the inferior pubic rami; its upper fibres are short and transverse, blending with the interpubic disc (Gray, 1858; Testut & Latarjet, 1928; Frick et al. 1991) and posterior pubic ligament (Luschka, 1864; Fick, 1904). The inferior pubic ligament is reported to be stronger than its superior counterpart (Knox, 1831; Testut & Latarjet, 1928) and, once again, a yellowish appearance has been observed (Knox, 1831; Gray, 1858). Quantitative data on the ligament are sparse. Its maximum width has been recorded as 25 mm in men and 35 mm in women, with a height of 10–12 mm in both sexes (Fick, 1904; Testut & Latarjet, 1928). A small gap exists between its sharp inferior edge and the anterior margin of the perineal membrane; this transmits the deep dorsal vein of the penis or clitoris (Fick, 1904; Standring, 2008).
Anterior pubic ligament
The anterior pubic ligament connects the pubic bones anteriorly and merges with their periosteum laterally (Knox, 1831; Luschka, 1864; Fick, 1904) (Fig. 5). This ligament is reported to be a thick resistant structure, second only to the interpubic disc in maintaining stability of the pubic symphysis (Fick, 1904; Testut & Latarjet, 1928). It is composed of several layers of collagen fibres that vary in their orientation: deeper layers have a more transverse alignment (Knox, 1831; Gray, 1858; Testut & Latarjet, 1928) and may blend with the interpubic disc (Aeby, 1858; Sutro, 1936), whereas more superficial fibres cross obliquely, interconnecting with the tendinous insertions of the rectus abdominis and oblique abdominal muscles (Gray, 1858; Luschka, 1864; Gamble et al. 1986), and pyramidalis (Fick, 1904; Testut & Latarjet, 1928). Several authors have also described contributions to the anterior pubic ligament from the tendinous insertions of the adductor muscles, specifically adductor longus, adductor brevis and gracilis (Luschka, 1864; Fick, 1904; Testut & Latarjet, 1928). Testut & Latarjet (1928) alone documented vertical fibres within the ligament connecting to the ischiocavernosus muscles and corpora cavernosa.
Fig. 5.
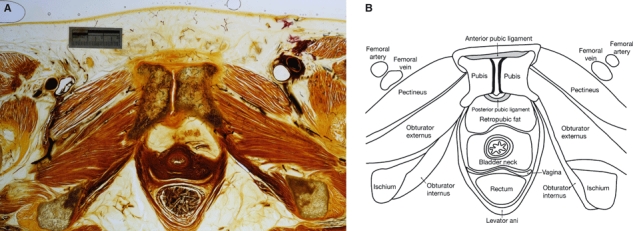
(A) E12 axial slice through a human female pubic symphysis (W.D. Trotter Anatomy Museum, Department of Anatomy & Structural Biology, University of Otago, Dunedin, New Zealand). (B) Illustration of the key anatomical features in Fig. 5A.
In a microdissection study of 17 elderly cadavers, Robinson et al. (2007) found that the adductor longus and rectus abdominis muscles attached to the anterior pubic ligament and interpubic disc in all cases. In nine specimens, adductor longus had both tendinous and muscular attachments and in the remaining eight specimens only muscle fibres were attached. Adductor brevis muscle fibres were also found to blend with the anterior aspect of the pubic symphysis in seven specimens but gracilis was attached in only one.
The mean thickness of the anterior pubic ligament has been reported to be between 5–12 mm (Aeby, 1858; Luschka, 1864; Fick, 1904; Testut & Latarjet, 1928). Two authors alluded to small perforating vessels within the ligament (Fick, 1904; Loeschcke, 1912).
Posterior pubic ligament
Relatively little is known about the posterior pubic ligament that spans the posterior aspect of the pubic symphysis and purportedly consists of only a few thin fibres (Aeby, 1858; Gray, 1858) (Fig. 5). Luschka (1864) described this ligament as blending with the periosteum of the pubic rami, whereas Testut & Latarjet (1928) were more specific about its attachment, referring to the posterior margins of the pubic articular surfaces. Superiorly, its transverse fibres blend with the superior pubic ligament and, inferiorly, oblique fibres cross over and merge with the inferior pubic ligament (Luschka, 1864; Testut & Latarjet, 1928). The ligament is thicker in multiparous women (Sutro, 1936; Putschar, 1976).
Interpubic disc and cleft
Interpubic disc
The pubic bones are united in the midline by a fibrocartilaginous interpubic disc (Fig. 4). Fibrocartilage is adapted to withstand compressive and tensional forces (Benjamin & Evans, 1990). Last (1954) described this connection as a broad mass of transversely running fibres, whereas Gray (1858) and Fick (1904) referred to fibrous elastic tissue, and Frick (1901) mentioned oblique fibres. Luschka (1864) noted that hyaline cartilage occasionally projects into the fibrous tissue in the centre of the joint.
The interpubic disc is usually wedge- or Y-shaped in axial cross-section with its apex directed posteriorly (Fick, 1904; Testut & Latarjet, 1928; Frick et al. 1991). The disc has a narrow waist and is broader superiorly and inferiorly (Weber, 1830; Luschka, 1864). It is wider and shorter in females (Gamble et al. 1986) and sometimes extends beyond the bony margins of the symphysis (Frick et al. 1991). A posterior bulge-like projection of the mid region of the disc, most prominent in multiparous females, is known as the retropubic eminence (Knox, 1831; Fick, 1904). The presence and extent of this projection are directly related to the existence and size of the interpubic cleft located within the disc (Loeschcke, 1912; Putschar, 1976) (see below).
Several authors have remarked on structural similarities between the interpubic and intervertebral discs (Weber, 1830; Luschka, 1864; Fick, 1904; Testut & Latarjet, 1928). Both Luschka (1864) and Fick (1904) considered that the interpubic disc has outer layers of obliquely running fibres that are thicker anteriorly, similar to the anulus fibrosus. This is supported by observations in the rat that reveal that the interpubic disc consists of an inner fibrocartilaginous core and an outer rim of dense fibrous tissue composed of collagen lamellae (da Rocha & Chopard, 2004). Testut & Latarjet (1928) noted that the peripheral parts of the disc are fused to the adjacent ligaments of the pubic symphysis.
Schmidt (1956) examined sectioned interpubic discs using polarized light microscopy and found that, in axial sections, collagen fibres form an arch posterior to the cleft before merging laterally with the adjacent periosteum. Anterior to the cleft, fibres cross at right angles between their periosteal attachments to the opposite pubic bones. In sagittal sections, fibre orientation was more complex; fibres pass forwards on each side of the cleft from its posterior aspect and then cross anterior to it before running in a cranial or caudal direction (Fig. 6). In coronal sections most fibres are vertically orientated.
Fig. 6.
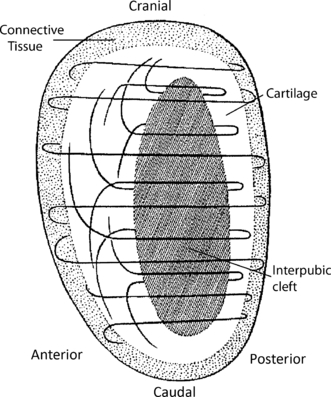
Sagittal section through the interpubic disc showing the arrangement of collagen fibres within the disc (adapted from Schmidt, 1956).
Interpubic cleft
A narrow, slit-like, oval-shaped cavity has frequently been described within the fibrocartilaginous interpubic disc. This is typically located within the superior and posterior part of the disc, occupying between one-third and one-half of the height of the disc (Knox, 1831; Zulauf, 1901; Fick, 1904; Putschar, 1976; Frick et al. 1991), covered posteriorly by a thin layer of fibrous tissue (Aeby, 1858; Fick, 1904). However, the cleft may extend the length and/or depth of the disc, particularly in women (Aeby, 1858; Testut & Latarjet, 1928; Sutro, 1936; Putschar, 1976) or it may be absent (Sutro, 1936; Putschar, 1976). Only rarely is the cleft situated centrally or anteriorly within the disc (Zulauf, 1901; Sutro, 1936). The walls of the cleft are rough and irregular (Frick, 1901; Testut & Latarjet, 1928), sometimes with small projections into the lumen. Reported variants include a single septum (Frick et al. 1991), irregular side branches (Aeby, 1858; Sutro, 1936), secondary clefts in multiparous women (Putschar, 1976), and a T-shaped cleft (Zulauf, 1901).
Both the lining and content of the interpubic cleft are controversial. According to some authors the cleft has no synovial lining (Frazer, 1920; Sutro, 1936; Last, 1954; Vix & Ryu, 1971), whereas others hold the opposite view (Knox, 1831; Gray, 1858; Frick et al. 1991). Testut & Latarjet (1928) described a rudimentary synovium but only within large clefts and Putschar (1976) reported a synovial lining in ‘secondary clefts’ in multiparous women. Aeby (1858) considered that the cleft was usually empty although adipose tissue or, occasionally, loose bodies from degenerating fibrocartilage have been reported within the cavity (Zulauf, 1901; Loeschcke, 1912).
There is also debate about the origin of the cleft. Zulauf (1901) stated that the cleft was present in 13 of 18 fetuses and newborns with a similar prevalence in both sexes. In contrast, others have suggested that the cleft develops in the second year of life (Putschar, 1976), at about 7–10 years of age (Aeby, 1858; Frazer, 1920), or at any age (Gray, 1858). Consequently, some have argued that the interpubic cleft is a degenerative phenomenon (Sutro, 1936) secondary to the ‘trauma’ of weight-bearing and walking (Loeschcke, 1912; Putschar, 1976). Nevertheless, there is general agreement that a cleft is present in most men and women, although larger and more prevalent in women (Knox, 1831; Aeby, 1858; Zulauf, 1901; Testut & Latarjet, 1928; Putschar, 1976). Thus, Putschar (1976) reported that a cleft was present in 80% of individuals before 16 years of age and in 88% of adult men and 97% of women.
Width of the pubic symphysis
Several studies have investigated the width of the normal pubic symphysis in an attempt to better understand the changes that occur in pregnancy (Table 2). There is reasonable consensus that the joint is wider anteriorly than posteriorly (Loeschcke, 1912; Vix & Ryu, 1971; Gamble et al. 1986; Björklund et al. 1996). In a study of adult cadavers, Loeschcke (1912) calculated mean joint widths to be 5 mm in men, 7.5 mm in nulliparous women, and 20 mm in multiparous women, but precise details of how these measurements were taken are lacking. Mean widths determined by imaging studies have yielded results ranging from 2.6 mm in nulliparous women (Roberts, 1934) to 12.6 mm measured at the most anterior part of the joint in women who had on average given birth to three children (Alicioglu et al. 2008) (Table 2).
Table 2.
Width of the adult pubic symphysis derived from imaging studies
| Mean symphyseal width (mm) | |||||
|---|---|---|---|---|---|
| Author (year) | Type of imaging study and no. of participants | Measurement plane/position of subjects | Site of measurements | Females | Males |
| Abramson et al.(1934) | Radiographic 33 nullipara 20 primipara 70 multipara 71♂ | Coronal/supine | Narrowest diameter between pubic bones | 4.1 (nullipara) 4.2 (primipara) 4.6 (multipara) | 4.4 |
| Barnes (1934) | Radiographic 130♂ 140♀ (non-pregnant) 81 primpara 99 multipara | Coronal/unknown | No details given | 4.0 (non-pregnant) No mean value provided for pregnant women | 4.2 |
| Roberts (1934) | Radiographic 59♀ | Coronal Supine or prone | Mid-point of pubic symphysis; no further details | 2.6 (nullipara) | |
| Vix & Ryu (1971) | Radiographic 200♂, 200♀ | Coronal/unknown | Greatest width in mid-third of symphysis anteriorly | 4.9 ± 1.1 | 5.9 ± 1.3 |
| Björklund et al.(1996) | Radiographic 15♀ Ultrasound 15♀ | Coronal/unknown Axial/unknown | Narrowest dimension of superior part of pubic symphysis; no further details | 2.9 ± 0.9 Parity unknown 3.2 ± 1.1 Parity unknown | |
| Sgambati et al.(1996) | Radiographic 64♂, 32♀ | Coronal/unknown | Width of superior, middle and inferior parts of pubic symphysis anteriorly | Superior: 4.3 Middle: 4.4 Inferior: 4.3 | Superior: 4.4 Middle: 4.9 Inferior: 4.6 |
| Wurdinger et al.(2002) | MRI 11♀ | Horizontal/supine | No details given | 5.0 ± 0.6 (nullipara) | |
| Alicioglu et al.(2008) | CT 264♂, 278♀ | Axial/supine | Width of anterior, middle and posterior parts of mid-pubic symphysis | Anterior: 12.6 Middle: 4.6 Posterior: 3.7 | Anterior: 11.8 Middle: 5.0 Posterior: 3.7 |
CT, computed tomography; MRI, magnetic resonance imaging.
Unfortunately, most of these studies are not directly comparable as the pubic symphysis was measured at different sites in different planes with different degrees of accuracy and with no indication of inter-rater or intrarater reliability. Furthermore, age, sex, parity and anthropometric indices were often not recorded. In the single CT study, Alicioglu et al. (2008) found no relationship between symphyseal width and parity or body mass index.
Blood supply and innervation
Few authors have investigated the blood supply of the pubic symphysis and even fewer its innervation. The joint is reportedly supplied by a pubic branch from the obturator artery and a branch of the inferior epigastric artery (Fick, 1904; Testut & Latarjet, 1928; Gamble et al. 1986; Frick et al. 1991). Lesser and more variable contributions are derived from branches of the external and internal pudendal arteries and the medial circumflex femoral artery (Fick, 1904; Gamble et al. 1986). Small blood vessels have been noted within the interpubic disc (Loeschcke, 1912) and these may become more prominent with advancing age (Putschar, 1976). In rats, the interpubic ligaments and fibrous rim of the disc are well vascularized from an anastomotic arterial circle fed by pubic branches of the obturator and inferior epigastric arteries (da Rocha & Chopard, 2004).
The innervation of the joint is variously described as coming from the pudendal and genitofemoral nerves (Gamble et al. 1986) and branches of the iliohypogastric, ilioinguinal and pudendal nerves (Standring, 2008). However, no further information is provided regarding the pattern of innervation or which branches supply specific parts of the joint.
Biomechanics
During everyday activities, the pubic symphysis is subjected to a variety of forces. These include traction on the inferior part of the joint and compression of the superior region when standing, compression when sitting, and shearing and compression during single-leg stance (Meissner et al. 1996). The healthy joint is highly resistant to separation although, on rare occasions, it may rupture during childbirth (Boland, 1933).
Given the location of this joint it is not surprising that few biomechanical studies have been undertaken. Moreover, the lack of consistency in methodology makes it difficult to compare different studies. In one study of 15 healthy young adults (six men, six nulliparous women and three multiparous women), steel pins were inserted into the superior pubic ramus on either side of the symphysis and horizontal, vertical, and sagittal movement in specific postures was measured (Walheim et al. 1984). Given the morphology of the joint, the magnitude of movement was small, with anteroposterior sagittal movements being similar in both sexes at around 0.6 mm, but greater (up to 1.3 mm) in multiparous women. In the supine position with the hips flexed 90° and maximally abducted, the mean lateral movement of each pin was 0.5 mm in men and 0.9 mm in women. When standing on alternate legs, the mean vertical descent of the pin on the contralateral side was 1 mm in men, 1.3 mm in nulliparous women, and 2.1 mm in multiparous women. Maximum symphyseal movement was observed in this direction. In a subsequent experimental study in 10 fresh cadavers, Meissner et al. (1996) estimated the forces required to produce such joint movements as 120 N in the vertical direction and 68 N in the sagittal direction. Similar values for vertical shift at the pubic symphysis were obtained from radiographic studies when mobility was again greatest in multiparous women (Garras et al. 2008).
Walheim et al. (1984) also investigated rotation at the pubic symphysis in healthy young adults. Less than 1° of rotation occurred at the joint in both a coronal plane about a sagittal axis and in a sagittal plane about a horizontal axis. In a further study of two healthy young adults, one male and one multiparous female, both investigated at different intervals after insertion of steel pins and implantation of tantalum balls into the pubic bone, rotation of up to 2° was recorded in the sagittal plane and up to 3° in the coronal plane in the young woman (Walheim & Selvik, 1984).
The strength of the pubic ligaments was investigated by forced separation of the pubic symphysis in one poorly documented study consisting of four groups of adult cadavers: male, nulliparous females, non-pregnant multiparous females, and, somewhat disturbingly, primigravidae in the last trimester of pregnancy (Ibrahim & El-Sherbini, 1961). In each group, a single pubic ligament was left intact and the others divided; the relative force required to rupture this remaining ligament was determined. The anterior ligament proved the strongest, followed by the inferior and then superior ligament. No data were provided for the posterior pubic ligament. Each ligament showed the same pattern of being strongest in men, slightly stronger in nulliparous compared with multiparous women, and weakest in primigravidae in the last trimester of pregnancy (Ibrahim & El-Sherbini, 1961).
Drawing on evidence from these studies it seems that small-magnitude, multidirectional movements can occur at the pubic symphysis, with the possibility of slightly greater ranges in women who have given birth.
Pregnancy-related changes
The pubic symphysis is capable of remarkable anatomical changes during pregnancy. The guinea-pig illustrates this spectacularly. The mean diameter of the fetal head is 20 mm, whereas the pelvic canal in early pregnancy is just 11 mm wide (Ruth, 1937). The pubic bones are united by hyaline cartilage but during late pregnancy and parturition they separate by up to 23 mm (Todd, 1923).
Widening of the interpubic gap and increased mobility at the symphysis also occur in humans, although to a lesser extent. In an early radiographic investigation of 111 mostly multiparous women in the last 2 months of pregnancy, Abramson et al. (1934) recorded a mean symphyseal width of 7.7 mm. This represented a mean increase of 3 mm when compared with 67 non-pregnant multiparous controls. The variability between pregnant women was striking, with widths ranging from 3 to 20 mm. Garagiola et al. (1989) reported a CT study of 14 multiparous women scanned within 24 h of uncomplicated term vaginal delivery. Mean symphyseal width was 6.5 mm (range 3–11 mm) compared with 4–6 mm in 15 controls matched for age and sex but not parity. Ultrasound has also been used to investigate pregnancy-related changes in the pubic symphysis. In one such study of 211 women, 56% of whom were primigravidae, pubic width increased from a mean of 4 mm at 8 weeks of gestation to 7 mm at term (Bahlmann et al. 1993). Unlike the guinea-pig, widening of the human pubic symphysis in pregnancy begins as early as 8–10 weeks’ gestation and appears to progress steadily (Bahlmann et al. 1993).
Other changes in the pubic symphysis during pregnancy have been described. During late pregnancy and soon after birth, gas can be detected radiographically in the joint in 30–40% of women (Camiel & Aaron, 1956; Vix & Ryu, 1971; Garagiola et al. 1989). It usually appears as a linear vertical streak near the centre of the joint. Interestingly, this phenomenon has also been observed in the sacroiliac joint in the absence of joint widening (Garagiola et al. 1989). The gas is typically asymptomatic and usually disappears within days of delivery (Williams, 1955). Radiographic studies have shown that osteoclastic resorption of the symphyseal margins of the pubic bones also occurs in pregnant women (Abramson et al. 1934; Vix & Ryu, 1971; Putschar, 1976) as reported in pregnant guinea-pigs (Ruth, 1937) and mice (Pinheiro et al. 2004). The articular cartilage has a higher water content during and after pregnancy (Wurdinger et al. 2002). Finally, the pubic ligaments become thicker and more vascular (Loeschcke, 1912), a retropubic eminence develops (Putschar, 1976), and the interpubic cleft increases in size (Testut & Latarjet, 1928) and may develop irregularities and secondary clefts (Sutro, 1936; Putschar, 1976). Bleeding into the pubic ligaments and interpubic cleft together with tears in the hyaline cartilage may be seen immediately after birth (Putschar, 1976).
Animal studies provide some insight into the mechanisms underlying widening of the pubic symphysis in humans. In mice, the pubic symphysis increases in width from about 0.2 mm to 3 mm during a gestation that lasts about 3 weeks (Pinheiro et al. 2004) (Fig. 7). Early in pregnancy, there is partial resorption of the medial ends of the pubic bones and articular cartilage. From day 12 onwards the interpubic cleft expands due to growth of an interpubic ligament consisting of tightly packed collagen fibres that run parallel to the long axis of the symphysis. Further widening of the symphysis from day 15 onwards is associated with untwisting and rupture of helical collagen fibrils and an increase in the water content of the extracellular matrix (Pinheiro et al. 2004). Relaxin (produced by the placenta during pregnancy) and oestrogen appear to be important hormonal mediators of this process; in relaxin-knockout mutant mice, interpubic ligament formation occurs but the later changes in the pubic symphysis do not (Zhao et al. 2000). Fibrocartilage from the murine pubic symphysis demonstrates strong immunohistochemical staining for α and β oestrogen and relaxin (leucine-rich repeat-containing G-protein-coupled receptor 7) receptors and quantitative gene expression studies in cultured chondrocytes from this site mirror these findings (Wang et al. 2009). These observations differ from those with meniscal fibrocartilage, reflecting differential responses to female hormones. Oestrogen and relaxin can also induce expression of matrix metalloproteinases in fibrocartilage (Wang et al. 2007), which may play an important role in collagen degradation and remodelling (Pinheiro et al. 2004). Further evidence that relaxin is involved in connective tissue remodelling in the pubic symphysis comes from studies in oophorectomized non-pregnant rats given exogenous synthetic human relaxin. This caused an increase in the length and weight of the interpubic fibrocartilage but a substantial decrease in total collagen content (Samuel et al. 1996).
Fig. 7.
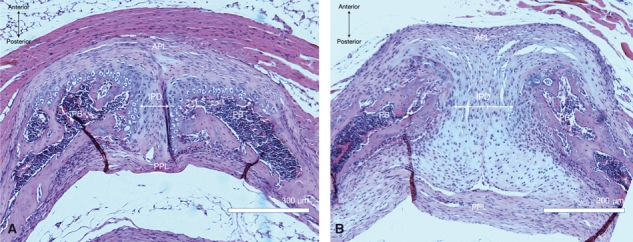
Photomicrographs of 5-μm axial sections through a mouse pubic symphysis. (A) Virgin adult female; (B) Multiparous. Haematoxylin & eosin, × 10 magnification. APL, anterior pubic ligament; PPL, posterior pubic ligament; PB, pubic bone; IPD, interpubic disc.
Studies on the relationship between widening of the pubic symphysis, symphyseal symptoms, and circulating concentrations of relaxin have yielded conflicting results. Björklund et al. (1999, 2000) found a significantly greater symphyseal width in women with severe pregnancy-related pelvic girdle pain compared with those with mild or no pain. However, pelvic girdle pain in this study also included low back pain and there was no control for parity. MacLennan et al. (1986) first reported higher serum relaxin concentrations in women with severe pelvic pain in late pregnancy and postpartum but other studies have failed to confirm this finding at 33–35 weeks’ gestation (Albert et al. 1997; Björklund et al. 2000). Kristiansson et al. (1996) found a significant but weak correlation (r = 0.15) between mean serum relaxin levels throughout pregnancy and symphyseal pain in late pregnancy and so perhaps chronic effects of relaxin are more important. Serum concentrations of relaxin peak in the 12th week of pregnancy and decrease to stable levels at around 50% of peak values from about the 20th week onwards (Kristiansson et al. 1996), whereas mean symphyseal width increases steadily throughout pregnancy (Bahlmann et al. 1993). Therefore, if symphyseal symptoms are related to joint widening it is not surprising that there is no direct correlation between circulating levels of relaxin and symptoms at a single time-point.
Clinical relevance
As alluded to in the introduction, symphyseal pain is a relatively common but poorly understood condition affecting athletes, patients with traumatic pelvic injuries, and pregnant women (Gibbon & Hession, 1997; Owens et al. 2002; Robinson et al. 2007; Ronchetti et al. 2008; Cheer & Pearce, 2010). Typical symptoms include pubic pain radiating to the upper or anterior thigh (Owens et al. 2002; Ronchetti et al. 2008), perineum and back, aggravated by weight bearing and associated with difficulty in walking (Jain et al. 2006). Symptoms are more likely if there is more than 10 mm horizontal and 5 mm vertical separation of the pubic bones (Hagen, 1974), although the relationship between the degree of separation and severity of symptoms is far from linear (Schwartz et al. 1985). In some women, symptoms are severe enough to require the use of crutches or to necessitate admission to hospital for bed rest. As many as one-fifth of affected women continue to have pain for up to 6 months after delivery with the prospect of recurrent symptoms in future pregnancies (Owens et al. 2002).
In side impact motor vehicle crashes the pubic symphysis is subjected to lateral compression, posterior bending and multidirectional shear forces (Li et al. 2007) that may result in rupture (Weber et al. 1999) and/or chronic and disabling joint laxity (Li et al. 2007). The joint may also rarely rupture during childbirth (Boland, 1933); iatrogenic rupture (symphysiotomy) for obstructed labour is rarely if ever performed these days although the operation was carried out in Ireland as recently as the early 1980s and continues to be a contentious issue among survivors (Buckley et al. 2010).
The pubic symphysis is also affected in a variety of other conditions including congenital disorders (e.g. bladder exstrophy and cleidocranial dysostosis), osteitis (inflammatory, infective, and traumatic), metabolic disorders (e.g. hyperparathyroidism and haemochromatosis), and rarely by neoplasms (mostly metastatic disease).
Concluding remarks
This review highlights the need for further detailed anatomical studies in order to clarify the morphology of the pubic symphysis. The precise attachments of surrounding ligaments, particularly the posterior pubic ligament, require further investigation. An integral feature of this joint is the interpubic disc, yet its composition and morphology are not well understood. Disparities are evident in descriptions of the prevalence, position, lining and content of its cleft(s). In addition, details regarding the width and movements of the ‘normal’ pubic symphysis are limited. Given the prevalence of symphyseal pain during and after pregnancy, it is imperative to gain a better understanding of the natural history of the anatomy of the pubic symphysis in this population. Carefully conducted longitudinal ultrasound studies could prove particularly valuable. A better understanding of the normal anatomy of the human pubic symphysis should improve our understanding of all pathologies affecting this joint and ultimately contribute to better treatments for patients suffering from symphyseal pain and dysfunction.
Author contributions
I.B.: design of the systematic review, literature search and acquisition of material, analysis and interpretation of literature, selection and acquisition of figures, writing of first draft and revised manuscript; S.J.W.: design of systematic review, critical review of final draft and revision of manuscript; and M.D.S.: design of systematic review, analysis of literature, figure selection, processing from first to final draft, and revision of manuscript.
References
- Abramson D, Roberts SM, Wilson PD. Relaxation of the pelvic joints in pregnancy. Surg Gynecol Obstet. 1934;58:595–613. [Google Scholar]
- Aeby C. Über die Symphysis Ossium Pubis des Menschen nebst Beiträgen zur Lehre vom hyalinen Knorpel und seiner Verknöcherung. Z Ration Med. 1858;4:1–25. [Google Scholar]
- Albert H, Godskesen M, Westergaard JG, et al. Circulating levels of relaxin are normal in pregnant women with pelvic pain. Eur J Obstet Gynecol. 1997;74:19–22. doi: 10.1016/s0301-2115(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Alicioglu B, Kartal O, Gurbuz H, et al. Symphysis pubis distance in adults: a retrospective computed tomography study. Surg Radiol Anat. 2008;30:153–157. doi: 10.1007/s00276-007-0295-0. [DOI] [PubMed] [Google Scholar]
- Bahlmann F, Merz E, Macchiella D, et al. Sonographische Darstellung des Symphysenspaltes zur Beurteilung eines Symphysenschadens in der Schwangerschaft und Post Partum. Z Geburtsh u Perinat. 1993;197:27–30. [PubMed] [Google Scholar]
- Barnes J. The symphysis pubis in the female. Am J Roentgenol. 1934;32:333–352. [Google Scholar]
- Benjamin M, Evans EJ. Fibrocartilage. J Anat. 1990;171:1–15. [PMC free article] [PubMed] [Google Scholar]
- Björklund K, Bergström S, Lindgren PG, et al. Ultrasonographic measurement of the symphysis pubis: a potential method of studying symphyseolysis in pregnancy. Gynecol Obstet Invest. 1996;42:151–153. doi: 10.1159/000291932. [DOI] [PubMed] [Google Scholar]
- Björklund K, Nordström M-L, Bergström S. Sonographic assessment of symphyseal joint distention during pregnancy and post partum with special reference to pelvic pain. Acta Obstet Gynecol Scand. 1999;78:125–130. doi: 10.1080/j.1600-0412.1999.780210.x. [DOI] [PubMed] [Google Scholar]
- Björklund K, Bergström S, Nordström M-L, et al. Symphyseal distension in relation to serum relaxin levels and pelvic pain in pregnancy. Acta Obstet Gynecol Scand. 2000;79:269–275. doi: 10.1080/j.1600-0412.2000.079004269.x. [DOI] [PubMed] [Google Scholar]
- Boland BF. Rupture of the symphysis pubis articulation during delivery. Surg Gynecol Obstet. 1933;57:517–522. [Google Scholar]
- Brooks S, Suchey JM. Skeletal age determination based on the os pubis: a comparison of the Acsadi-Nemeskeri and Suchey-Brooks methods. Hum Evol. 1990;5:227–238. [Google Scholar]
- Buckley C, Byrne J, Caherty T, et al. Childbirth operation inquiry. Irish Times. 2010 Tuesday, February 23, 2010. http://www.irishtimes.com/newspaper/letters/2010/0223/1224265032353.html (accessed on 24 February 2010) [Google Scholar]
- Camiel MR, Aaron JB. The gas or vacuum phenomenon in the pubic symphysis during pregnancy. Radiology. 1956;66:548–551. doi: 10.1148/66.4.548. [DOI] [PubMed] [Google Scholar]
- Cheer K, Pearce S. Osteoarticular infection of the symphysis pubis and sacroiliac joints in active young sportsmen. BMJ. 2010;339:362–364. doi: 10.1136/bmj.b5019. [DOI] [PubMed] [Google Scholar]
- Eastman NJ. Pelvic mensuration: a study in the perpetuation of error. Obstet Gynecol Surv. 1948;3:301–329. [PubMed] [Google Scholar]
- Federative Committee on Anatomical Terminology. Terminologia Anatomica. Stuttgart: Thieme; 1998. p. 30. [Google Scholar]
- Fick R. Handbuch der Anatomie und Mechanik der Gelenke unter Berücksichtigung der bewegenden Muskeln. Erster Teil: Anatomie der Gelenke. Jena: Verlag von Gustav Fischer; 1904. [Google Scholar]
- Frazer J. The Human Anatomy of the Skeleton. 2nd edn. London: J & A Churchill; 1920. [Google Scholar]
- Frick R. Bemerkungen über die Höhlenbildung am Schamfugenknorpel. Anat Anz. 1901;19:307–312. [Google Scholar]
- Frick H, Leonhardt H, Starck H. Human Anatomy 1. 3rd edn. Stuttgart: Georg Thieme Verlag; 1991. [Google Scholar]
- Gamble JG, Simmons SC, Freedman M. The symphysis pubis, anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;203:261–272. [PubMed] [Google Scholar]
- Garagiola DM, Tarver RD, Gibson L, et al. Anatomic changes in the pelvis after uncomplicated vaginal delivery: a CT study on 14 women. AJR Am J Roentgenol. 1989;153:1239–1241. doi: 10.2214/ajr.153.6.1239. [DOI] [PubMed] [Google Scholar]
- Garras DN, Carothers JT, Olson SA. Single-leg-stance (Flamingo) radiographs to assess pelvic instability: how much motion is normal? J Bone Joint Surg Am. 2008;90:2114–2118. doi: 10.2106/JBJS.G.00277. [DOI] [PubMed] [Google Scholar]
- Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis. Am J Roentgenol. 1997;169:849–853. doi: 10.2214/ajr.169.3.9275910. [DOI] [PubMed] [Google Scholar]
- Gilbert BM, McKern TW. A method for aging the female os pubis. Am J Phys Anthropol. 1973;38:31–38. doi: 10.1002/ajpa.1330380109. [DOI] [PubMed] [Google Scholar]
- Gray H. Anatomy: Descriptive and Surgical. London: John W Parker; 1858. [Google Scholar]
- Hagen R. Pelvic girdle relaxation from an orthopaedic point of view. Acta Orthop Scand. 1974;45:550–563. doi: 10.3109/17453677408989178. [DOI] [PubMed] [Google Scholar]
- Hunter W. Remarks on the symphysis ossis pubis. Med Observ Inquiries. 1762;2:333–339. [Google Scholar]
- Ibrahim A, El-Sherbini A. The different ligaments of the symphysis pubis in the pregnant and the non-pregnant state. J Obstet Gynaecol Br Emp. 1961;68:592–596. doi: 10.1111/j.1471-0528.1961.tb02775.x. [DOI] [PubMed] [Google Scholar]
- Jain S, Eedarapalli P, Jamjute P, et al. Symphysis pubis dysfunction: a practical approach to management. Obstet Gynaecol (Lond) 2006;8:153–158. [Google Scholar]
- Knox R. A System of Human Anatomy: On the Basis of the “Traite d'anatomie descriptive” of M.H. Cloquet. 2nd edn. Edinburgh: MacLachlan and Stewart; 1831. [Google Scholar]
- Kristiansson P, Svärdsudd K, von Schoultz B. Serum relaxin, symphyseal pain, and back pain during pregnancy. Am J Obstet Gynecol. 1996;175:1342–1347. doi: 10.1016/s0002-9378(96)70052-2. [DOI] [PubMed] [Google Scholar]
- Last RJ. Anatomy. Regional and Applied. 1st edn. London: J & A Churchill; 1954. [Google Scholar]
- Li Z, Kim JE, Davidson JS, et al. Biomechanical response of the pubic symphysis in lateral pelvic impacts: a finite element study. J Biomech. 2007;40:2758–2766. doi: 10.1016/j.jbiomech.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. Untersuchungen über die Entstehung und Bedeutung der Spaltbildungen in der Symphyse, sowie über physiologische Erweiterungsvorgaenge am Becken Schwangerer und Gebärender. Archiv Gynaek. 1912;96:525–560. [Google Scholar]
- Luschka H. Die Anatomie des Menschen in der Rücksicht auf die Bedürfnisse der praktischen Heilkunde. Tuebingen: Verlag der H. Laupp'schen Buchhandlung Laupp & Siebeck; 1864. [Google Scholar]
- MacLennan AH, Nicolson R, Green RC, et al. Serum relaxin and pelvic pain of pregnancy. Lancet. 1986;2:243–245. doi: 10.1016/s0140-6736(86)92069-6. [DOI] [PubMed] [Google Scholar]
- McMinn RMH. Last's Anatomy. Regional and Applied. 9th edn. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- Meissner A, Fell M, Wilk R, et al. Biomechanics of the pubic symphysis. Which forces lead to mobility of the symphysis in physiological conditions? Unfallchirurg. 1996;99:415–421. [PubMed] [Google Scholar]
- Owens K, Pearson A, Mason G. Symphysis pubis dysfunction – a cause of significant obstetric morbidity. Eur J Obstet Gynecol Reprod Biol. 2002;105:143–146. doi: 10.1016/s0301-2115(02)00192-6. [DOI] [PubMed] [Google Scholar]
- Pinheiro MC, Moraes SG, Battlehner CN, et al. Histochemical and ultrastructural study of collagen fibers in mouse pubic symphysis during late pregnancy. Micron. 2004;35:685–693. doi: 10.1016/j.micron.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Putschar WGJ. The structure of the human symphysis pubis with special consideration of parturition and its sequelae. Am J Phys Anthropol. 1976;45:589–594. doi: 10.1002/ajpa.1330450324. [DOI] [PubMed] [Google Scholar]
- Putz R, Mueller-Gerbl M. Anatomische Besonderheiten des Beckenrings. Unfallchirurg. 1992;95:164–167. [PubMed] [Google Scholar]
- Roberts RE. Discussion on the physiology and pathology of the pelvic joints in relation to child-bearing. A radiological investigation. Proc R Soc Med. 1934;27:1217–1225. [PMC free article] [PubMed] [Google Scholar]
- Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. Am J Roentgenol. 2007;188:W440–W445. doi: 10.2214/AJR.06.1238. [DOI] [PubMed] [Google Scholar]
- da Rocha RC, Chopard RP. Nutrition pathways to the symphysis pubis. J Anat. 2004;204:209–215. doi: 10.1111/j.0021-8782.2004.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti I, Vleeming A, van Wingerden JP. Physical characteristics of women with severe pelvic girdle pain after pregnancy. Spine. 2008;33:E145–E151. doi: 10.1097/BRS.0b013e3181657f03. [DOI] [PubMed] [Google Scholar]
- Rosse C, Gaddum-Rosse P. Hollinshead's Textbook of Anatomy. 5th edn. New York: Lippincott-Raven; 1997. [Google Scholar]
- Ruth EB. Metamorphosis of the pubic symphysis. III Histological changes in the symphysis of the pregnant guinea pig. Anat Rec. 1937;67:409–421. [Google Scholar]
- Samuel CS, Butkus A, Coghlan JP, et al. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology. 1996;137:3884–3890. doi: 10.1210/endo.137.9.8756561. [DOI] [PubMed] [Google Scholar]
- Schmidt H. Die funktionelle Struktur der Symphyse im Erwachsenenalter. Anat Anz. 1956;103:135–152. [PubMed] [Google Scholar]
- Schwartz Z, Katz Z, Lancet M. Management of puerperal separation of the symphysis pubis. Int J Gynaecol Obstet. 1985;23:125–128. doi: 10.1016/0020-7292(85)90056-6. [DOI] [PubMed] [Google Scholar]
- Sgambati E, Stecco A, Capaccioli L, et al. Morphometric evaluation of the symphysis pubis. Ital J Anat Embryol. 1996;101:195–201. [PubMed] [Google Scholar]
- Standring S. Gray's Anatomy: the Anatomical Basis of Clinical Practice. 40th edn. New York: Churchill Livingstone Elsevier; 2008. [Google Scholar]
- Sutro CJ. The pubic bones and their symphysis. Arch Surg. 1936;32:823–841. [Google Scholar]
- Tague RG. Pubic symphyseal synostosis and sexual dimorphism of the pelvis in Presbytis cristata and Presbytis rubicunda. Int J Primatol. 1993;14:637–654. [Google Scholar]
- Testut J, Latarjet A. Traite d'Anatomie Humaine. 8th edn. Paris: Gaston Doin & C; 1928. [Google Scholar]
- Todd TW. Age changes in the pubic bone: I. The white male pubis. Am J Phys Anthropol. 1920;3:467–470. [Google Scholar]
- Todd TW. The pubic symphysis of the guinea-pig in relation to pregnancy and parturition. Am J Anat. 1923;31:345–357. [Google Scholar]
- Todd TW. Age changes in the pubic bone. VIII: Roent-genographic differentiation. Am J Phys Anthropol. 1930;14:255–271. [Google Scholar]
- Vesalius A. On the Fabric of the Human Body. Book 1: The Bones and Cartilages. San Francisco: Norman Publishing; 1543. A translation of De Humani Corporis Fabrica Libri Septem by Richardson WF, Carman JB (1998) [Google Scholar]
- Vix VA, Ryu CY. The adult symphysis pubis: normal and abnormal. Am J Roentgenol Radium Ther Nucl Med. 1971;112:517–525. doi: 10.2214/ajr.112.3.517. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Albert HB, Ostgaard HC, et al. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17:794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walheim G, Selvik G. Mobility of the pubic symphysis. In vivo measurements with an electromechanic method and a Roentgen stereophotogrammetric method. Clin Orthop Relat Res. 1984;191:129–135. [PubMed] [Google Scholar]
- Walheim G, Olerud S, Ribbe T. Mobility of the pubic symphysis. Acta Orthop Scand. 1984;55:203–208. doi: 10.3109/17453678408992338. [DOI] [PubMed] [Google Scholar]
- Wang W, Hayami T, Kapila S. Estrogen/relaxin induce while progesterone represses MMP expression in TMJ fibrochondrocytes. J Dent Res. 2007;86:1279. (Abstract) [Google Scholar]
- Wang W, Hayami T, Kapila S. Female hormone receptors are differentially expressed in mouse fibrocartilages. Osteoarthritis Cartilage. 2009;17:646–654. doi: 10.1016/j.joca.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber EH. Handbuch der Anatomie des Menschen – Beschreibung des Knochensystems, des Muskelsystems under der Haut. 4th edn. Braunschweig: Verlag der Schulbuchhandlung; 1830. [Google Scholar]
- Weber K, Vock B, Müller W, et al. Rupture of the pubic symphysis: diagnosis, treatment, and clinical outcome. Ann Saudi Med. 1999;19:544–546. doi: 10.5144/0256-4947.1999.544. [DOI] [PubMed] [Google Scholar]
- White TD, Folkens PA. The Human Bone Manual. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- Williams JL. Gas in symphysis pubis during and following pregnancy. Am J Roentgenol. 1955;73:403–409. [PubMed] [Google Scholar]
- Wurdinger S, Humbsch K, Reichenbach JR, et al. MRI of the pelvic ring joints postpartum: normal and pathological findings. J Magn Reson Imaging. 2002;15:324–329. doi: 10.1002/jmri.10073. [DOI] [PubMed] [Google Scholar]
- Zhao L, Samuel CS, Tregear GW, et al. Collagen studies in late pregnant relaxin null mice. Biol Reprod. 2000;63:697–703. doi: 10.1095/biolreprod63.3.697. [DOI] [PubMed] [Google Scholar]
- Zulauf C. Die Höhlenbildung im Symphysenknorpel. Archiv Anat Physiol. 1901;1:95–116. [Google Scholar]


