Abstract
Comparison of the early development of the mandibular symphysis between primates and modern humans is of particular interest in human palaeontology. Using geometric morphometric methods, we explored and compared the ontogenetic shape changes of 14 chimpanzee mandibles (Pan troglodytes) against 66 human CT-scanned mandibles over the age range from fetal life to the complete emergence of the deciduous dentition in a visualization incorporating the deciduous tooth arrangement. The results reveal that the symphysis is anteriorly inclined in the youngest chimpanzee fetuses but develops an increasingly vertical orientation up until birth. At the same time, the anterior teeth reorient before a vertical emergence, and a symphyseal tuber appears on the labial side. When the deciduous canine emerges, the symphysis inclines anteriorly again, exhibiting the adult characteristic slope. These two phases are characterized by a repositioning of the simian shelf. Unlike chimpanzees, the human symphysis remains vertical throughout fetal development. However, the combination of morphological changes observed in chimpanzee fetuses is similar to that of modern humans after birth, as the mental region projects forward. By elongating the alveolar process, the inclination of the chimpanzee symphysis could be a key event for emergence of the deciduous canine, as space is lacking at the alveolar ridge in a vertical symphysis once the deciduous incisors and molars have emerged. The repositioning of the simian shelf suggests that the suprahyoid muscles have a significant influence on the anterior growth of the symphysis. The anteroposterior positioning of the basal symphysis in both species may be related to hyoid bone position during ontogeny.
Keywords: chimpanzees, deciduous teeth, early ontogeny, geometric morphometrics, humans, mandibular symphysis
Introduction
Mandibular remains are frequent in the hominoid fossil record and some aspects of their external morphology help us diagnose or describe fossil taxa. The symphyseal region is of systematic value in hominid palaeontology. In particular, some authors claim that a protruding mental region is a valid character defining modern humans (Stringer et al. 1984). Schwartz & Tattersall (2000) describe the modern human chin as a vertical keel lying along the midline, flanked on each side by a mental fossa, also called the anterior buccal groove (Arensburg et al. 1989), and expanding laterally along the distended inferior margin of the mandible (Fig. 1). The labial surface of the mental region outlines an inverted T-shaped relief, well-delineated in human fetuses and babies (Schwartz & Tattersall, 2000). After birth, though still visible, the inverted T-relief rapidly becomes smoothed at the same time as the mental region projects forward. Schwartz & Tattersall (2000) consider the presence of this inverted T-shaped relief a typical modern human feature in comparison with any form of protrusion of the basilar region of the symphysis that can also be identified in various extinct hominids (e.g. Frayer et al. 1993; Lieberman, 1995; Rosas, 1995). The protrusion leads to ambiguity in an evolutionary context. This character is substantially variable and there is no empirical data, from analysis of either human or non-human primates, to argue that the different sorts of protrusions observed in these extinct hominoids could have emerged from a common developmental pathway (Lieberman, 1995, 1999).
Fig. 1.
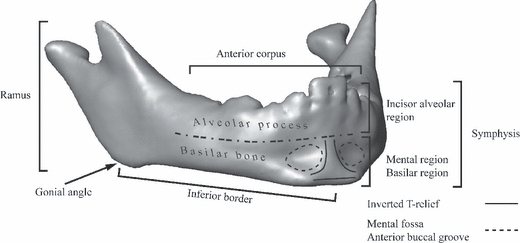
Mandible of a 1-year-old modern human labelled with the name of the anatomical regions that are used in the text.
Comparison of ontogenetic changes between human and non-human primates is also important for inferences about the evolutionary significance of variations observed in extinct hominoids. While adult chimpanzees have symphyses inclined anteriorly, modern humans have symphyses inclined posteriorly, as the mental region is positioned forward relative to the incisor alveolar process (DuBrul & Sicher, 1954). However, Bolk (1924) observed that at birth the symphysis is oriented vertically relative to the inferior border in both modern humans and chimpanzees (Fig. 2A), suggesting that discrepancies in the forward growth of the basilar and the alveolar regions might be related to anterior symphyseal inclination in chimpanzees and prominence of the mental region in modern humans (Fig. 2B,C). According to Bolk, because the first and second permanent molars emerge before the replacement of the deciduous dentition in chimpanzees, the forward growth of the alveolar region is faster than that of the basilar region, hence the anterior inclination of the symphysis (Fig. 2C). In humans, on the other hand, when the first permanent molar has emerged and the deciduous dentition is being replaced, the forward alveolar growth slows relative to the basilar growth, hence the posterior inclination (Fig. 2B). Bolk's suggestion that the difference in the period of tooth emergence between humans and chimpanzees is a strong component to explain the final symphyseal configurations (Fig. 2B,C) has been criticized (see DuBrul & Sicher, 1954). However, the growth discrepancy at the symphysis between the alveolar and basilar regions is a well-accepted mechanism. For instance, as we explain below, this mechanism may be supported by the bone remodeling reversal at the labial surface of the symphysis which occurs during the emergence of the deciduous dentition (Johnson et al. 1976; Kurihara et al. 1980; Enlow, 1990). The inclination of the symphysis is likely to happen earlier than when Bolk (1924) suggested – earlier, in fact, than the observations permitted by his sample (human and chimpanzee neonates and specimens with a fully emerged deciduous dentition).
Fig. 2.
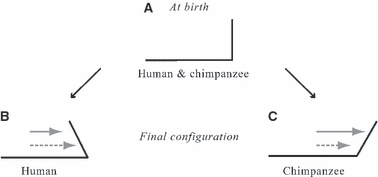
Bolk's ‘shifting theory’. Black line: lateral profile of the anterior corpus. Grey arrow: forward growth of the alveolar region. Dashed gray arrow: forward growth of the basilar region. (A) Shape at birth. (B) Final shape of the chimpanzee symphysis. (C) Final shape of the modern human symphysis.
Since Bolk's study, comparative growth of the chimpanzee and modern human symphysis during early development has been neglected. How and when humans and chimpanzees develop a vertical symphysis during fetal life is unknown. Likewise, the postnatal changes of this configuration towards an anteriorly inclined symphysis in chimpanzees vs. a prominent mental region in humans have received little attention. Moreover, the two species differ in their sequence of deciduous tooth emergence (Robinow et al. 1942; Nissen & Riesen, 1945; Meredith, 1946), which must bear implications for the mandible. The present study explores the 3D-morphological change of the human and chimpanzee (Pan troglodytes) mandibles and the development of the deciduous dentition in order to provide useful insights about growth and development of the symphysis.
Patterns of mandibular growth during early ontogeny
The deformation of the mandibular surface during growth has been investigated in two different ways: first, by mapping the bone depository and resorptive fields to identify the polarity of growth in different surface areas (Enlow & Harris, 1964; Mauser et al. 1975; Johnson et al. 1976; Kurihara et al. 1980; Enlow, 1990); secondly, by studying the development of muscles and teeth (Moss & Young, 1960; Moss, 1962; Moss & Salentijn, 1969a,b;), which stimulate the activity of osteoblasts and osteoclasts (Moss, 1997a,b;). Figure 1 sets out the anatomical regions of the mandible as we will refer to them here.
Bone depository and resorptive fields of the modern human mandible are known for many developmental stages (Mauser et al. 1975; Kurihara et al. 1980; Enlow, 1990). The forward growth of the symphysis is associated with bone deposition on the labial side of the symphysis from fetal stages to the emergence of the second deciduous molar (dm2; Kurihara et al. 1980; Enlow, 1990) at approximately 2.3 year (Smith et al. 1994). From the time dm2 emerges, the mental region continues to grow forwards while the incisor alveolar process displaces backwards as the labial side of this region becomes resorptive (the phenomenon known as ‘bone remodeling reversal’). In chimpanzees, there appear to be no data on bone depository and resorptive fields for fetuses. During early postnatal life and before the emergence of the deciduous canine (dc), the whole labial surface of the symphysis is depository, as in modern humans (Johnson et al. 1976). However, in chimpanzees the labial side of the basal part of the mental region becomes resorptive after dc emergence. In both species, the bone remodeling reversal may be consistent with the transition from a vertical symphysis to the contrasting final configurations: a symphysis inclined anteriorly in chimpanzees but a protrusive mental region in modern humans. These final configurations (Fig. 2B,C) may appear prior to the emergence of the permanent first molar (M1).
Growth of the alveolar process is associated with dental development (Enlow, 1990). The anterior portion of the chimpanzee mandible remains narrow across postnatal development, whereas both the deciduous and the permanent anterior teeth are large (Aiello & Dean, 1990; Dean & Beynon, 1991). Once the alveolar process has elongated enough via growth of the posterior region of the corpus, the incisors and the canines can come into occlusion (Aiello & Dean, 1990). The order of emergence of dc and dm2 differs between the species: dc emerges after dm2 in chimpanzees but not in humans (Robinow et al. 1942; Nissen & Riesen, 1945; Meredith, 1946). Thus, one can ask if the anterior region of the corpus has sufficient room for dc to come into occlusion once the deciduous incisors and molars have emerged. If not, do the deciduous molars move posteriorly along with the posterior elongation of the alveolar process, and thus create space for the dc emergence?
The functional loadings transmitted by the muscles to bone tissue participate in the regulation of the activity of the bone cells by mechanisms such as mechanotransduction (for review see Moss, 1997a,b;). Experiments on rats have demonstrated that resection of the suprahyoid muscles (digastrics and mylohyoid), binding the lingual side of the basilar region of the symphysis with the hyoid bone, substantially reduce the forward growth of the symphysis (Spyropoulos et al. 2002). During growth in humans and chimpanzees, the position of the hyoid bone relative to the mental region changes along the anteroposterior and superoinferior axes (Negus, 1949; King, 1952; Lieberman & Crelin, 1971; Falk, 1975), and the spatial orientation of the muscle vectors moves accordingly. Taking into consideration that reorientation of muscle vectors is also a factor controlling growth (Hohl, 1983; van Spronsen et al. 1997), there might be covariation between the repositioning of the hyoid bone during ontogeny and the anterior growth and form of the basilar region of the symphysis. No empirical data on such a correlation appear to exist.
Aim of the study
This study explores the mandibular shape changes of humans and chimpanzees (Pan troglodytes) from fetal life to the complete emergence of the deciduous dentition. Using geometric morphometric methods, we aim to visualize how these two species develop a vertical symphysis during fetal life and how this configuration changes towards a prominent chin in humans but towards an anteriorly inclined symphysis in chimpanzees. We incorporate deciduous tooth positions and orientations in this geometric framework over the same growth interval to visualize how space is created within the jaw while the mineralizing teeth are erupting into the dental arch. In our study, there is no particular hypothesis to test. Instead, we search for complex phenotypic interactions to detect shared and divergent developmental patterns in these two species, a subject which until now has remained obscure in the literature. In our discussion, we will consider how findings of the present study alter the understanding of Bolk's comments of long ago.
Material and methods
CT-scanned sample
The postnatal human sample comprises computed tomography (CT) scans of 58 humans (28 females and 30 males) of mixed ethnicity from France (Table 1). This sample ranges from birth to the near-full emergence of M1. The CT scans were provided by the Pellegrin Hospital (Bordeaux; n = 35), the Necker Hospital (Paris; n = 6), and the Clinique Pasteur (Toulouse; n = 17). Pixel size ranges from 0.23 to 0.66 mm and slice thickness from 0.30 to 0.70 mm. These individuals had been referred for cranial trauma, inflammation of maxillary sinuses or neonatal distress but were found to be free of reportable abnormalities. The CT scans were anonymized by the medical institutes, except for information about age and gender. The use of these data for our present purpose was approved by French institutional boards.
Table 1.
Human and chimpanzee sample. DS1: dental stage 1. DS2: dental stage 2. BPD: biparietal diameter. The fetuses are listed with their reference number
| Specimens | DS1 | DS2 | BPD (mm) | Gestational age (weeks) |
|---|---|---|---|---|
| Postnatal specimens | ||||
| Modern humans | 32 | 26 | ||
| Chimpanzees | 4 | 5 | ||
| Fetuses | ||||
| Modern humans (Mh) | ||||
| Mh01 | 84.5 | 33 | ||
| Mh02 | 78.0 | 30 | ||
| Mh03 | 77.4 | 29 | ||
| Mh04 | 74.3 | 28 | ||
| Mh05 | 75.0 | 28 | ||
| Mh06 | 68.5 | 27 | ||
| Mh07 | 43.6 | 19 | ||
| Mh08 | 26.4 | 13 | ||
| Chimpanzees (Ch) | ||||
| Ch01 | 78.9 | neonate | ||
| Ch02 | 72.0 | neonate | ||
| Ch03 | 69.2 | 31 | ||
| Ch04 | 68.1 | 29 | ||
| Ch05 | 64.8 | 27 | ||
| Ch06 | 61.0 | 25 | ||
| Ch07 | 44.9 | 18 | ||
The chimpanzee sample included seven CT-scanned juvenile Pan troglodytes gathered from the Senckenberg Museum of Frankfurt (Germany; n = 2), the Department of Anthropology at the University of Vienna (Austria; n = 1), and the Royal Museum of Central Africa of Tervuren (Belgium; n = 4). Pixel size ranges from 0.15 to 0.40 mm and slice thickness from 0.33 to 0.80 mm. None of the specimens has a fully erupted M1.
Because no precise information on age at death was available for the chimpanzee specimens, we assigned the chimpanzee mandibles to one of two dental stages: DS1, prior to the full emergence of the dc, and DS2, after the full emergence of dc and prior to the full emergence of M1. The human sample was split into similar dental stages: DS1, prior to the full emergence of dm2; and DS2, after the full emergence of dm2 and prior to the full emergence of M1. Although the emergence of a different tooth in each species was used as a stage boundary, we considered these stages equivalent because (i) sequence of emergence is different between the species (Robinow et al. 1942; Nissen & Riesen, 1945; Meredith, 1946), (ii) both species have accomplished approximately one-third of their neural growth at the time of the emergence of dc in chimpanzees and dm2 in humans (Lieberman & McCarthy, 1999), and (iii) a bone reversal remodeling of the buccal surface of the symphysis begins at the transition between DS1 and DS2 (Johnson et al. 1976; Kurihara et al. 1980).
As we focused on early developmental characteristics of mandibular growth, our human sample also included CT-scanned fetal specimens (n = 8) provided by the Musée de l’Homme (Paris). Their sex is unknown. Pixel size ranges from 0.18 to 0.25 mm and slice thickness is 0.25 mm. The age of these fetuses was documented either in units of gestational weeks (g.w.) or gestational months. To homogenize the information across the species, we measured each individual's biparietal diameter (BPD) from the CT scans and assigned the equivalent age in gestational weeks by the formula of Chitty et al. (1994). Thus, the ages of the human fetuses were estimated to range from g.w. 13 to g.w. 33.
The chimpanzee sample was augmented by seven CT-scanned formaldehyde-fixed Pan troglodytes fetuses, provided by the Laboratoire d’Anatomie Comparée of the Musée National d’Histoire Naturelle (Paris). Pixel size ranges from 0.20 to 0.29 mm and slice thickness is 1 mm. The age and sex of these specimens were also unknown. We measured BPD and estimated ages in g.w. by the formula of Bourry et al. (2006). Five specimens ranged from about g.w. 18 to 31, while two specimens were identified as having actually been liveborn and thus reassigned to DS1.
Reconstruction of the mandibular surfaces
The half-maximum height protocol (Spoor et al. 1993) was used to reconstruct each mandibular surface from the CT scans using the software package amira 5.2 (Mercury Computer Systems, Chelmsford, MA, USA). This protocol samples the Hounsfield values on either side of the transition between two adjacent tissues and takes the value halfway between them as the threshold value. The youngest specimens had areas with different mineralization levels, requiring local adjustments of the threshold value. The reconstructed mandibular halves of the youngest specimens that showed incomplete ossification of the symphysis were fused virtually by cubic interpolation of the surface from each side of the symphyseal cartilage (amira 5.2).
Landmarks and semi-landmarks of the mandibular surfaces
Using the open-source software edgewarp3d (Bookstein & Green, 2002), a 3D-template of 415 landmarks and semilandmarks created to capture the mandibular surface, was warped onto each mandible. Figure 3 and Table 2 present the template on the right hemimandible. It corresponds to a human, aged 1 year.
Fig. 3.
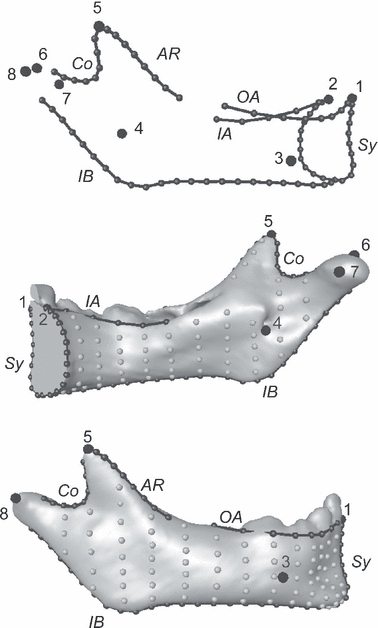
Mandibular template, right hemimandible. Top: landmarks (big black dots) and curve semilandmarks (black dots and lines). Middle and bottom: right hemimandible of a specimen aged about 1 year with landmarks, curve semilandmarks, and surface semi-landmarks (gray dots). Names of the landmarks and curves are as in Table 2.
Table 2.
List of landmarks and curve semi-landmarks shown in Fig. 3
| Landmarks and curves | Label in Fig. 3 |
|---|---|
| Landmark points | |
| Infradentale | 1 |
| Linguale | 2 |
| Right mental foramina | 3 |
| Right mandibular foramina | 4 |
| Tip of the right coronoid | 5 |
| Top of the right condyle | 6 |
| Medial extremity of the right condyle | 7 |
| Lateral extremity of the right condyle | 8 |
| Landmark curves | |
| Midsymphysis | Sy |
| Right outer alveolar | OA |
| Right inner alveolar | IA |
| Right anterior ramus | AR |
| Right coronoid | Co |
| Right inferior border | IB |
After digitization, semilandmarks were allowed to slide along curves and surfaces to minimize the bending energy of the thin-plate spline interpolation function computed between each specimen and the sample Procrustes average (Bookstein, 1997; Gunz et al. 2005).
After sliding, landmarks and semilandmarks were treated as homologous points and converted to shape coordinates by generalized Procrustes analysis (GPA: Rohlf & Slice, 1990; Bookstein, 1991). This involves translating, rescaling, and rotating the configurations relative to each other so as to minimize the overall sum of squared distances between corresponding (semi)landmarks. The rescaling adjusts the landmark coordinates so that each configuration has a unit centroid size [CS: square root of the summed squared Euclidean distances from all (semi)landmarks to their centroid; Dryden & Mardia, 1998].
Analyses of the mandibular surfaces
Principal component analysis (PCA) of the matrix of shape coordinates augmented by a column of the natural logarithm of centroid size (LnCS) – corresponding to PCA in form space (Mitteroecker et al. 2004; Mitteroecker & Bookstein, 2005) – was carried out on the whole sample of mandibular surfaces. Form-space principal component 1 (PC1) was highly related to size as LnCS had by far the largest loading, 0.992, on this component. PC1 thus represents a ‘growth axis’.
To describe size-related shape variations throughout growth, we constructed sequences of mandibular surfaces in each species. We first estimated several sets of mandibular coordinates within species via quadratic regression of the Procrustes shape coordinates (one by one) upon LnCS. The mandibular surfaces corresponding to the regression estimates were computed using the triangulated surfaces of human and chimpanzee mean shapes and the thin-plate spline as an interpolation function (TPS: Bookstein, 1991).
Analysis of the deciduous dental configuration
In addition to the statistical analysis of the mandibular surfaces, we visualized the deciduous dental configurations, as their positions could be either causes or consequences (or both) of changes in mandibular form. The deciduous teeth were reconstructed using the half-maximum height protocol (Spoor et al. 1993) for nine specimens: three fetuses (one human, two chimps), four specimens at DS1 (two humans, two chimps) and two specimens at DS2 (one human, one chimp). In each group, the specimens were selected as those at the smallest Procrustes form distances from the (semi)landmark configurations calculated by the quadratic regression. The intra-species variability of tooth position and orientation throughout growth is not presented in this paper owing to the differential preservation of the teeth of the dried vs. non-dried specimens.
To obtain the position of the developing teeth, we digitized cusp tip(s) and the deepest point of di1, di2, dc, dm1, and dm2 alveolar chambers (there are no roots developed in the first stages) using a plane parallel to the inferior border. To analyze the dental configurations along with allometric shape changes, we used the TPS interpolation function to warp the dental coordinates of each specimen into the space of each Procrustes configuration on the regression curves.
Results
Form-space PCA
The form-space PCA is summarized in Fig. 4. The ontogenetic trajectories between humans and chimpanzees already fail to overlap even at late fetal stages, and the divergence increases steadily from birth onwards. This result is in line with previous findings on the cranium (Cobb & O’Higgins, 2004; Mitteroecker et al. 2004; Strand Vidarsdottir & Cobb, 2004). PC1 (94.5% of the total Procrustes form variance) expresses overall size increase as well as allometric shape changes, while PC2 (3.2% of the total form variance) separates the species. This second axis depicts some of the typical morphological differences between the species: the anterior corpus of the chimpanzee is longer and without a chin, relatively narrower anteriorly, and the condyles are relatively closer to each other than in the human mandible. PC3 accounted for only 0.5% of the total variance and hence is not visualized by surfaces here.
Fig. 4.
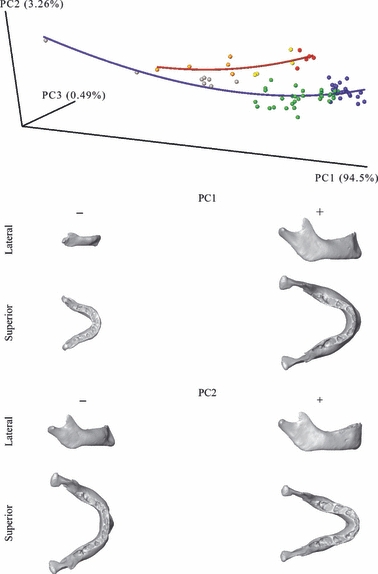
Form-space principal component analysis. The first three components account for 98% of the total form-space variance. Surfaces correspond to the shapes represented by negative and positive extremes of PC1 and PC2. Line: blue, human ontogenetic trajectory of mandibular growth; red, chimpanzee ontogenetic trajectory of mandibular growth. Spheres: gray, human fetuses; green, humans at DS1; blue, humans at DS2; orange, chimpanzee fetuses; yellow, chimpanzees at DS1; red, chimpanzees at DS2.
We illustrate the ontogenetic shape changes of the whole mandibles and the midsagittal symphyseal sections in Figs 5–7. These shapes are estimates from the separate regressions species by species, scaled to CS = 1.0 in order to focus on the shape changes over growth. As supplementary information, we provide videos to help the reader to visualize the 3D shape changes of the mandibular surface from stage to stage.
Fig. 5.
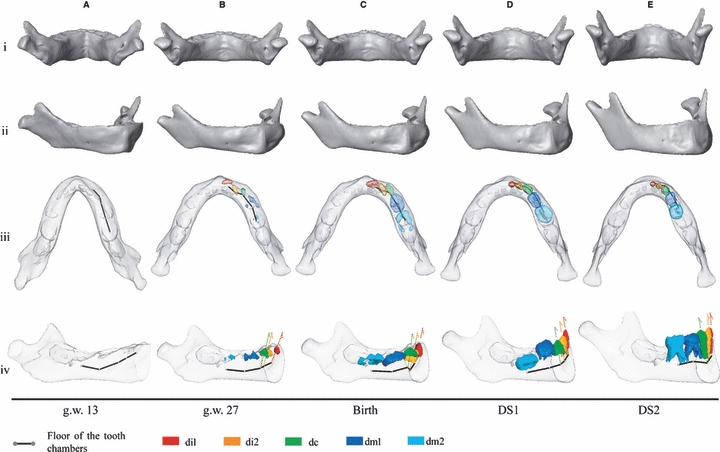
Morphs of the human mandibular surfaces from g.w. 13 to DS2. According to the regression in Figure 3, these are scaled to constant overall mandibular centroid size. Translucent surfaces let us see the position and orientation of deciduous teeth in selected specimens. Arrows: tooth orientations. This analysis does not include landmarks on the tooth alveolar chambers (translucent) and therefore the surface morphs do not take their changes into account.
Fig. 7.
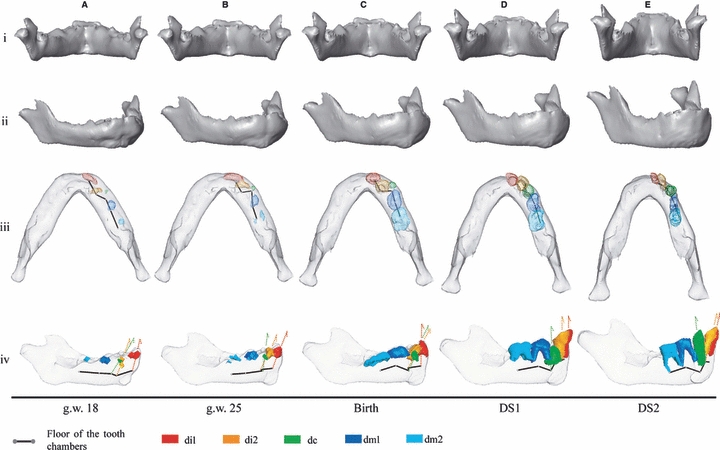
Morphs of the chimpanzee mandibular surfaces from g.w. 18 to DS2. According to the regression in Fig. 3, these are scaled to constant overall mandibular centroid size. Translucent surfaces enable visualization of the position and orientation of deciduous teeth in selected specimens. Arrows: tooth orientations. This analysis does not include landmarks on the tooth alveolar chambers (translucent) and therefore the surface morphs do not take their changes into account.
Humans
At g.w. 13, the human mandible is V-shaped (Fig. 5A.iii) and the rami run obliquely from medial (gonial angle) to lateral (condyle) (Fig. 5A.i). The symphysis is vertically oriented from g.w. 13 to birth (Fig. 5A.iv).
During the 2nd trimester (g.w. 13–27), the basilar bone of the mandible maintains a V-shape, while its alveolar process changes to a shallow U-shape (Fig. 5A.iii–B.iii, see supporting information Video S1–S3). Simultaneously the configuration of the tooth chambers changes to a curved line with a flexion at dc (Fig. 5A.iv–B.iv). The rami become more vertical (Fig. 5A.i–B.i) and the gonial angles become more prominent and project downwards (Fig. 5A.iv–B.iv). The chin and the mental fossae simultaneously emerge in this stage. The chin has an inverted T-shape as described by Schwartz & Tattersall (2000) (Fig. 5A.ii–B.ii) but each side of the vertical bar is still directed backwards (Fig. 5A.iii–B.iii). The mental fossae develop beneath the floor of the anterior tooth chambers (Fig. 5A.iv–B.iv).
During the 3rd trimester (g.w. 27 to birth) the shallow U-shape of the alveolar process and the V-shape of the basilar bone persist despite a slight widening at the basal symphysis (see Supporting Information Video S1–S3). The rami rotate forward (Fig. 5B.iv–C.iv). From g.w. 13 to birth, Fig. 6A illustrates a thickening as well as an anterior shift of the basal symphysis relative to the incisor alveolar process. The posterior profile of the basal symphysis – where the digastric and the geniohyoid muscles insert – is flat, but the anterior surface is rounded.
Fig. 6.
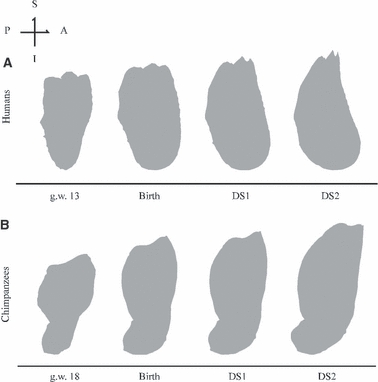
Human and chimpanzee symphysis in midsagittal plane section according to regression in Fig. 3 and scale as in Figs 5 and 7. A, anterior (labial); P, posterior (lingual); I, inferior; S, superior.
From birth onwards, the symphysis inclines backwards, and the chin becomes more prominent at the transition between DS1 and DS2 (Fig. 5C.iv–E.iv). The mandible changes to a well-rounded, wide U-shape. The lower border turns outward relative to the alveolar process, and at the symphysis, each side of the T keel projects forward (Fig. 5C.iii–E.iii, see Supplementary Information Video S4) and the mental fossae become less concave (Fig. 5C.ii–E.ii). As Fig. 6A highlights, the prominence of the chin is associated with the forward shift and the thickening of the basal symphysis relative to the alveolar process when the incisors erupt. This leads to a shallow depression, called an ‘incurvatio mandibularis’ (Hublin & Tillier, 1981), below the alveolar process. Simultaneously, the profile of the basal symphysis becomes evenly rounded from the lingual side to the labial side, showing how the forward translation of the basal symphysis is combined with a lingual expansion of the area where the digastric and mylohyoid muscles insert. Simultaneously, the anteroposterior width of the basal symphysis increases (Fig. 6A). The orientation of di1, di2, and dc changes to a vertical orientation that corresponds to the actual axis of eruption and later functional position (Fig. 5C.iv–E.iv).
Chimpanzees
At g.w. 18 the chimpanzee mandible has a V-shape (Fig. 7A.iii) with a prominent gonial angle projecting inferiorly (Fig. 7A.iv). The incisor alveolar process is protrusive and large anteroposteriorly relative to the basilar region; the symphysis is thus inclined anteriorly (Figs 6B and 7A.iv). The inferior border of this area is located slightly above the inferior border of the lateral corpus. Two bilateral anterior buccal grooves delineate a bulbous mental region (Fig. 7A.ii). The anterior buccal grooves are located beneath the floor of the anterior tooth chambers as in humans (Fig. 7A.iv).
From g.w. 18 to birth, the chimpanzee mandible maintains a V-shape but the rami move relatively nearer to each other (Fig. 7A.i–C.i, A.iii–C.iii, see Supporting Information Video S5–S7). The bilateral anterior buccal grooves disappear simultaneously with the forward shift of the inferior border underneath the central incisors (Fig. 7A.iv–C.iv) and the emergence of both a symphyseal tuber (downward expansion of the bone at the basilar region of the symphysis) at the labial side (Fig. 7A.ii–C.ii) and the simian shelf at the lingual side of the inferior border (Fig. 6B). In addition to these coordinated events, the di1 reorients as the basal symphysis shifts anteriorly. At birth, the midsagittal tip of the simian shelf aligns with the inferior border of the lateral corpus (Fig. 7A.iv–C.iv). The deepest points of the deciduous tooth chambers are arranged in a Z-shape along the length of the tooth row (Fig. 7A.iii–C.iii). In addition to the mesial displacement of the molars, the relatively advanced mineralization of di2 and dm1 drastically reduces the horizontal space into which the dc elongates its crown, and whereas the alveolar process does not lengthen in the anteroposterior direction, it does slightly widen mesiodistally (Fig. 7A.iii–C.iii). At birth, the symphysis is oriented vertically as in humans (Fig. 7C.iv).
From birth onwards, while the ramal length increases along the superoposterior direction, the chimp mandibular corpus elongates along its anteroposterior axis (Fig. 7C.iv–E.iv), and the relative mesiodistal width of the symphysis narrows (Fig. 7C.iii–E.iii). From birth to DS1, the midsagittal symphysis maintains its vertical configuration, the incisors emerge upwards (Fig. 7C.iii–D.iii), and the symphyseal tuber attenuates (Fig. 7C.ii–D.ii). From DS1 to DS2, the symphysis inclines anteriorly, the symphyseal tuber disappears, and the simian shelf drifts posteriorly (Figs 6B and 7D.iv–E.iv). Moreover, the midsagittal tip of the simian shelf is now above the inferior border of the lateral corpus (Fig. 7D.iv–E.iv, see Supporting Information Video S8). The dc emerges simultaneously. By DS2, the chimpanzee mandible has reached a shape very similar to the adult ‘squared’ U-shape with a relatively narrow anterior corpus and the condyles relatively close together (Fig. 7E.i,iii).
Discussion
We have described the major ontogenetic shape changes undergone by the mandibular surface relative to the developing deciduous teeth in humans and chimpanzees. We showed that the human symphysis remains vertical across fetal stages until birth, while it reorients from upward-forward to vertical in chimpanzees. Humans, like chimpanzees, have a V-shaped mandible during the earlier fetal stages (Figs 5A.iii and 7A.iii). The profound reshaping of the human mandible during the 2nd trimester includes apposition of a shallow U-shaped alveolar process to a V-shaped basilar bone and formation of the mental fossae (anterior buccal grooves) and the inverted T-relief of the mental region (Fig. 5). In contrast to humans, the chimpanzee mandible maintains its V-shape during fetal development, and the basal symphysis modifies to simultaneously form the simian shelf and a symphyseal tuber (Fig. 7). After birth, the adult final configurations – the anteriorly inclined symphysis of the chimpanzee (Fig. 2B) and the prominent mental region of the modern human (Fig. 2C) – are established prior to the emergence of M1, like the upper midface (Ackermann & Krovitz, 2002; Bulygina et al. 2006). To summarize our findings, Fig. 8 shows the period of tilting of the modern human and chimpanzee symphysis; this contrasts with Bolk's (1924) comments illustrated in Fig. 2.
Fig. 8.
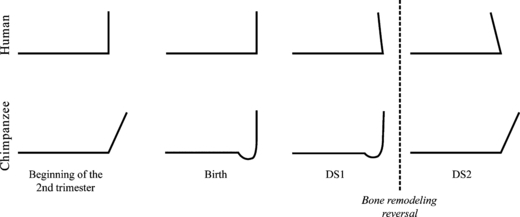
Summary of the findings to contrast with Bolk's (1924) comments in Fig. 2. Black line: lateral profile of the anterior corpus. Arrows of the forward growth at the alveolar process and at the basilar region are not represented, as this study does not have data on growth rate.
Our analysis shows that modern humans and chimpanzees share a complex combination of phenotypic changes that occur at different times: at least from g.w. 17 to DS1 in chimpanzees and from birth to DS2 in modern humans. Those phenotypic changes occur at the symphysis and simultaneously include (Figs 5–7): (i) the forward shift of the basal symphysis associated with the emergence of a symphyseal tuber and a prominent chin at the labial side in chimpanzees and humans, respectively; (ii) the anterior buccal grooves (mental fossae in humans) that become less concave in both species; (iii) the backwards expansion of the surface at the lingual side leading to the simian shelf in chimpanzees and contributing to an evenly rounded symphyseal profile in humans; and (iv) the reorientation of the incisors before eruption in both species.
The 3D visualization of the V-shaped mandible in the earlier fetal stages of the two species (Figs 5A.iii and 7A.iii) is consistent with previous observations on the ossifying V-shaped Meckel's cartilage during embryonic development (Radlanski et al. 1994, 2003). Blechschmidt (1973, 2004) associates the downward projection of the gonial angles (Fig. 5A.iv–C.iv), which in our study derive from the reorientation of the rami to a vertical position (Fig. 5.A.i–C.i), with the descent of the hyoid bone into the neck that starts at the beginning of the second trimester. Our results also agree with Schwartz & Tattersall's (2000) observation that the mental fossae and the inverted T-chin appear during the 2nd trimester of human gestation (Fig. 5A.ii–B.ii). Earlier, Arensburg et al. (1989) likewise observed that the mental fossae become less concave during human postnatal ontogeny (Fig. 5C.ii–E.ii).
Overall, the symphyseal shape changes seem consistent with the bone remodeling patterns on the labial side of the symphysis, as observed in other studies (Johnson et al. 1976; Kurihara et al. 1980; Enlow, 1990). The posterior drift of the chimpanzee mental region as well as the loss of the symphyseal tuber at the transition between DS1 and DS2 (Figs 7D.iv–E.iv and 8) are consistent with bone remodeling reversal at the time of the emergence of dc, the labial side becoming resorptive (Johnson et al. 1976). In humans, the mental region becomes more prominent after the transition between DS1 and DS2 (Figs 5D.iv–E.iv and 8), in coordination with bone remodeling reversal at the incisor alveolar process (Kurihara et al. 1980; Enlow, 1990). However, these morphological changes occur gradually from birth – the symphyseal tuber in chimpanzees and the chin in modern humans attenuate or project, respectively, throughout DS1 (Fig. 8). Differential rates of bone deposition at the labial side may exist between the mental region and the alveolar process before the initiation of the remodeling reversal. Bone remodeling is nonlinearly related to tissue stress stimulus (Beaupré et al. 1990). Bone deposition in the incisor alveolar region (humans) and the basilar region (chimpanzees) respectively may decrease along with the intensity of the stimulus before the areas become resorptive.
As mentioned earlier, Bolk (1924) assumed that the forward inclination of the chimpanzee symphysis is related to the development of the permanent molars that emerge while the deciduous dentition is still in occlusion. But we have found that the forward inclination of the chimpanzee symphysis occurs earlier, simultaneously with the emergence of dc (Fig. 7D.iv–E.iv). After birth, the mesial displacement of the deciduous molars and the relatively advanced development of di2 and dm1 reduce the space available for dc (Fig. 7C.iv–D.iv), and the symphysis remains narrow mediolaterally, as reported by Aiello & Dean (1990). Owing to growth in the posterior mandibular corpus, it has been assumed that only when the alveolar process has sufficiently elongated is there room for the incisors and canines to come into occlusion (Aiello & Dean, 1990). With respect to the deciduous dentition, Fig. 7D.iv clearly shows that the lower third of the dc crown has insufficient room between di2 and dm1 to reach the alveolar ridge if the symphysis is vertically oriented, even if the corpus elongates backwards. The anterior inclination of the symphysis elongates the alveolar process as well, in particular between the distal side of di2 and the mesial side of dm1 as the incisors incline, too (Fig. 7E.iv), which could be a key event helping dc to erupt into its proper position in the dental arch.
At g.w. 18 in chimpanzees, the prominence of the incisor alveolar process relative to the inferior border is apparently associated with the way the deciduous incisors are packed in the alveolar bone, di2 developing behind di1 (Figs 6B and 7A.iii–iv). During fetal development, the displacement of the inferior border underneath the incisor alveolar process combines with the downward and forward development of the simian shelf. At birth, the midsagittal tip of the simian shelf aligns with the inferior border of lateral corpus. During postnatal ontogeny, while the symphysis inclines anteriorly, the simian shelf develops backwards and upwards so that the midsagittal tip of the simian shelf lies above the inferior border of lateral corpus at DS2 (Fig. 7B.iv–E.iv). Because the development of this feature is associated with the suprahyoid muscles that insert upon it, the repositioning of the simian shelf suggests a significant influence of these muscles on the anterior growth of the symphysis; this has also been seen in experimental studies (Spyropoulos et al. 2002). In addition, the spatial orientation of the muscle force also influences growth (Hohl, 1983; van Spronsen et al. 1997), and so the reorientation of the muscles, linked to the relocation of the hyoid bone relative to the inferior border of the mandible, correlates with the reshaping of the symphysis. In neonate chimpanzees, the hyo-laryngeal structures lie at their adult level relative to the cervical vertebrae (Negus, 1949; Nishimura, 2006). In stillborn specimens, Falk (1975) showed that the hyoid bone is located underneath the inferior border, the body lying anterior to the gonial angles. In this position, the suprahyoid muscles pull the simian shelf slightly downward. After birth, as the mandible grows, the hyoid bone aligns with the inferior border of the mandible but more posteriorly than at birth, in the vicinity of the gonial angles (Falk, 1975). Postnatally, the suprahyoid muscles exert a less downward force on the simian shelf than at birth. Therefore, during ontogeny the reorientation of the suprahyoid muscle is consistent with the superoinferior repositioning of the simian shelf.
As noted earlier, the development of the vertical symphysis in chimpanzees combines phenotypic changes at the lingual and labial sides shared with modern humans during early postnatal life. A comparison of the way in which the hyo-laryngeal components are organized relative to the basilar bone of the mandible in both modern humans and chimpanzees could provide useful insights into the anteroposterior positioning of the mental region. From birth onwards in humans, the position of the hyoid bone relative to the basilar region of the symphysis changes while the mandible grows and the hyoid and larynx descend to the adult level relative to the cervical vertebrae at about 2 years of age (Negus, 1949; Carlsöö & Leijon, 1960; Roche & Barkla, 1965; Westhorpe, 1987). At birth, the hyoid body is located slightly above the basal symphysis and directly below the gonial angles. During postnatal ontogeny, the hyoid body repositions farther forward relative to the gonial angles and drops below the basal symphysis (King, 1952; Lieberman & Crelin, 1971). In neonate chimpanzees, Falk (1975) observed that the position of the hyoid bone is anterior to the gonial angles, as in humans after 2 years. In both species, the forward and downward positioning of the hyoid bone relative to the inferior border of the symphysis seems to be coordinated with the vertical orientation of the symphysis in chimpanzees and the posterior inclination of the symphysis in modern humans. In comparison with modern humans, the ethmomaxillary complex of chimpanzees develops much farther forward relative to the cranial base during postnatal ontogeny. According to the counterpart principle (Enlow, 1990), the anterior corpus would follow this displacement, especially its alveolar process, to afford occlusion during emergence of the teeth. As the hyoid bone repositions farther backward relative to the symphysis, the mental region may develop less forwardly than the incisor alveolar process. The combination of those two events, in coordination with the emergence of dc, might be associated with the forward inclination of the chimpanzee symphysis. This merits further exploration.
Why humans have a protruding mental region is still discussed in relation to the mechanical effect of reduction of the teeth (Hrdlicka, 1911; Riesenfeld, 1969), the articulation of speech (Walkhoff, 1904; DuBrul & Sicher, 1954; Ichim et al. 2007), and the effects of masticatory stresses (Howells, 1959; White, 1977; Daegling, 1993; Dobson & Trinkaus, 2002; Ichim et al. 2006). Biomechanical studies show that the mental region of the adult modern human has a minor effect on the functional aspects of mastication (Dobson & Trinkaus, 2002; Ichim et al. 2006). There is a general view that evolution of humans led to a shift in functional demands that allowed the reduction of the dental arcade, along with reduced tooth size, and called at the same time for other morphological changes, for instance the shortening of the mandible and the protrusion of the mental region (Hrdlicka, 1911; Riesenfeld, 1969). The shortening of the human lower jaw is concomitant with a positioning of the face beneath the anterior braincase and the associated reduction of the anteroposterior dimension of the vocal tract (Lieberman et al. 2000; McCarthy & Lieberman, 2001). In contrast, the relative size of the human tongue does not seem to have reduced (Negus, 1949). During the first 2 years of life, the tongue changes from a long and flat (ape-like) shape to a rounded shape (Negus, 1949; Lieberman, 1984). Concomitantly, the hyoid bone displaces into the neck, along a downward-forward direction (King, 1952), so that the position and orientation of the muscles of the floor of the mouth change relative to the basilar region of the symphysis. The forward displacement of the mental region may provide space for those muscles, contributing to a spatial packing that preserves the airways from obstruction. This hypothesis is also in line with that of DuBrul & Sicher (1954), who proposed that the forward displacement of the mental region accommodates the lack of space for the tongue. The extent to which growth and repositioning of the tongue and suprahyoid muscles contribute to the reshaping of the primate symphysis requires further study. Recently, Ichim et al. (2007) found that the limits of strain concentrations produced by the oblique contractions of the genioglossus muscle during speech outline the inverted T-relief over the labial surface of an adult prominent mental region. The ability to speak is established by the way the tongue is packed relative to the larynx and by the horizontal dimension of the oral cavity (Negus, 1949; Lieberman, 1984, Lieberman et al. 2001). We believe that investigation of the spatial packing of the supralaryngeal structures is important if we aim to comprehend the orientation of the symphysis and the development of the morphological traits at the mental region of primates.
During fetal ontogeny and in contrast to humans, the shape change of the chimpanzee mandible does not show an apposition of a shallow U-shaped alveolar process to a V-shaped basilar bone. Instead, the chimpanzee alveolar process maintains a V-shape and the central incisors occupy the most anterior part of the alveolar process (Fig. 7A.iii–C.iii). To assess the effect of the anterior tooth size on the shape of the mandible, Riesenfeld (1969) extracted the central incisors from the lower jaw of 10-day-old rats and observed that the V-shaped alveolar process became parabolic during growth. He concluded that reduction of anterior tooth size during evolution may have led to such transitions from V-shaped to U-shaped. If the anterior buccal grooves and the inverted T-relief of the mental region result merely from the apposition of a shallow U-shaped alveolar process to a V-shaped basilar bone, there is no reason to think that this is restricted to modern human fetuses and neonates. For instance, the size of the deciduous anterior teeth in Neanderthals is slightly larger than those of the contemporary modern human (Tillier, 1979; Bailey & Hublin, 2006), and in infant Neanderthals such as the 10-month-old Amud 7, the alveolar process has a shallow U-shape and the symphysis is vertical before the emergence of the deciduous teeth (Rak et al. 1994; Schwartz & Tattersall, 2000, 2002). The symphysis of Amud 7 has no anterior buccal grooves or inverted T-relief, and the labial surface is smooth from side to side across the midline (Schwartz & Tattersall, 2000). However, the mandibles of Neanderthal neonates such as Le Moustier 2 (Maureille, 2002) or Mezmaiskaya (Golovanova et al. 1999; Ponce de León et al. 2008) have not been analyzed yet to reject the hypothesis that during fetal development a Neanderthal could develop a mental region similar to that of modern human fetuses with both anterior buccal grooves and an inverted T-shaped relief.
Conclusions
Our data highlight the developmental changes of the mandible and deciduous dentition from fetal ontogeny through early childhood of modern humans and Pan troglodytes. The development of a vertical symphysis is coordinated with the reorientation of the anterior teeth for a vertical emergence, and the postnatal anterior inclination of the symphysis with the emergence of the deciduous canine. In chimpanzees, the repositioning of the simian shelf during ontogeny is evidence of the developmental relationship between the supra-hyoid muscles and the symphysis. An equivalent combination of morphological changes exists between chimpanzees and modern humans with respect to the reshaping of the symphysis: this may be evidence of complex phenotypic interactions between the suprahyoid muscles, the symphyseal bone and the anterior teeth in both species. Further investigations focusing on the biomechanical and developmental relationships between those units would help our understanding of the evolutionary shape change of the human symphysis.
Acknowledgments
Research was supported by the EU FP6 Marie Curie Actions MRTN-CT-2005-019564 (EVAN; http://www.evan.at). We thank J. Treil (Clinique Pasteur, Toulouse), F. Brunelle, N. Boddaert, J.-M. Debaets, C. Leroy and D. Gustave (AP-HP Necker, Paris), V. Dousset, C. Douws, C. Thibaut and E. Gatuing (C.H.U. Pellegrin, Bordeaux), P. Mennecier, A. Fort, V. Laborde (Musée de l’Homme, Paris), O. Kullmer (Senckenberg Museum, Frankfurt), and the Laboratoire d’Anatomie Comparée du Musée National d’Histoire Naturelle (Paris) for access to their CT-datasets and collections. We are grateful to M. Bastir, M. Frelat, P. Gunz, K. Kupcik, P. Mitteröcker, S. Senck, A. Stadlmayr and B. Viola for discussions. We also thank G. Morris-Kay, Dan Lieberman and the two anonymous reviewers for their helpful comments on an earlier draft of this manuscript. We thank A. Lassere and A. G. Drake for editing the English language.
Author's contribution
M. Coquerelle designed the study, provided CT-scans, digitization, data analysis, data interpretation, drafting of the manuscript. F. L. Bookstein was responsible for checking of the statistical analysis, critical revision of the manuscript and approval of the article. J. Braga provided CT-scans, interpretation of data, critical revision of the manuscript and approval of the article. D. J. Halazonetis provided interpretation of data, critical revision of the manuscript and approval of the article. G. W. Weber designed the study, provided interpretation of data, critical revision of the manuscript and approval of the article.
Supporting information
Additional Supporting Information may be found in the online version of this article: They are movies (MPEG file format), creating in the software AMIRA. The surface morph is computed by interpolating the shape estimates from the regression analyses.
Video S1. Mandibular shape change in modern human from g.w. 13 to birth, right side.
Video S2. Mandibular shape change in modern human from g.w. 13 to birth, inferior view.
Video S3. Mandibular shape change in modern human from g.w. 13 to birth, superior view.
Video S4. Mandibular shape change in modern human from birth to DS2, anterolateral view.
Video S5. Mandibular shape change in chimpanzee from g.w. 18 to birth, right side.
Video S6. Mandibular shape change in chimpanzee from g.w. 18 to birth, inferior view.
Video S7. Mandibular shape change in chimpanzee from g.w. 18 to birth, superior view.
Video S8. Mandibular shape change in chimpanzee from birth to DS2, anterolateral view.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Ackermann RR, Krovitz GE. Common patterns of facial ontogeny in the hominid lineage. Anat Rec (New Anat) 2002;269:142–147. doi: 10.1002/ar.10119. [DOI] [PubMed] [Google Scholar]
- Aiello L, Dean C. An introduction to human evolutionary anatomy. New York: Academic Press; 1990. [Google Scholar]
- Arensburg B, Kaffe I, Littner MM. The anterior buccal mandibular depressions: ontogeny and phylogeny. Am J Phys Anthropol. 1989;78:431–437. doi: 10.1002/ajpa.1330780311. [DOI] [PubMed] [Google Scholar]
- Bailey SE, Hublin J-J. Dental remains from the Grotte du Renne at Arcy-sur-Cure (Yvonne) J Hum Evol. 2006;50:485–508. doi: 10.1016/j.jhevol.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Beaupré GS, Orr TE, Carter DR. An approach for time-dependent bone modeling and remodeling – Theorical development. J Orthop Res. 1990;8:651–661. doi: 10.1002/jor.1100080506. [DOI] [PubMed] [Google Scholar]
- Blechschmidt E. Die Entstehung des Unterkiefers Eine funktionelIe Differenzierung im 1. Fertilisationsmonat. Fortschr Kieferorthop. 1973;34:337–358. [Google Scholar]
- Blechschmidt E. The ontogenetic basis of human anatomy: the biodynamic approach to development from conception to birth. Berkeley, CA: North Atlantic Books; 2004. [Google Scholar]
- Bolk L. Die Entstehung des Menschen Kinnes, ein Beitrag zur Entwicklungsgeschichete des Unterkiefers. Tweede sectie 25 (5) Amsterdam: Koninklijke Akademie; 1924. [Google Scholar]
- Bookstein FL. Morphometric tools for landmark data: geometry ad biology. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Bookstein FL. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal. 1997;1:225–243. doi: 10.1016/s1361-8415(97)85012-8. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Green WDK. 2002. Users Manual, EWSH3.19. ftp://brainmap.med.umich.edu/pub/ewsh.3.19.manual.
- Bourry O, Ouwe-Missi-Oukem-Boyer O, Anne Blanchard A, et al. Fetal ultrasonography: biometric data from four African primate species. J Med Primatol. 2006;35:38–47. doi: 10.1111/j.1600-0684.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- Bulygina E, Mitteroecker P, Aiello L. Ontogeny of facial dimorphism and patterns of individual development within one human population. Am J Phys Anthropol. 2006;131:432–443. doi: 10.1002/ajpa.20317. [DOI] [PubMed] [Google Scholar]
- Carlsöö S, Leijon G. A radiographic study of the position of the hyo-laryngeal complex in relation to the skull and the cervical column in man. Trans R Sch Dent Umea (Stockh) 1960;5:13–35. [Google Scholar]
- Chitty LS, Altman DG, Henderson A, et al. Charts of fetal size: 2, head measurements. Br J Obstet Gynaecol. 1994;101:35–43. doi: 10.1111/j.1471-0528.1994.tb13007.x. [DOI] [PubMed] [Google Scholar]
- Cobb SN, O’Higgins P. Hominins do not share a common postnatal facial ontogenetic shape trajectory. J Exp Zool (Mol Dev Evol) 2004;302B:302–321. doi: 10.1002/jez.b.21005. [DOI] [PubMed] [Google Scholar]
- Daegling DJ. Functional morphology of the human chin. Evol Anthropol. 1993;1:170–177. [Google Scholar]
- Dean MC, Beynon AD. Tooth crown heights, tooth wear, sexual dimorphism and jaw growth in hominoids. Z Morphol Anthropol. 1991;78:425–440. [PubMed] [Google Scholar]
- Dobson SD, Trinkaus E. Cross-sectional geometry and morphology of the mandibular symphysis in Middle and Late Pleistocene Homo. J Hum Evol. 2002;43:67–87. doi: 10.1006/jhev.2002.0563. [DOI] [PubMed] [Google Scholar]
- Dryden IL, Mardia KV. Statistical shape analysis. New York: Wiley; 1998. [Google Scholar]
- DuBrul LE, Sicher H. The adaptive chin. Springfield, IL: Charles C Thomas; 1954. [Google Scholar]
- Enlow DH. Facial growth. 3rd edn. Philadelphia: Saunders; 1990. [Google Scholar]
- Enlow DH, Harris DB. A study of the postnatal growth of the human mandible. Am J Orthod. 1964;50:25–50. [Google Scholar]
- Falk D. Comparative anatomy of the larynx in man and the chimpanzee: implications for language in Neanderthal. Am J Phys Anthropol. 1975;43:123–132. doi: 10.1002/ajpa.1330430116. [DOI] [PubMed] [Google Scholar]
- Frayer DW, Wolpoff MH, Thorne AG, et al. Theories of modern human origins: the paleontological test. Am Anthropol. 1993;95:14–50. [Google Scholar]
- Golovanova LV, Hoffecker JF, Kharitonov VM, et al. Mezmaiskaya cave: a Neanderthal occupation in the northern Caucasus. Curr Anthropol. 1999;40:77–86. [Google Scholar]
- Gunz P, Mitteroecker P, Bookstein FL. Semi-landmarks in three dimensions. In: Slice DE, editor. Modern morphometrics in physical anthropology. New York: Kluwer Academic/Plenum; 2005. pp. 73–98. [Google Scholar]
- Hohl T. Masticatory muscle transposition in primates: effects on craniofacial growth. J Maxillo Fac Surg. 1983;11:149–156. doi: 10.1016/s0301-0503(83)80038-1. [DOI] [PubMed] [Google Scholar]
- Howells WW. Garden City, NY: Doubleday; 1959. Mankind in the Making. [Google Scholar]
- Hrdlicka A. Human dentition and teeth from the evolutionary and racial standpoint. Dominion Dent J. 1911;23:403–417. [Google Scholar]
- Hublin J-J, Tillier A-M. The Mousterian juvenile mandible form Irhoud (Morocco): a phylogenetic interpretation. In: Stringer CB, editor. Aspects of Human Evolution. London: Taylor & Francis; 1981. pp. 167–185. [Google Scholar]
- Ichim I, Swain VM, Kieser J. Mandibular stiffness in humans: numerical predictions. J Biomech. 2006;39:1903–1913. doi: 10.1016/j.jbiomech.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Ichim I, Kieser J, Swain M. Tongue contractions during speech may have led to the development of the bony geometry of the chin following the evolution of human language: a mechanobiological hypothesis for the development of the human chin. Med Hypotheses. 2007;69:20–24. doi: 10.1016/j.mehy.2006.11.048. [DOI] [PubMed] [Google Scholar]
- Johnson PA, Atkinson PJ, Moore WJ. The development and structure of the chimpanzee mandible. J Anat. 1976;122:467–477. [PMC free article] [PubMed] [Google Scholar]
- King E. A roentgenographic study of laryngeal growth. Angle Orthod. 1952;22:23–37. [Google Scholar]
- Kurihara S, Enlow DH, Rangel RD. Remodeling reversals in anterior parts of the human mandible and maxilla. Angle Orthod. 1980;50:98–106. doi: 10.1043/0003-3219(1980)050<0098:RRIAPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lieberman DE. Testing hypotheses about recent human evolution from skulls: integrating morphology, function, development and phylogeny. Curr Anthropol. 1995;36:159–197. [Google Scholar]
- Lieberman DE. Homology and hominid phylogeny: problems and potential solutions. Evol Anthropol. 1999;7:142–151. [Google Scholar]
- Lieberman P, Crelin ES. On the speech of Neanderthal man. Ling Inq. 1971;2:203–222. [Google Scholar]
- Lieberman DE, McCarthy RC. The ontogeny of cranial base angulation in humans and chimpanzees and its implications for reconstructing pharyngeal dimensions. J Hum Evol. 1999;36:487–517. doi: 10.1006/jhev.1998.0287. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Ross CF, Ravosa MJ. The primate cranial base: ontogeny, function, and integration. Am J Phys Anthropol. 2000;113:117–169. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McCarthy RC, Hiiemae KM, et al. Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Biol. 2001;46:117–128. doi: 10.1016/s0003-9969(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Lieberman P. The biology and evolution of language. Cambridge, Mass: Harvard University Press; 1984. [Google Scholar]
- Maureille B. A lost Neanderthal neonate found. Nature. 2002;419:33–34. doi: 10.1038/419033a. [DOI] [PubMed] [Google Scholar]
- Mauser C, Enlow DH, Overman DO, et al. Growth and remodeling of the human fetal face and cranium. In: McNamara JA, editor. Determinants of mandibular form and growth. Ann Arbor: Center for Human Growth and Development, University of Michigan; 1975. pp. 243–275. Monograph 5. Craniofacial Growth Series. [Google Scholar]
- McCarthy RC, Lieberman DE. Posterior maxillary (PM) plane and anterior cranial architecture in primates. Anat Rec. 2001;264:247–260. doi: 10.1002/ar.1167. [DOI] [PubMed] [Google Scholar]
- Meredith HV. Order and age of eruption for the deciduous dentition. J Dent Res. 1946;25:43–66. doi: 10.1177/00220345460250010901. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Bookstein FL. Heterochrony and geometric morphometrics: a comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evol Dev. 2005;7:244–258. doi: 10.1111/j.1525-142X.2005.05027.x. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Bernhard M, et al. Comparison of cranial ontogenetic trajectories among great apes and humans. J Hum Evol. 2004;46:679–698. doi: 10.1016/j.jhevol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix. In: Krauss BS, Riedel CA, editors. Vistas in Orthodontics. Philadelphia: Lea & Febiger; 1962. pp. 85–98. [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 1. The role of the mechanotransduction. Am J Orthod Dentofacial Orthop. 1997a;112:8–11. doi: 10.1016/s0889-5406(97)70267-1. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 2. The role of an osseous connected cellular network. Am J Orthod Dentofacial Orthop. 1997b;112:221–226. doi: 10.1016/s0889-5406(97)70249-x. [DOI] [PubMed] [Google Scholar]
- Moss ML, Salentijn L. The primary role of functional matrices in facial growth. Am J Orthod. 1969a;55:566–577. doi: 10.1016/0002-9416(69)90034-7. [DOI] [PubMed] [Google Scholar]
- Moss ML, Salentijn L. The capsular matrix. Am J Orthod. 1969b;56:474–490. doi: 10.1016/0002-9416(69)90209-7. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–291. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Negus VE. The comparative anatomy and physiology of the larynx. New York: Hafner; 1949. [Google Scholar]
- Nishimura T. Descent of the larynx in chimpanzees: Mosaic and multiple-step evolution of the foundations for human speech. In: Matsuzawa T, Tomonaga M, Tanaka M, editors. Cognitive development in chimpanzees. Tokyo: Springer; 2006. pp. 75–95. [Google Scholar]
- Nissen HW, Riesen AH. The deciduous dentition of chimpanzee. Growth. 1945;9:265–274. [Google Scholar]
- Ponce de León MS, Golovanova L, Doronichev V, et al. Neanderthal brain size at birth provides insights into the evolution of human life history. Proc Natl Acad Sci U S A. 2008;105:13764–13768. doi: 10.1073/pnas.0803917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlanski RJ, Kjaer I, Vastardis H, et al. Morphometrische Untersuchungen zur Fetalentwicklung der menschlichen Mandibula. Fortschr Kieferorthop. 1994;55:77–83. doi: 10.1007/BF02174360. [DOI] [PubMed] [Google Scholar]
- Radlanski RJ, Renz H, Klarkowski MC. Prenatal development of the human mandible. 3D reconstructions, morphometry, and bone remodeling pattern, stages 12–117 mm CRL. Anat Embryol (Berl) 2003;207:221–232. doi: 10.1007/s00429-003-0343-4. [DOI] [PubMed] [Google Scholar]
- Rak Y, Kimbel WH, Hovers E. A Neanderthal infant from Amud Cave, Israel. J Hum Evol. 1994;26:313–324. [Google Scholar]
- Riesenfeld A. The adaptative mandible: an experimental study. Acta Anat (Basel) 1969;72:246–262. doi: 10.1159/000143250. [DOI] [PubMed] [Google Scholar]
- Robinow M, Richards TW, Anderson M. The eruption of deciduous teeth. Growth. 1942;6:127–133. [Google Scholar]
- Roche AF, Barkla DH. The level of the larynx during childhood. Ann Otol Rhinol Laryngol. 1965;74:645–654. doi: 10.1177/000348946507400307. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- Rosas A. Seventeen new mandibular specimens from Atapuerca/Ibeas Middle Pleistocene hominids sample (1985–1992) J Hum Evol. 1995;28:533–559. [Google Scholar]
- Schwartz JH, Tattersall I. The human chin revisited: what is it and who has it? J Hum Evol. 2000;38:367–409. doi: 10.1006/jhev.1999.0339. [DOI] [PubMed] [Google Scholar]
- Schwartz JH, Tattersall I. The human fossil record. Terminology and craniodental morphology of genus Homo (Africa and Asia) New York: Wiley-Liss; 2002. [Google Scholar]
- Smith BH, Crummett TL, Brandt KL. Ages of eruption of primate teeth: a compendium for aging individuals and comparing life histories. Am J Phys Anthropol. 1994;37(S19):177–231. [Google Scholar]
- Spoor F, Zonneveld F, Macho GA. Linear measurements of cortical bone and dental enamel by computed tomography: applications and problems. Am J Phys Anthropol. 1993;91:469–484. doi: 10.1002/ajpa.1330910405. [DOI] [PubMed] [Google Scholar]
- van Spronsen PH, Koolstra JH, van Ginkel FC, et al. Relationships between the orientation and moment arms of the human jaw muscles and normal craniofacial morphology. Eur J Orthod. 1997;19:313–328. doi: 10.1093/ejo/19.3.313. [DOI] [PubMed] [Google Scholar]
- Spyropoulos MN, Tsolakis AI, Alexandridis C, et al. Role of suprahyoid musculature on mandibular morphology and growth orientation in rats. Am J Orthod Dentofacial Orthop. 2002;122:392–400. doi: 10.1067/mod.2002.125992. [DOI] [PubMed] [Google Scholar]
- Strand Vidarsdottir U, Cobb S. Inter- and intra-specific variation in the ontogeny of the hominoid facial skeleton: testing assumptions of ontogenetic variability. Ann Anat. 2004;186:423–428. doi: 10.1016/s0940-9602(04)80076-1. [DOI] [PubMed] [Google Scholar]
- Stringer CB, Hublin J-J, Vandermeersch B. The origin of anatomically modern humans in western Europe. In: Smith FH, Spencer F, editors. The Origins of Modern Humans: A World Survey from the Fossil Evidence. New York: Alan R. Liss; 1984. [Google Scholar]
- Tillier A-M. La dentition de l’enfant moustérien Chateauneuf 2 découvert à l’abri de Hauteroche (Charente) L’Anthropologie. 1979;83:417–438. [Google Scholar]
- Walkhoff O. Die menschliche Sprache in ihrer Bedeutung für die funktionelle Gestalt des Unterkiefers. Anat Anz. 1904;24:129. [Google Scholar]
- Westhorpe RN. The position of the larynx in children and its relationship to the ease of intubation. Anaesth Intensive Care. 1987;15:384–388. doi: 10.1177/0310057X8701500405. [DOI] [PubMed] [Google Scholar]
- White TD. The anterior mandibular corpus of early African hominidae: Functional significance of shape and size. Ann Arbor: University Microfilms, University of Michigan; 1977. PhD. Dissertation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


