Abstract
The main aim of the present work is to synthesize the information obtained from our dissections of the pectoral and forelimb muscles of representative members of the major extant taxa of limbed amphibians and reptiles and from our review of the literature, in order to provide an account of the comparative anatomy, homologies and evolution of these muscles in the Tetrapoda. The pectoral and forelimb musculature of all these major taxa conform to a general pattern that seems to have been acquired very early in the evolutionary history of tetrapods. Although some muscles are missing in certain taxa, and a clear departure from this general pattern is obviously present in derived groups such as birds, the same overall configuration is easily distinguishable in these taxa. Among the most notable anatomical differences between the groups, one that seems to have relevant evolutionary and functional implications, concerns the distal insertion points of the forearm musculature. In tetrapods, the muscles of the radial and ulnar complexes of the forearm are pleisomorphically mainly inserted onto the radius/ulna or onto the more proximal carpal bones, but in mammals some of these muscles insert more distally onto bones such as the metacarpals. Interestingly, a similar trend towards a more distal insertion of these muscles is also found in some non-mammalian tetrapod taxa, such as some anurans (e.g. Phyllomedusa). This may be correlated with the acquisition of more subtle digital movement abilities in these latter taxa.
Keywords: amphibians, anatomy, evolution, homologies, pectoral and forelimb muscles, reptiles, tetrapods
Introduction
In a recent paper, Diogo et al. (2009a) summarized the results of their long-term study of the comparative anatomy, homologies and evolution of the pectoral and forelimb muscles of sarcopterygians (the group comprising tetrapods and bony fish such as coelacanths and dipnoans). The paper was mainly based on dissections of numerous sarcopterygians, and on a review of the literature. The goal of the authors was to present the homologies and evolution of the pectoral and forelimb muscles of the Sarcopterygii as a whole, thus providing a background for more detailed morphological and taxon-based analyses. Of the seven sarcopterygian taxa featured in the tables of that paper, only two, the urodele Ambystoma ordinarium and the lepidosaur Timon lepidus, were non-mammalian tetrapods. In the present work we thus focus on the comparative anatomy, evolution and homologies of the pectoral and forelimb muscles of the major extant clades of limbed amphibians and reptiles, that is, urodeles, anurans, lepidosaurs, crocodylians, birds, and turtles (caecilian amphibians, amphisbaenians, and snakes usually lack limbs, their pectoral and forelimb musculature being extremely reduced; these taxa will not be discussed in the present paper: see Carroll, 2007; Diogo, 2007).
Many anatomical works have provided information about the pectoral and forelimb muscles of amphibians and reptiles (e.g. Mivart, 1869; Humphry, 1872a,b; Fürbringer, 1876; Ecker, 1889; Gaupp, 1896; McMurrich, 1903a,b; Ribbing, 1907, 1938; Romer, 1922, 1924, 1944; Howell, 1935, 1936a,b; Haines, 1939, 1950; Straus, 1942; Sullivan, 1962, 1967; Grim, 1971; Hudson et al. 1972; Walker, 1973; Holmes, 1977; Ghetie et al. 1981; Duellman & Trueb, 1986; Russell, 1988; Manzano, 1996; Burton, 1998; Dilkes, 2000; Wyneken, 2001; Meers, 2003; Walthall & Ashley-Ross, 2006; Maxwell & Larsson, 2007; Russell & Bauer, 2008). However, most of these works have focused on a specific group within the Tetrapoda and/or a specific pectoral or forelimb region, and none of them has actually provided detailed information about the homologies of all the pectoral and forelimb muscles of amphibians and reptiles. The present account on the comparative anatomy, homologies and evolution of the forelimb and pectoral muscles of these groups is based on the results of our own dissections, combined with an exhaustive literature review.
As stressed by Diogo et al. (2008a,b, 2009a,b), among others, one of the major communicative problems researchers face when they compare the muscles of a certain tetrapod taxon with those of other taxa is the use of different names to designate the same muscle in the members of different clades, and even of the same clade. To reconcile the different nomenclatures we propose a unifying nomenclature for the pectoral and forelimb muscles of the Tetrapoda as a whole. In fact, we should note that as we were working on this paper, we were informed (e.g. M. Fabrezi, pers. comm.) about ambitious, new, and clearly needed, ontological projects that are now being developed in different biological disciplines. Such ontologies are extremely important, and are becoming increasingly popular because they provide a vocabulary for representing and communicating knowledge about a certain topic and a set of relationships that hold among the terms in that vocabulary. Although we did not have in mind to build an ontology when we began the aforementioned project, the fact is that we did it, and still do, in each of our papers about vertebrate myology (e.g. Diogo, 2004a,b, 2007, 2008, 2009; Abdala & Moro, 2006; Diogo & Abdala, 2007; Abdala et al. 2008; Diogo et al. 2008a,b, 2009a,b). Therefore, we hope that the information provided in this specific paper might help to pave the way for developing an ontology of the pectoral and forelimb musculature of amphibians and reptiles.
Materials and methods
We begin by setting out the phylogenetic framework for the discussions provided in this paper, which is shown in Fig. 1. Within tetrapods, Amphibia is the sister group of Amniota, which includes the Mammalia and the Reptilia. Amphibia includes three main extant groups: caecilians (Gymnophiona or Caecilia), frogs (Anura, including Rhinella), and salamanders (Caudata or Urodela, including Ambystoma), the two latter groups being possibly more closely related to each other than to the caecilians (see the recent review of Carroll, 2007). The Reptilia includes four main extant groups: the Testudines (or Chelonia, including Trachemys), the Lepidosauria (including Timon), the Crocodylia (including Caiman), and Aves (including Gallus; see Modesto & Anderson, 2004 for a current phylogenetic definition of the Reptilia). The Lepidosauria comprises the Rhynchocephalia, which includes a single extant genus, Sphenodon, and the Squamata, which according to the recent study of Conrad (2008) includes amphisbaenians, mosasaurs, snakes and ‘lizards’ (as explained by this author, ‘lizards’ do not form a monophyletic group, because some ‘lizards’ are more closely related to taxa such as snakes than to other ‘lizards’: see Conrad, 2008 for more details on the interrelationships of squamates). The Crocodylia and Aves are included in the Archosauria, and this latter group is currently commonly included with the Lepidosauria in the clade Diapsida. Turtles are thus commonly considered to be non-diapsid reptiles: this is the working hypothesis followed in most of the recent works on the muscle homologies of reptiles, and is also the main working hypothesis that we follow in the present paper, when we analyze and discuss the homologies of the tetrapod muscles (Fig. 1; see Benton, 1985; Gauthier et al. 1988; Dawkins, 2004; Tsuihiji 2007; Holliday & Witmer, 2007; Holliday, 2009). However, it should be noted that some authors have defended the placement of lepidosaurs as more closely related to turtles than to archosaurs (e.g. Rieppel, 1994, 2000; De Braga & Rieppel, 1997; Rieppel & Reisz, 1999; Müller, 2003; Hill, 2005), whereas others have defended the classification of turtles as the closest living relatives of crocodylians (e.g. Hedges & Poling, 1999; Mannen & Li, 1999; Cao et al. 2000). As explained above, the main working hypothesis followed in the present work is that turtles are the extant sister-group of the other living reptiles, but we consider that it is useful to also show the alternative hypotheses defended by these latter authors in the tree of Fig. 1; we will also address this subject in the discussion below.
Fig. 1.
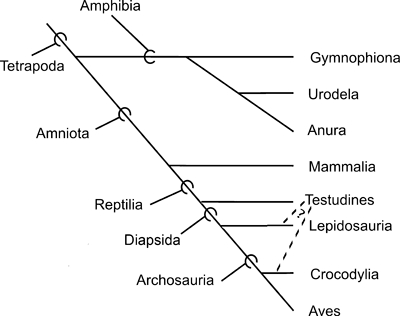
Phylogenetic framework for the discussions provided in the present paper (for more details, see the Materials and methods section).
We dissected numerous specimens of urodeles, anurans, turtles, lepidosaurs, crocodylians and birds for the present project. The dissected specimens are from the personal collection of Anthony Herrel (AH), the ‘Colección Mamíferos Lillo’ of the Universidad Nacional de Tucumán (CML), the herpetological collection of ‘Diamante-CONICET-Argentina’ (DIAM), the ‘Fundación Miguel Lillo of Argentina’ (FML), the herpetological collection of the Hebrew University of Jerusalem-Israel (HUJ), the ‘Museo de Zoologia of the San Pablo University-Brasil’ (MZUSP), the Tupinambis Project Tucumán-Argentina (PT), the personal collection of Richard Thomas in Puerto Rico University (RT), the San Diego State University (SDSU), the Smithsonian National Museum of Natural History (USNM), the Peabody Museum of Natural History of Yale University (YPM), and the ‘Museo Nacional de Ciencias Naturales de Madrid’ (MNCN). The list of alcohol-preserved amphibian and reptilian specimens examined for the present work is given below (note: in this list, ‘sp.’ means specimen per species).
We use the definition of pectoral and forelimb muscles proposed by Jouffroy (1971) and mainly follow the nomenclature proposed by Diogo et al. (2009a). Therefore, hypobranchial muscles such as the sternohyoideus and branchial muscles such as the trapezius, which are head and neck muscles sensuDiogo et al. (2008a,b);, are not included in our work. When cited papers use a nomenclature that differs from that followed here, the respective synonymy is given. When we refer to the anterior, posterior, dorsal and ventral regions of the body, we do so in the sense the terms are used for pronograde tetrapods (e.g. the forelimb is anterior to the hind limb, and the sternum is ventral to the thoracic part of the vertebral column). Note that in this work we follow the interpretation that has been commonly supported in the studies of fossils and of hox genes, and thus consider that the three digits that are usually present in adult birds are digits 1, 2 and 3, and not digits 2, 3 and 4 as is often suggested by the authors of embryological studies (for recent reviews on this subject, see Galis et al. 2003, 2005; Vargas & Fallon, 2005a,b; Vargas et al. 2008; Kundrát, 2009). However, to make this clear, we always also state, between round brackets, the number of the digit according to most embryologists. So, for instance, if we refer to the most radial digit of adult chickens, we state ‘digit 1 (i.e. digit 2 according to most embryologists)’ (Figs 2 and 3). We consider that this is a clear, simple, and also neutral, way of referring to the avian digits.
Fig. 2.
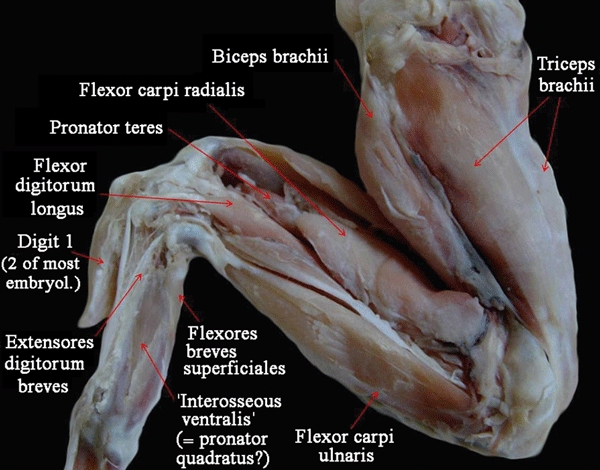
Gallus domesticus (Reptilia, Aves): ventral view of the superficial musculature of the wing. embryol., embryologists.
Fig. 3.
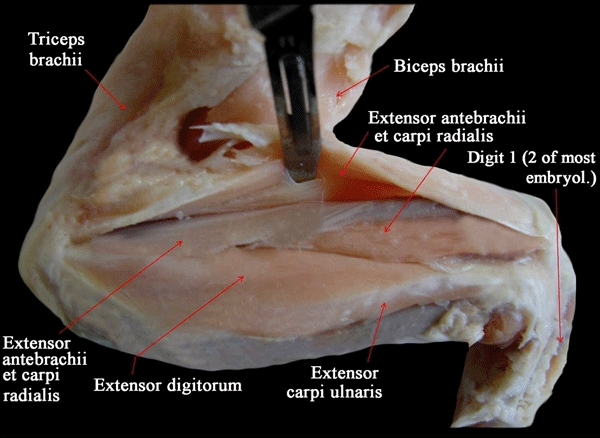
Gallus domesticus (Reptilia, Aves): Dorsal view of the deep musculature of the wing. embryol., embryologists.
The definition of homology and its use in systematics and comparative anatomy have been discussed by several authors (e.g. Patterson, 1988; de Pinna, 1991; Agnarsson & Coddington, 2007). The simplest meaning of homology is equivalence of parts (e.g. De Pinna, 1991). In the present work we follow the phylogenetic definition of homology, as proposed by Patterson (1988): homology is equal to synapomorphy. Therefore, following De Pinna (1991), we recognize two main types of muscular homology. ‘Primary homology’ hypotheses are conjectures or hypotheses about common origin of muscular characters that are established after a careful analysis of criteria such as function, topology and ontogeny (i.e. after the so-called test of similarity). In this study we follow the same methodology that we have employed and explained in previous works (e.g. Diogo, 2007, 2008; Diogo et al. 2008a,b, 2009a) and thus take into account all the lines of evidence obtained either from our dissections or gleaned from the literature in order to formulate such ‘primary homology’ hypotheses (e.g. the innervation of the muscles, when this information is available; their relationships with other muscular structures; their relationships with hard tissues; the configuration/orientation of their fibers; their development; their function; etc.). This is because, as pointed out by Edgeworth (1935), no single criterion is sufficient. For instance, although the innervation of a muscle generally remains constant and corresponds to its segment of origin, there are cases in which the same muscle has different innervations in different taxa (e.g. although wholly of mandibular origin, the intermandibularis of dipnoans is innervated by the Vth and/or the VII nerve; Edgeworth, 1935). Also, there are cases in which the same muscle may be ontogenetically derived from different regions and/or segments of the body in different taxa and in which ‘an old structure or group of structures may be transformed’ (e.g. the levator hyoideus ‘may be transformed, either partially or wholly, into a depressor mandibulae’; Edgeworth 1935: 224).
Following De Pinna (1991), the ‘primary homology’ hypotheses have, however, to pass the second, or ‘hard’, test of homology, i.e. the test of phylogenetic conjunction and congruence (agreement in supporting the same phylogenetic relationships) before they can actually be considered solid hypotheses of homology, i.e. ‘secondary homology’ hypotheses. The important point is, thus, that under the phylogenetic definition of homology it is the test of phylogenetic conjunction and congruence that ultimately determines whether a hypothesis can be considered a solid hypothesis of homology. So if for instance a muscle A of a taxon X and a muscle B of a taxon Y have a similar innervation, function, topology and development, but the phylogenetic data available strongly support the idea that muscles A and B were the result of convergent evolution (i.e. that they were acquired independently in evolution and do not correspond to a structure that was present in the last common ancestor of A and B), then the phylogenetic criterion has priority over the other criteria. In the specific case of the present work the phylogenetic framework that we use to investigate and discuss the evolution and homologies of the pectoral and forelimb musculature of the taxa listed in Tables 1–3 was provided in the first paragraph of this section. So, following the methodology explained above, if an analysis of the data provided by some lines of evidence (e.g. innervation, function and relationships with other muscular and hard structures) indicates that muscles C and D could be homologous (‘primary homology’ hypothesis), but within all reptiles muscle C is only present in testudines and muscle D in a specific subgroup of birds, then we would consider that muscles C and D were likely independently acquired in that specific subgroup of birds and in the testudines, respectively (see Fig. 1). Thus, these muscles C and D are likely not homologous (i.e. the ‘primary homology’ hypothesis did not pass the ‘hard’ test of homology, that is, the test of phylogenetic conjunction and congruence; see Diogo, 2007, 2008; Diogo et al. 2008a,b;, 2009a, for more details on this subject). So, the hypotheses of homology that are shown in Tables 1–3 are hypotheses that are phylogenetically congruent with the phylogenetic framework that was set out in the first paragraph of this section, i.e. they are ‘secondary homology’ hypotheses sensuDe Pinna (1991).
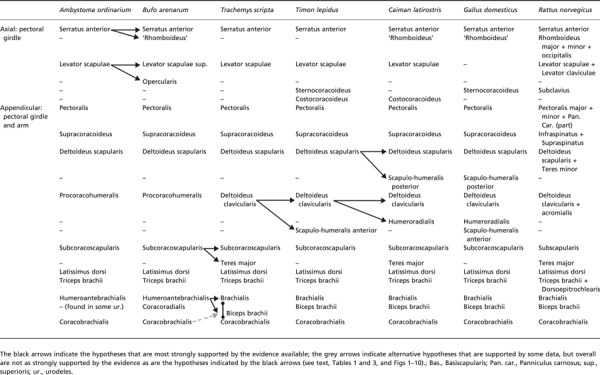
Table 1.
Pectoral and forelimb muscles of adults of representative amphibian and reptilian taxa
| Amphibia (Caudata): Ambystoma ordinarium (Michoacan stream salamander) | Amphibia (Anura): Rhinella arenarum (Argentine common toad) | Reptilia (Testudines): Trachemys scripta (Red-eared slider turtle) | Reptilia (Lepidosauria): Timon lepidus (Ocellated ‘lizard’) | Reptilia (Crocodylia): Caiman latirostris (Brown-snouted caiman) | Reptilia (Aves): Gallus domesticus (Chicken) |
|---|---|---|---|---|---|
| Serratus anterior (part of serrati sensuHowell, 1937a) | Serratus anterior (part of serrati sensuHowell, 1935, 1937b) | Serratus anterior | Serratus anterior (serratus ventralis sensuKardong & Zalisko, 1998 and Kardong, 2002) | Serratus anterior (serratus ventralis sensuMeers, 2003) | Serratus anterior |
| – | ‘Rhomboideus’ | – | – | ‘Rhomboideus’ | ‘Rhomboideus’ |
| Levator scapulae (thoracico-scapularis sensuJouffroy, 1971; levator scapulae superioris sensu Heterington & Tugaoen 1990; opercularis sensuWalthall & Ashley-Ross, 2006 and Piekarski & Olsson, 2007; includes the collumellaris and the opercularis sensuDuellman & Trueb, 1986 and Carroll, 2007) | Levator scapulae superioris (levator scapulae sensuHowell, 1935, 1937b) | Levator scapulae | Levator scapulae | Levator scapulae | – |
| – | Opercularis (colummelaris sensuCarroll, 2007) | – | – | – | – |
| – | – | – | Sternocoracoideus (sternocoracoid superior and inferior sensuHowell, 1937b) | – [according to authors such as Fürbringer, 1876; Walker, 1973; Holmes, 1977 and Dilkes, 2000; the sternocoracoideus is not present as an independent muscle in turtles and in crocodylians] | Sternocoracoideus [according to authors such as Dilkes, 2000; the sternocoracoideus is present in birds] |
| – | – | – [according to Walker, 1973 the costocoracoideus is not present as a distinct muscle in turtles] | Costocoracoideus (costoscapularis sensuHowell, 1936a, 1937b and Holmes, 1977; costosternocoracoideus sensuHolmes, 1977 and Dilkes, 2000) | Costocoracoideus (costosternocoracoideus sensuHolmes, 1977 and Dilkes, 2000) | – [according to authors such as Dilkes, 2000; the costocoracoideus is not present as an independent muscle in birds] |
| Pectoralis | Pectoralis | Pectoralis (pectoralis major sensuWyneken, 2001) | Pectoralis [according to authors such as Fürbringer, 1876; Walker 1973, Holmes, 1977 and Dilkes, 2000; the pectoralis is present in all the major extant reptilian clades] | Pectoralis | Pectoralis (includes pectoralis pars thoracica sensuMaxwell & Larsson, 2007) |
| Supracoracoideus (coracohumeralis sensuHowell, 1935, 1937b) | Supracoracoideus (coracohumeralis sensuHowell, 1935, 1937b) | Supracoracoideus | Supracoracoideus (coracohumeralis sensuHowell, 1936a) | Supracoracoideus | Supracoracoideus (pectoralis secundus sensuJollie, 1962) |
| Deltoideus scapularis [the deltoideus scapularis sensuJouffroy, 1971 corresponds to the dorsalis scapulae sensuSmith, 1926; Howell, 1937b; Romer, 1944; Walthall & Ashley-Ross, 2006; Diogo & Abdala, 2007; and Diogo, 2007] | Deltoideus scapularis (dorsalis scapulae sensuHowell, 1935, 1937b) | Deltoideus scapularis (dorsal, or scapular, part of deltoideus sensuWalker, 1973 and Wyneken, 2001) | Deltoideus scapularis (dorsalis scapulae sensuHowell, 1936a; Diogo & Abdala, 2007; and Diogo, 2007) [according to Holmes, 1977 and Dilkes, 2000 the deltoideus scapularis is present in turtles, crocodylians, birds and lepidosaurs] | Deltoideus scapularis (dorsalis scapulae sensuFürbringer, 1876) | Deltoideus scapularis (part of acromialis sensuRibbing, 1938; part of deltoideus sensuJollie, 1962) |
| – | – | – | – [but see text] | Scapulo-humeralis posterior (scapulo-humeralis caudalis sensuMeers, 2003) | Scapulo-humeralis posterior (scapulo-humeralis caudalis sensuDilkes, 2000 and Maxwell & Larsson, 2007) |
| Procoracohumeralis [see text] | Procarocohumeralis | Deltoideus clavicularis (ventral, or clavicular, part of deltoideus sensuWalker, 1973 and Wyneken, 2001) | Deltoideus clavicularis (procoracohumeralis sensuDiogo & Abdala, 2007 and Diogo, 2007) | Deltoideus clavicularis (scapularis inferior sensuFürbringer, 1876) | Deltoideus clavicularis (part of acromialis sensuRibbing, 1938) |
| – | – | – | – [but see text] | Humeroradialis | Humeroradialis (deltoides propatagialis sensuRomer, 1944; tensor patagii sensuJollie, 1962; tensor propatagii sensuSullivan, 1962, 1967; tensor propatagialis sensuMeers, 2003) |
| – | – | – | Scapulo-humeralis anterior | – | Scapulo-humeralis anterior (scapulohumeralis cranialis sensuDilkes, 2000 and Maxwell & Larsson, 2007) |
| Subcoracoscapularis [present in urodeles according to Smith, 1926; Romer, 1944 and Kardong, 2002; but see text] | Subcoracoscapularis (subscapularis sensuEcker,1889) | Subcoracoscapularis (subscapularis sensuWalker, 1973; Holmes, 1977; Dilkes, 2000 and Wyneken, 2001) | Subcoracoscapularis (subscapularis + subcoracoideus sensuHolmes, 1977 and Dilkes, 2000) | Subcoracoscapularis (subscapularis sensu Dilkes and Meers, 2003) | Subcoracoscapularis (subscapularis + subcoracoideus sensuSullivan, 1962, 1967, Dilkes, 2000 and Maxwell & Larsson, 2007) |
| – | – | Teres major | – [but see text] | Teres major | – |
| Latissimus dorsi | Latissimus dorsi | Latissimus dorsi | Latissimus dorsi | Latissimus dorsi | Latissimus dorsi |
| Triceps brachii | Triceps brachii | Triceps brachii | Triceps brachii | Triceps brachii | Triceps brachii |
| Humeroantebrachialis (part of biceps sensuRomer, 1944 and of brachialis sensuHowell, 1937b) | Humeroantebrachialis | Brachialis (brachialis inferior sensuRomer, 1944; Walker, 1973; Holmes, 1977; Dilkes, 2000 and Wyneken 2001) | Brachialis (brachialis inferior sensuRomer, 1944; Holmes, 1977 and Dilkes, 2000) | Brachialis (brachialis inferior sensuRomer, 1944; Holmes, 1977 and Dilkes, 2000) | Brachialis (brachialis inferior sensuHolmes, 1977 and Dilkes, 2000) |
| – [but coracoradialis present in other urodeles, see text] | Coracoradialis | Biceps brachii | Biceps brachii (humeroantebrachialis sensuDiogo & Abdala, 2007; and Diogo, 2007; short and long heads of biceps brachii sensuJouffroy, 1971) | Biceps brachii | Biceps brachii |
| Coracobrachialis (corresponds to the coracobrachialis longus/superficialis sensuWalthall & Ashley-Ross, 2006; Diogo & Abdala, 2007; and Diogo, 2007) | Coracobrachialis | Coracobrachialis (coracobrachialis magnus + coracobrachialis brevis sensuWalker, 1973 and Wyneken, 2001; which correspond respectively to the coracobrachialis longus + coracobrachialis brevis sensuHolmes, 1977 and Dilkes, 2000) | Coracobrachialis (coracobrachialis superficialis/longus + coracobrachialis profundus/ brevis sensuHowell, 1936a; Romer, 1944; Jollie, 1962; Holmes, 1977 and Dilkes, 2000) | Coracobrachialis (coracobrachialis brevis sensuHolmes, 1977 and Dilkes, 2000) | Coracobrachialis (coracobrachialis longus + coracobrachialis brevis, or coracobrachialis cranialis plus coracobrachialis caudalis, or coracobrachialis anterior + coracobrachialis posterior, sensuJollie 1962, Holmes, 1977 and Dilkes, 2000) |
| Pronator quadratus (pronator profundus sensuRibbing, 1907; Walthall & Ashley-Ross, 2006; Diogo & Abdala, 2007; and Diogo, 2007) | Pronator quadratus (pronator profundus sensuRibbing, 1907) [Ribbing, 1907 states that the ‘abductor pollicis’sensuGaupp, 1896 corresponds to the pronator quadratus, and not to the abductor pollicis brevis, sensu the present work] | Pronator quadratus (probably corresponds to part of the pronator profundus sensuWalker, 1973; the other part corresponding to the pronator accessorius sensu the present work; pronator profundus sensuAbdala et al. 2008) | Pronator quadratus (pronator profundus sensuMoro & Abdala, 2004; Abdala & Moro, 2006; Diogo & Abdala, 2007; and Diogo, 2007) | Pronator quadratus (pronator profundus sensuStraus, 1942) | Pronator quadratus |
| – | – | Pronator accessorius (probably corresponds to part of the pronator profundus sensuWalker, 1973; the other part corresponding to the pronator quadratus sensu the present work | Pronator accessorius | – | – |
| Contrahentium caput longum (ulnocarpalis sensuMcMurrich, 1903a,b;, Straus, 1942; and Bunnell, 1942) | Contrahentium caput longum (ulnocarpalis + intercarpalis sensuGaupp, 1896) | – | – [interestingly, our dissections indicated that a few ‘lizards’ may eventually have a small muscle that somewhat resembles the contrahentium caput longum of Ambystoma: see text] | – | – |
| Flexor accessorius lateralis (caput dorsale of flexor palmaris profundus sensuStraus, 1942; seemingly corresponds to the palmaris profundus III sensuMcMurrich, 1903a) | Flexor accessorius (sensuRibbing, 1907; palmaris profundus sensuGaupp, 1896; Straus, 1942 and Manzano et al. 2008b) | – [absent as an independent muscle in extant amniotes: see text] | – | – | – |
| Flexor accessorius medialis (caput volare of flexor palmaris profundus sensuStraus, 1942) | – | – [absent as an independent muscle in extant amniotes: see text] | – | – | – |
| Flexor digitorum communis (palmaris superficialis sensuMcMurrich, 1903a; flexor primordialis communis sensuRibbing, 1907) | Flexor digitorum communis (flexor primordialis communis sensuRibbing, 1907; flexor digitorum communis longus sensuManzano, 1996 and Manzano et al. 2008b) | Flexor digitorum longus (flexor primordialis communis + flexor accessorius communis sensuRibbing, 1907) | Flexor digitorum longus (palmaris communis sensuHolmes, 1977 and Dilkes, 2000; flexor digitorum communis sensuDiogo & Abdala, 2007; and Diogo, 2007; it probably also includes the ‘pronator radii teres’sensuMcMurrich, 1903a and Holmes, 1977; at least in taxa such as Sphenodon and ‘lizards’) | Flexor digitorum longus (palmaris communis sensuHolmes, 1977 and Dilkes, 2000) | Flexor digitorum longus (flexor accessorius communis sensuRibbing, 1938; palmaris communis sensuHolmes, 1977 and Dilkes, 2000) |
| – | – [but see text] | ‘Palmaris longus’ | – [but some lepidosaurs do have a ‘palmaris longus’] | – | – |
| Flexor carpi ulnaris (part of flexor antebrachii et carpi ulnaris sensuDiogo & Abdala, 2007; and Diogo, 2007) | Flexor carpi ulnaris | Flexor carpi ulnaris (part of the flexor carpi ulnaris sensuWalker, 1973 and Abdala et al. 2008) | Flexor carpi ulnaris (part of flexor antebrachii et carpi ulnaris sensuDiogo & Abdala, 2007 and Diogo, 2007) [according to Dilkes, 2000; the flexor carpi ulnaris is present in all major extant groups of reptiles] | Flexor carpi ulnaris | Flexor carpi ulnaris |
| Epitrochleoanconeus (flexor antebrachii ulnaris sensuMcMurrich, 1903a and Ribbing, 1907) | Epitrochleoanconeus (epitrochleo-cubitalis sensuGaupp, 1896 and Manzano et al. 2008b; flexor antebrachii ulnaris sensuMcMurrich, 1903a and Ribbing, 1907) | Epitrochleoanconeus (flexor antebrachii ulnaris sensuRibbing, 1907, 1938; part of the flexor carpi ulnaris sensuWalker, 1973 and Abdala et al. 2008) | Epitrochleoanconeus | Epitrochleoanconeus | Epitrochleoanconeus (flexor antebrachii ulnaris sensuRibbing, 1938; entepicondylo-ulnaris sensuMaxwell & Larsson, 2007) |
| Flexor antebrachii et carpi radialis (flexor carpi radialis sensuMcMurrich, 1903a,b;) | Flexor carpi radialis | Flexor carpi radialis [as described by Walker, 1973; Holmes, 1977 and Abdala et al. 2008; in turtles the pronator teres (which Holmes designates as a ‘head of the flexor carpi radialis’) and the flexor carpi radialis are usually present as distinct muscles] | Flexor carpi radialis (part of flexor carpi radialis sensuHolmes, 1977 and Dikes 2000) | Flexor carpi radialis (part of flexor carpi radialis sensuHolmes, 1977 and Dilkes, 2000 and of pronator teres sensuMeers, 2003) | Flexor carpi radialis (part of flexor carpi radialis sensuHolmes, 1977 and Dilkes, 2000; seems to correspond to the pronator superficialis sensuSullivan, 1962; Shellswell & Wolpert, 1977; Meyers, 1996 and Dilkes, 2000) |
| – | Pronator teres (flexor antebrachii radialis sensuRibbing, 1907; seems to correspond to the flexor antebrachii lateralis superficialis and profundus sensuGaupp, 1896; although Ribbing, 1907 stated that it actually corresponds to the flexor antebrachii medialis sensuGaupp, 1896) | Pronator teres (pronator radii teres Holmes, 1977) | Pronator teres (flexor antebrachii radialis sensuRibbing, 1907; part of flexor carpi radialis sensuHolmes, 1977 and Dilkes 2000) | Pronator teres (part of flexor carpi radialis sensuHolmes, 1977 and Dilkes, 2000 and of pronator teres sensuMeers, 2003) | Pronator teres (part of flexor carpi radialis sensuHolmes, 1977 and Dilkes, 2000; seems to correspond to the pronator profundus sensuSullivan, 1962; Shellswell & Wolpert, 1977; Meyers, 1996 and Dilkes, 2000) |
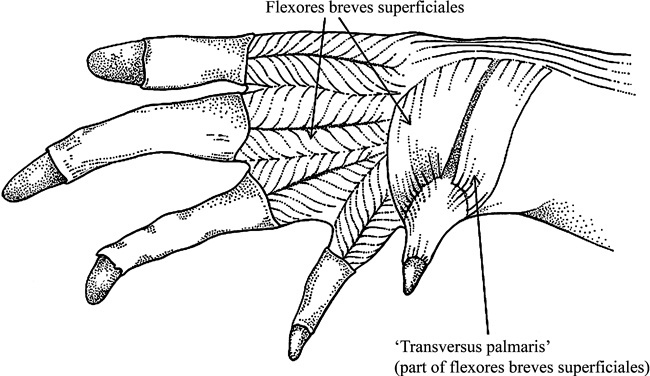
| Flexores breves superficiales (flexores digitorum breves superficiales sensuMcMurrich, 1903a; b) | Flexores breves superficiales | Flexores breves superficiales (flexor brevis superficialis sensuWalker, 1973; flexores digiti brevis superficiales sensuAbdala et al. 2008) | Flexores breves superficiales (part of flexores digitorum breves superficiales sensuMcMurrich, 1903a,b; flexores breves sublimes sensuHolmes, 1977; flexores digiti brevis superficialis sensuAbdala & Moro, 2006) | Flexores breves superficiales (part of flexores breves sublimes sensuHolmes, 1977; flexores digitorum breves superficiales and probably also flexor digiti quinti and/or transversus palmaris sensuMeers, 2003) | Flexores breves superficiales [see text] |
| – [but according to authors such as McMurrich, 1903a,b;, the lumbricales are present in other urodeles, such as Ambystoma tigrinum] | Lumbricales | Lumbricales (lumbricalis sensuAbdala et al. 2008) | Lumbricales | Lumbricales | – [see text] |
| Contrahentes digitorum (part of flexores digitorum breves medii sensuMcMurrich, 1903a,b;) | Contrahentes digitorum (part of flexores digitorum breves medii sensuMcMurrich, 1903a,b; probably includes the adductor pollicis sensuManzano et al. 2008b) | Contrahentes digitorum (includes adductor digiti minimi sensuWalker, 1973) | Contrahentes digitorum (part of flexores digitorum breves medii sensuMcMurrich, 1903a,b; and Lewis, 1989; include the flexor digitorum V transversus I and II of e.g. Abdala & Moro, 2006) | Contrahentes digitorum (part of flexores digitorum breves medii sensu authors such as McMurrich, 1903a,b; flexores digitorum intermedii sensuHolmes, 1977 and Meers, 2003) | Contrahentes digitorum |
| Flexores breves profundi (flexores digitorum breves profundi sensuMcMurrich, 1903a,b;) | Flexores breves profundi | Flexores breves profundi | Flexores breves profundi (flexores digitorum breves profundi sensuMcMurrich, 1903a,b; flexores digiti brevis profundus sensuAbdala & Moro, 2006) | Flexores breves profundi (flexores digitorum profundus, and possibly also flexor digitorum intermedius digiti V, sensuMeers, 2003; : see contrahentes digitorum) | Flexores breves profundi (flexores digitorum breves profundi sensuRibbing, 1938 and Holmes, 1977) |
| – [seemingly absent in Ambystoma ordinarium; see text] | Abductor pollicis brevis [see text] | Abductor pollicis brevis | Abductor pollicis brevis (abductor brevis pollici sensuAbdala & Moro, 2006) | Abductor pollicis brevis (abductor metacarpi I sensuMeers, 2003) | Abductor pollicis brevis [the ‘abductor alulae’sensuMeyers, 1996 corresponds to the ‘abductor indicis’sensuSullivan, 1962 and Shellswell & Wolpert, 1977 and goes to digit 1 (i.e. digit 2 according to embryology), thus seemingly corresponding to the abductor pollicis brevis sensu the present work] |
| Abductor digiti minimi (extensor lateralis digiti IV sensuWalthall & Ashley-Ross, 2006 and Diogo et al. 2009a) | Abductor digiti minimi (abductor primus digiti V sensuGaupp, 1896) | Abductor digiti minimi (abductor digitorum V sensuAbdala et al. 2008) | Abductor digiti minimi (abductor digitorum V sensuAbdala & Moro, 2006) | Abductor digiti minimi (abductor metacarpi V sensuMeers, 2003) | – |
| Intermetacarpales | Intermetacarpales (transversi metacarpi sensuGaupp, 1896; transversi metacarporum sensuBurton, 1998; transversus metacarpi superficialis + transversus metacarpi profundus profundus sensuManzano, 1996) | Intermetacarpales | Intermetacarpales (intermetacarpales I and II sensuAbdala & Moro, 2006; Diogo & Abdala, 2007; and Diogo, 2007) | Intermetacarpales (part of interossei dorsalae sensuMeers, 2003) | Intermetacarpales |
| – [see text] | Dorsometacarpales | Dorsometacarpales (possibly correspond to part of interossei dorsales sensuWalker, 1973; see intermetacarpales above) | Dorsometacarpales | Dorsometacarpales (part of interossei dorsalae sensuMeers, 2003) | Dorsometacarpales |
| Extensor antebrachii et carpi radialis (including extensor radialis longus + extensor radialis brevis sensuStraus, 1941a,b;) | Extensor antebrachii et carpi radialis | Extensor antebrachii et carpi radialis (extensor radialis superficialis, extensor carpi intermedius and extensor radialis profundus sensuHaines, 1939 and Walker, 1973; extensor carpi radialis sensuAbdala et al. 2008) | Extensor antebrachii et carpi radialis (including extensor radialis longus + extensor radialis brevis sensuStraus, 1941a,b; extensor carpi radialis sensuAbdala & Moro, 2006) | Extensor antebrachii et carpi radialis (extensor radialis superficialis, extensor carpi intermedius and extensor radialis profundus sensuHaines, 1939) | Extensor antebrachii et carpi radialis (including extensor carpi radialis sensuRibbing, 1938) [the extensor antebrachii et carpi radialis sensu the present work includes, very likely, the ‘extensor metacarpi radialis’ and the ‘supinator’sensuMeyers, 1996] |
| – [not present as an independent muscle in Ambystoma; see text] | – | Brachioradialis (tractor radii sensuHaines, 1939; Walker, 1973; Wyneken, 2001; and Abdala et al. 2008) | – [but seemingly present in Sphenodon; see text] | Brachioradialis (seems to correspond to the supinator sensuMeers, 2003) | – [but see text] |
| Extensor antebrachii et carpi ulnaris (extensor ulnaris sensuStraus, 1941a,b;) | Extensor carpi ulnaris | Extensor antebrachii et carpi ulnaris (extensor ulnaris sensuHaines, 1939; extensor carpi ulnaris sensuWalker, 1973 and Abdala et al. 2008) | Extensor antebrachii et carpi ulnaris (extensor carpi ulnaris + extensor antebrachii ulnaris sensuHaines, 1939; extensor ulnaris sensuStraus, 1941a,b; extensor carpi ulnaris + anconeus quartus sensuHolmes, 1977) | Extensor antebrachii et carpi ulnaris (flexor ulnaris sensuMeers, 2003; extensor carpi ulnaris + anconeus quartus sensuHolmes, 1977) | Extensor carpi ulnaris (seemingly corresponds to the extensor metacarpi ulnaris sensuShellswell & Wolpert, 1977 and Meyers, 1996) |
| – [but according to authors such as Haines, 1939; the anconeus is present in at least some urodeles, such as Salamandra: see text] | Anconeus (epicondylo-cubitalis sensuGaupp, 1896; extensor antebrachii ulnaris sensuRibbing, 1907) | – [but the anconeus is seemingly present in at least some other turtles, such as Testudo, Pelomedusa, Chelodina and Emys: see text] | – [but the anconeus is seemingly present in at least some lepidosaurs, such as Sphenodon: see text] | – | Anconeus (anconeus quartus sensuHolmes, 1977; seemingly corresponds to the ectepicondylo-ulnaris sensuMeyers, 1996 and Maxwell & Larsson, 2007) |
| Extensor digitorum (extensor digitorum communis sensuRibbing, 1907 and Walthall & Ashley-Ross, 2006; Diogo & Abdala, 2007; and Diogo, 2007; humerodorsalis sensuHaines, 1939) | Extensor digitorum (extensor digitorum communis longus sensuGaupp, 1896 and Manzano et al. 2008b; extensor digitorum communis sensuRibbing, 1907; humerodorsalis sensuHaines, 1939) | Extensor digitorum (humerodorsalis sensuHaines, 1939; extensor digitorum communis sensuWalker, 1973; extensor digitorum longus sensuAbdala et al. 2008) | Extensor digitorum (humerodorsalis sensuHaines, 1939; extensor digitorum longus sensuAbdala & Moro, 2006; extensor digitorum communis sensuDiogo & Abdala, 2007; and Diogo, 2007) | Extensor digitorum (extensor digitorum longus sensuHolmes, 1977 and Dilkes 2000; extensor carpi ulnaris longus sensuMeers, 2003) | Extensor digitorum (extensor digitorum longus sensuRibbing, 1938; Holmes, 1977 and Dilkes, 2000; extensor longus communis sensuDilkes, 2000) |
| Extensores digitorum breves | Extensores digitorum breves [probably correspond to the extensores breves superficiales, and, possibly, also to the extensores breves medii, sensuGaupp, 1896] | Extensores digitorum breves (extensores digiti brevis sensuAbdala et al. 2008) | Extensores digitorum breves (extensor digitorum brevis communis sensuHolmes, 1977; extensores digiti brevis sensuAbdala & Moro, 2006) | Extensores digitorum breves (extensor digitorum brevis communis sensuHolmes, 1977) | Extensores digitorum breves (extensores breves digitorum superficiales and seemingly also extensores breves digitorum profundi sensuRibbing, 1938; extensor digitorum brevis communis sensuHolmes, 1977) |
| Abductor et extensor digiti I (supinator manus sensuBrooks, 1889; Ribbing, 1907; Haines, 1939; Howell, 1936a,b; and Straus, 1941a,b;) | Abductor pollicis longus (abductor indicis longus sensuGaupp, 1896 and Manzano et al. 2008; supinator manus sensuHaines, 1939) | Abductor pollicis longus (supinator manus sensuHaines, 1939 and Walker, 1973) [according to Haines, 1939; Walker, 1973 and Abdala et al. 2008; turtles usually have an abductor pollicis longus running from the ulna to the metacarpal I] | Abductor pollicis longus (supinator manus sensuHaines, 1939 and Holmes, 1977; abductor longus pollicis sensuAbdala & Moro, 2006; abductor et extensor digiti I sensuDiogo & Abdala, 2007 and Diogo, 2007) | Abductor pollicis longus (supinator manus sensuHolmes, 1977) | Abductor pollicis longus (abductor digit 1 sensuRibbing, 1938; supinator manus sensuHolmes, 1977) |
The nomenclature of the muscles shown in bold follows that of the text; in order to facilitate comparisons, in some cases names often used by other authors to designate a certain muscle/bundle are given in front of that muscle/bundle, between round brackets; additional comments are given between square brackets. Data compiled from evidence provided by our own dissections and comparisons and by an overview of the literature (see text, Tables 2 and 3, and Figs 1–10).
Table 3.
Scheme illustrating the hypotheses regarding the homologies of the forearm and hand muscles of adults of representative amphibian and reptilian taxa, as well as of the mammalian Rattus norvegicus. It should be noted that many of these hypotheses have been previously proposed by the authors of the works that we reviewed to prepare the present paper; that is, the information shown in this table is based on the works of those authors and on our own dissections and comparisons (see text). The nomenclature of the muscles follows that used in the text
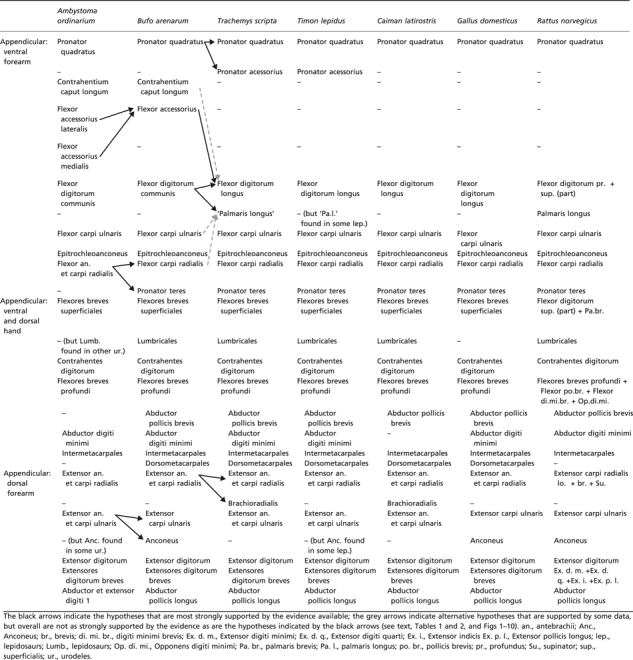 |
Fig. 8.
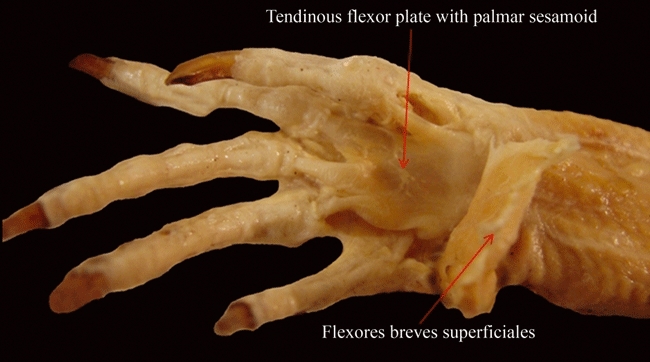
Tupinambis meriane (Reptilia, Lepidosauria): ventral view of the flexor plate after resection of the superficial layer of flexores breves superficiales.
Fig. 9.
Caiman latirostris (Reptilia, Crocodylia): ventral view of the palm of the hand after resection of part of the aponeurotic tissues covering it.
Fig. 10.
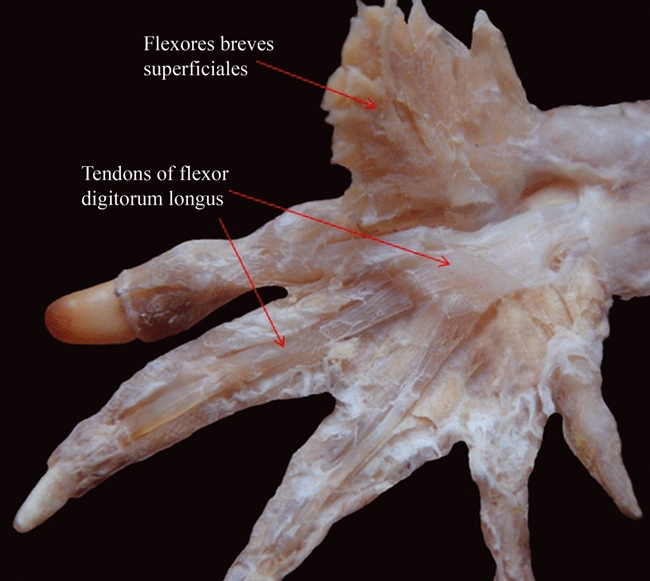
Caiman latirostris (Reptilia, Crocodylia): ventral view of the palm of the hand after resection of the layer of the flexores breves superficiales, showing that the expanded tendon of the flexor digitorum longus does not form a flexor plate.
Amphibia: Ambystoma mexicanum: MNCN, uncatalogued, 2 sp. Ambystoma ordinarium: MNCN, uncatalogued, 2 sp. Ambystoma texanum: FML 03402, 1 sp. Rhinella arenarum: FML 01352-1, 1 sp. Litoria caerulea: DIAM 0313, 1 sp. Phyllomedusa sauvagi: FML 04899, 2 sp, and DIAM 0337, 1 sp. Telmatobius laticeps: FML 3960, 1 sp. Aves: Cairina moschata: FML w/d, 1 sp. Coturnix coturnix: FML w/d, 2 sp. Gallus domesticus: FML w/d, 3 sp. Nothura sp. FML w/d 1 sp. Pitangus sulphuratus: FML w/d, 1 sp. Thraupis sayaca: FML w/d, 1 sp. Crocodylia: Caiman latirostris: FML w/d, 1 sp., and CCyTTP w/d, 4 sp. Lepidosauria: Ameiva ameiva: FML 03637, 4 sp. Amphisbaena alba: FML uncatalogued, 2 sp. Anisolepis longicauda: UNNEC no number, 1 sp. Basiliscus vittatus: SDSU 02097, 1 sp. Bogertia lutzae: MZUSP 54747, 1 sp. Briba brasiliana: MZUSP 73851, 1 sp. Callopistes maculatus: MZUSP 58107, 1 sp. Calyptommatus leiolepis: MZUSP 71339, 1 sp. Chalcides chalcides: FML 03712, 1 sp. Cnemidophorus ocellifer: FML 03389, 2 sp, FML 03409, 4 sp: without data, 1 sp, and FML 17606, 1 sp. Cordylus tropidosternon: AH no number, 1 sp. Crocodilurus lacertinus: MZUSP 12622, 1 sp. Dicrodon guttulatum: FML 02017, 1 sp. Diplolaemus bibroni: MACN 35850, 1 sp. Dracaena paraguayensis: MZUSP 52369, 1 sp. Echinosaura horrida: MZUSP 54452, 1 sp. Enyalius iheringii: MZUSP 74901, 1 sp. Garthia gaudichaudii: MZUSP 45329, 1 sp. Garthia penai: MZUSP 60937, 1 sp. Gekko vittatus: AH no number, 2 sp. Gerrohsaurus major: AH no number, 1 sp. Gymnodactylus geckoides: MZSP 48128, 1 sp. Hemidactylus garnoti: AH no number, 2 sp. Hemidactylus mabouia: FML 02142, 1 sp., and FML 02421, 1 sp. Homonota fasciata: FML 02137, 1 sp., and FML 00915, 2 sp. Leiosaurus paronae: MACN 4386, 1 sp. Liolaemus cuyanus: FML 02021, 7 sp. Mabuya frenata: FML 00277, 1 sp., and FML 01713, 1 sp. Microlophus theresioides: FML 03674, 1 sp. Phelsuma madagascariensis: AH no number, 2 sp. Phyllodactylus gerrophygus: FML 01563, 2 sp. Phyllopezus pollicaris: FML 02913, 2 sp. Phymaturus sp.: FML 13834-13844, 3 sp. Phymaturus punae: FML 2942, 4 sp. Podarcis sicula: FML 03714, 1 sp. Polychrus acutirostris: MZUSP 48151, 1 sp. MZUSP 08605, 1 sp. Pristidactylus achalensis: MACN 32779, 1 sp. Proctoporus guentheri: FML 02010, 1 sp. Teius teyous: FML 00290, 2 sp. Stenocercus caducus: FML 00260, 1 sp., and FML 00901, 1 sp. Thecadactylus rapicauda: MZUSP 11476, 1 sp. Tropidurus etheridgei: FML 03562, 2 sp. Tropidurus hygomi: FML 08796, 1 sp. Tropidurus oreadicus: FML 08771, 1 sp. Tropidurus spinulosus: FML 00129, 2 sp., and FML 03559, 2 sp. Tupinambis rufescens: PT 0084, 1 sp., PT 0085, 1 sp., FML 06412, 1 sp, FML 06425, 1 sp., and FML 07420, 1 sp. Vanzoia klugei: MZUSP 59130, 1 sp. Varanus sp.: AH no number, 1 sp. Xantusia sp.: AH no number 1, 1 sp. Amphisbaenidae: Zonosaurus sp.: AH no number, 1 sp. Testudines: Cuora amboinensis: YPM R 14443 1 sp. Cuora galbinifrons: YPM R 12735, 1 sp. Geochelone chilensis: DIAMR-038, 2 sp., DIAMR-039, 2 sp., DIAMR-040, 1 sp., FML 16879, 1 sp., FML 16880, 1 sp., FML16595, 1 sp., FML 00005, 1 sp., and FML 16978, 1 sp. Glyptemys insculpta: YPM R 5952, 1 sp. Mauremys caspica rivulata: YPM R 16233-36, 2 sp. Phrynops hilarii: DIAMR-044, 1 sp., DIAMR-042, 1 sp., DIAMR-041, 1 sp., DIAMR-043, 1 sp., DIAMR-037, 1 sp., DIAMR-005, 1 sp., DIAMR-006 1 sp., and DIAMR-007, 1 sp. Podocnemys unifilis: DIAMR-078, 6 sp. Rhinoclemmys pulcherrima: AH uncatalogued, 1 sp. Sacalia bealei: YPM R 14670-71 2 sp. Terrapene carolina: YPM R 13624 1 sp. YPM R 13622 1 specimen. Testudo graeca: HUJ-R 22843; HUJ-R 22845 2 sp. Trachemys scripta: RT uncatalogued, 2 sp.
Results
The results of our observations and comparisons are summarized in Tables 1–3, which present the best supported hypotheses of homology for the muscles discussed in the present paper. In the Tables, the muscles that we interpret as homologous structures are listed on the same line with the same name. Because it is not possible, due to space limitations, to provide an extensive discussion of the homologies and evolution for each of the pectoral and forelimb muscles of all taxa, we pay special attention only to issues that remain particularly controversial among morphologists. The muscles listed in these Tables are those that are usually present in adults of the respective taxa, and are listed in the same order as used by Diogo et al. (2009a).
Pectoral muscles derived from the postcranial axial musculature
Amphibian and reptilian taxa have six muscles derived from the axial musculature: serratus anterior, ‘rhomboideus’, levator scapulae, opercularis, sternocoracoideus and costocoracoideus. These six muscles mainly connect the axial skeleton to the pectoral girdle, and thus are associated with the movements of this girdle (Diogo et al. 2009a). Holmes (1977), Dilkes (2000) and Tsuihiji (2007) described a ‘serratus superficialis’ and a ‘serratus profundus’ in lepidosaurs, crocodylians and birds, the latter structure corresponding to the serratus anterior sensu this work, according to Holmes (1977). Howell (1935, 1937b) and Duellman & Trueb (1986) describe a ‘rhomboideus anterior’ and a ‘rhomboideus posterior’ in anurans such as Rana; according to Howell (1935, 1937b) urodeles do not have rhomboid muscles. Our dissections confirmed the presence of these two rhomboid structures in other frogs, such as Rhinella (Tables 1 and 2), and they were also described by Escariz de Peverelli in Bufo arenarum (1965). Authors such as Kardong (2002) suggest that reptiles do not have a ‘rhomboideus’, but Howell (1935, 1936a, 1937b), Sullivan (1962, 1967), Hudson et al. (1972), Dilkes (2000) and Meers (2003) argue that crocodylians and birds also have ‘rhomboid’ muscles; for instance, Sullivan (1962, 1967) report a ‘rhomboideus superficialis’ and a ‘rhomboideus profundus’ in birds. These descriptions are also confirmed by our dissections (Tables 1 and 2). To our knowledge, a ‘rhomboideus’ has never been described in lepidosaurs or turtles, nor were we able to find this structure in our own dissections of these reptiles (Tables 1 and 2). Jouffroy (1971) states that the mammalian rhomboideus is homologous to the ‘basiscapularis’ of ‘lower tetrapods’. However, the ‘basiscapularis’ muscle described in urodeles by authors such as Smith (1926), which runs from the occipital region to the scapula and is often innervated by the ventral rami of spinal nerves 1 and/or 2, clearly seems to correspond to the urodele levator scapulae sensuHowell (1935). In fact, Smith (1926) did list the ‘basiscapularis’ as a synonym of the urodele levator scapulae. Our dissections and comparisons pointed out that the overall configuration and the proximal and distal attachments of the ‘rhomboideus’ of anurans, crocodylians and birds are similar to those of the rhomboideus of mammals. In all these taxa, the ‘rhomboideus’ is mainly horizontal, originating proximally from the axial skeleton and inserting distally onto the scapula. However, it should be noted that in view of the phylogenetic framework we are using in this paper, it is cladistically more parsimonious to consider that the ‘rhomboideus’ was independently acquired in anurans, archosaurs and mammals (three evolutionary steps) than to consider that it was present in the last common ancestor (LCA) of tetrapods and then secondarily lost in urodeles (considering that the ‘subscapularis’ of some authors does not correspond to the ‘rhomboideus’ of other tetrapods), turtles and lepidosaurs (four evolutionary steps; see Fig. 1). In this specific case, this cladistically most parsimonious hypothesis implies that anurans, archosaurians and mammals independently acquired a muscle with a similar origin, insertion, orientation and function. The secondary loss of the muscle in turtles required by the alternative hypothesis would actually not be unsound, due to the presence of the carapace and the rigid connection between the skeletal elements of the axial and of the shoulder girdle in these reptiles. However, one would have to explain why this muscle would also have been secondarily lost in lepidosaurs and (in case it does not correspond to the ‘basiscapularis’ of some authors) in urodeles. In our opinion, what is missing here is a detailed study of the dorsal pectoral muscles of a greater number of taxa of tetrapod groups such as lepidosaurs and urodeles, and particularly of the most plesiomorphic extant members of these groups. For the moment, following the phylogenetic framework shown in Fig. 1, we prefer to prudently write ‘rhomboideus’ in Tables 1 and 2 (in the columns concerning anurans and archosaurs) to indicate that the ‘rhomboideus’ of archosaurs might actually not be homologous to the ‘rhomboideus’ of anurans and to the rhomboideus of mammals.
Table 2.
Scheme illustrating the hypotheses regarding the homologies of the pectoral and arm muscles of adults of representative amphibian and reptilian taxa, as well as of the mammal Rattus norvegicus. It should be noted that many of these hypotheses have been previously proposed by the authors of the works that we reviewed in order to prepare the present paper; that is, the information shown in this table is based on the works of those authors and on our own dissections and comparisons (see text). The nomenclature of the muscles follows that used in the text
 |
The levator scapulae is a voluminous muscles that, in urodeles, connects the cranium (often the cartilaginous operculum) to the pectoral girdle (e.g. Walthall & Ashley-Ross, 2006). Hetherington & Tugaoen (1990) noted that the structure of urodeles such as Ambystoma that is often named ‘opercularis’ corresponds, topologically, to part of the levator scapulae sensu the present work, which, in anurans such as Rana, is completely differentiated into two distinct muscles, the levator scapulae superioris and the opercularis sensu the present work (Tables 1 and 2). Therefore, the name opercularis should only be used for anurans. Piatt (1938), based on his developmental study of Ambystoma, suggests that the levator scapulae of this taxon derives from somites 2–4, together with the hypobranchial muscles. The recent ontogenetic work of Piekarski & Olsson (2007) makes clear that in Ambystoma the levator scapulae derives mainly from somite 3, being innervated by the first spinal nerve and also by the hypoglossal nerve. This is somewhat unexpected because this latter nerve is usually associated with the hypobranchial muscles (see Diogo et al. 2009a). However, Piekarski & Olsson (2007) show that the development and innervation of the levator scapulae are different from the innervation and development of the branchial muscle protractor pectoralis (‘cucullaris’), thus contradicting that the levator scapulae of urodeles derive from the protractor pectoralis, as was often suggested in the older literature (for more details about this subject, see Piekarski & Olsson, 2007). In squamata, the levator scapulae mainly run from the transverse process of the atlas to the suprascapula, scapula and clavicle (e.g. Russell & Bauer, 2008; this work). According to authors such as Holmes (1977) and Dilkes (2000), in lepidosaurs, including Sphenodon, the levator scapulae are usually divided into superficial and deep heads. The Crocodylia have mainly undivided levator scapulae (e.g. Holmes, 1977; Dilkes, 2000; Meers, 2003; Tsuihiji, 2007); Holmes (1977) argued that the ‘levator scapulae profundus’ portion of the ‘collothoraciscapularis profundus’sensuFürbringer (1876) is probably part of the serratus musculature, and not of the levator scapulae sensu the present work). As explained by Dilkes (2000), in birds the levator scapulae is not present as an independent muscle. The levator scapulae are thus consistently present in all major tetrapod extant taxa, except birds. In spite of some contradictory information regarding its development and innervation, the topology, function, orientation and taxonomic distribution of the levator scapulae indicates that this muscle is homologous across amphibians, reptiles and mammals (Tables 1 and 2; see also Diogo et al. 2009a).
Regarding the sternocoracoideus and costocoracoideus, the former muscle is present in various lepidosaurs and birds, while the latter is found in various lepidosaurs and crocodylians, where it is often subdivided into a ‘pars superficialis’ and a ‘pars profundus’ (e.g. Fürbringer, 1876; Romer, 1924; Howell, 1937b; Walker, 1973; Holmes, 1977; Dilkes, 2000; Tables 1 and 2). Mivart (1869) suggested that the subclavius could be part of the procoracohumeralis of amphibians, but according to Romer (1924) this latter muscle gives rise to mammalian muscles such as the teres minor and the deltoideus clavicularis instead. Howell (1937b) corroborated the idea that the sternocoracoideus and costocoracoideus are not present as distinct muscles in urodeles and anurans, that reptiles such as Iguana have a costocoracoideus and a sternocoracoideus superior and inferior, that monotreme mammals such as Ornithorhynchus have a costocoracoideus, and a sternocoracoideus, and that placental mammals have a ‘costoscapularis’ and a subclavius, the latter muscle thus seemingly corresponding to the sternocoracoideus of other tetrapods. As explained by Holmes (1977) and Dilkes (2000), in lepidosaurs such as Iguana and Sphenodon the sternocoracoideus is often divided into superficial and deep heads. As noted by Howell (1937b), Holmes (1977) and Dilkes (2000), in these lepidosaurs the costocoracoideus is also often divided into a ‘pars superficialis’ and a ‘pars profunda’, as is usually the case in crocodylians (see Meers, 2003). The homologies of the reptilian costocoracoideus and sternocoracoideus and of the mammalian subclavius have recently been discussed in detail by Diogo et al. (2009a).
Appendicular muscles of the pectoral girdle and arm
The pectoralis muscle of amphibians and reptiles is an intrinsic, fan-shaped muscle of the forelimb that usually runs from the sternum, clavicle and/or adjacent structures to the humerus and/or the scapula (e.g. Romer, 1944; Russell & Bauer, 2008; Diogo et al. 2009a). In salamanders it can originate from the fascia of the rectus abdominis, and usually inserts onto the humerus (e.g. Duellman & Trueb, 1986). The pectoralis muscle in amphibians and reptiles is usually divided into superficial and deep heads (e.g. Russell & Bauer, 2008). Our dissections show three heads of the pectoralis in anurans such as Rhinella. Manzano (1996) also described three heads of this muscle in pseudid frogs, which she designated as ‘epicoracoideus’, ‘esternalis’ and ‘abdominalis’. Interestingly, in Ambystoma, as well as in other urodeles such as Taricha (Walthall & Ashley-Ross, 2006), the pectoralis is mainly undivided. According to authors such as Romer (1944) and Kardong (2002), the plesiomorphic condition for reptiles is that in which the pectoralis is also mainly undivided, as is often the case in lepidosaurs and in turtles (Walker, 1973; this work). In crocodylians, the pectoralis is, however, usually subdivided into two or three heads: ‘cranial’ and ‘caudal’, or ‘cranial’, ‘caudal’ and ‘deep’sensuMeers (2003). In birds the pectoralis is often divided into a ‘pectoralis superficialis’ and a ‘pectoralis profundus’ (e.g. Dilkes, 2000), although authors such as Hudson et al. (1972) refer to a ‘pars thoracica’, a ‘pars propatagialis’ and a ‘pars abdominalis’. The avian ‘pectoralis profundus’ seemingly does not correspond to the ‘entopectoralis’ of some mammals. Instead, according to Diogo et al. (2009a) it corresponds to part of the mammalian ‘ectopectoralis’, i.e. of the pectoralis major sensuDiogo et al. (2009a), which is also often divided, in the mammalian literature, into ‘profundus’ (abdominal head sensuDiogo et al. 2009a) and ‘superficialis’ (sternocostal and/or clavicular head sensuDiogo et al. 2009a). The three divisions of the mammalian ‘entopectoralis’, i.e. the pectoralis abdominalis, pectoralis minor and ‘pectoralis tertius’sensuDiogo et al. (2009a), thus seem to be absent as distinct structures in birds and in most, if not all, non-mammalian tetrapods (Tables 1, 2; see also Diogo et al. 2009a). In summary, it can be said that all the major groups of tetrapods shown in the tree of our Fig. 1 have a pectoralis. Regarding the division of this muscle, the hypothesis proposed by Romer (1944) and Kardong (2002) is supported by the phylogenetic scenario shown in Fig. 1: the muscle was mainly undivided in the LCA of tetrapods, and then became divided into bundles in the lineage leading to anurans, in the lineage leading to birds + crocodylians, and in the lineage leading to mammals, independently; this requires three evolutionary steps; to consider that the division was acquired in the LCA of tetrapods and then secondarily lost in urodeles, turtles and lepidosaurs would require four evolutionary steps. Moreover, in urodeles, in mammals, and in birds + crocodylians, the pectoralis has different configurations and a different number of divisions, as explained above, supporting the idea that these divisions were effectively acquired independently in evolution (Tables 1 and 2; Figs 2 and 3).
It is now accepted that the mammalian supraspinatus and infraspinatus, which usually connect the dorsal region of the pectoral girdle to the proximal region of the arm, derive from the supracoracoideus (Tables 1 and 2), a muscle that lies ventral, not dorsal, to the pectoral girdle in most other extant tetrapods (e.g. Kardong, 2002; Diogo et al. 2009a). In a few non-mammalian taxa, such as chameleons, the supracoracoideus does also occupy a more dorsal space, as in mammals, thus leading some authors to propose that these reptiles have an ‘infraspinatus’ and a ‘supraspinatus’ (Jouffroy, 1971). However, this idea was not accepted by authors such as Romer (1922, 1924, 1944), who argued that the dorsal position of the supracoracoideus of chameleons is autapomorphic. According to Walker (1973), in turtles the supracoracoideus often consists of ‘anterior’ and ‘posterior’ bundles, and according to Meers (2003) in crocodylians this muscle is often divided into three heads (‘longus’, ‘intermedius’ and ‘brevis’).
The deltoideus scapularis is consistently present in amphibians and reptiles (Tables 1 and 2). It is a muscle that usually mainly connects the suprascapula, scapula and/or occasionally the clavicle to the humerus. In urodeles, it commonly originates from the suprascapular cartilage, and inserts onto the humerus. In anurans the ‘pars scapularis of the deltoides’sensuDuellman & Trueb (1986), which corresponds to the deltoideus scapularis sensu the present work, usually runs from the lateral end of the clavicle and the anterior and ventral surfaces of the scapula to the humerus. According to Dilkes (2000), the deltoideus scapularis probably corresponds to the muscle that is often designated as ‘deltoideus major’ in birds, and not to both the avian ‘deltoideus major’ and ‘deltoideus minor’. As explained by Romer (1944), the ‘longus’ head of the amphibian procoracohumeralis corresponds to the deltoideus clavicularis plus humeroradialis of reptiles such as Sphenodon, birds and crocodylians, and the ‘brevis’ head of the amphibian procoracohumeralis corresponds to the scapulo-humeralis anterior of reptiles such as lepidosaurs and birds (Tables 1 and 2; see also Diogo et al. 2009a). The deltoideus clavicularis is present in turtles, crocodylians, lepidosaurs and birds (e.g. Holmes, 1977; Dilkes, 2000; this work). Dilkes (2000) stated that in turtles the deltoideus clavicularis is partially fused with the deltoideus scapularis; these two structures are described as ‘part of the deltoideus’ by Walker (1973) and Wyneken (2001). Walker (1973) states that in some turtles, such as trionychids, the ‘deltoideus’ is undivided, i.e. the ‘dorsal, or scapular, head’ is not differentiated in these turtles. According to Dilkes (2000), in birds the deltoideus clavicularis is sometimes divided into a ‘pars cranialis’ and a ‘pars caudalis’; as stated by this author, the ‘deltoideus minor’ of birds probably corresponds to part or all of the deltoideus clavicularis of other tetrapods, and not to part of the deltoideus scapularis, as suggested by Romer (1944; see above). In the case of reptiles, using the name deltoideus clavicularis, as do most authors working with amniotes, is justified because this muscle does not correspond directly to the procoracohumeralis of amphibians such as Ambystoma. It corresponds only to part of the procoracohumeralis; the other part of the amphibian procoracohumeralis corresponds to the scapulo-humeralis anterior of reptiles such as Timon.
In turtles, the deltoid musculature has been described as one of the most variable of the shoulder muscles (Walker, 1973). According to Romer (1944), Jollie (1962), Jouffroy (1971) and Holmes (1977) the scapulo-humeralis posterior is present in Sphenodon, crocodylians and birds, and absent in turtles and all ‘lizards’ except Agama; according to Dilkes (2000) this muscle is effectively present in at least some squamates. Jouffroy (1971) argued that the reptilian scapulo-humeralis posterior might be homologous to the mammalian teres minor, because both these muscles derive from the deltoideus scapularis (see Table 2). However, authors such as Holmes (1977) argued that the scapulo-humeralis anterior and scapulo-humeralis posterior were acquired during the evolution of reptiles, i.e. that these muscles were not differentiated in the LCA of extant reptiles, and, thus, that the mammalian teres minor cannot be directly homologous to the scapulo-humeralis posterior of some reptilian taxa. As the deltoideus scapularis is present in reptiles and amphibians, and also in mammals (Diogo et al. 2009a), and has basically the same topology and function (mainly to elevate and rotate the humerus) in all these taxa, it is likely that this muscle had a similar topology and function in the LCA of tetrapods. According to Meers (2003) the humeroradialis is mainly a flexor of the antebrachium that is only present in living archosaurs and that was probably derived from the dorsal musculature, being perhaps developmentally related with the deltoid muscles (e.g. it is innervated by the axillary nerve). Authors such as Romer (1944), Jollie (1962) and Sullivan (1962, 1967) did support the idea that the humeroradialis is related to the deltoid group, and specifically to the deltoideus clavicularis, thus corresponding to part of the procoracohumeralis longus of amphibians. The humeroradialis does not seem to be present as a distinct muscle in Timon (Tables 1 and 2); however, contrary to the statements of Meers (2003), authors such as Romer (1944) and Jollie (1962) stated that the humeroradialis is also present in the lepidosaur Sphenodon. Regarding the scapulo-humeralis anterior, Holmes (1977) and Dilkes (2000) argued that this muscle is not present as a separate structure in turtles. Fürbringer (1876), Romer (1944), Jollie (1972), Holmes (1977), Dilkes (2000) and Meers (2003) also stated that the scapulo-humeralis anterior is not present as an independent muscle in crocodylians, but is present in birds, ‘lizards’ and Sphenodon.
The subcoracoscapularis is consistently present in amphibians and reptiles. This muscle was not described in Taricha torosa by Walthall & Ashley-Ross (2006), and our dissections did not allow us to discern appropriately if it is present in urodeles such as Ambystoma ordinarium (Table 1). However, according to authors such as Romer (1944) and Kardong (2002), the subcoracoscapularis is found in various urodeles. As explained by Walker (1973), the subcoracoscapularis is usually undivided in turtles, but may be divided into a shorter, ‘medial head’ and a longer, ‘lateral head’ in taxa such as sea turtles, Testudo and Hydromedusa. The subcoracoscapularis is mainly undivided in turtles and crocodylians, corresponding to the muscle that is often designated, in these two groups, as ‘subscapularis’. In Sphenodon, squamates and birds, the subcoracoscapularis is divided into a ‘subscapularis’ and a ‘subcoracoideus’, each of these two structures being in turn often subdivided into two heads in various birds (see Holmes, 1977; Dilkes, 2000). The mammalian teres major, another muscle that mainly connects the scapula to the humerus, is probably derived from the subcoracoscapularis (Tables 1 and 2; Diogo et al. 2009a). According to Dilkes (2000), there is a ‘teres major’ in turtles, crocodiles and many ‘lizards’, but not in lepidosaurs such as Sphenodon, Iguana and in birds. Jouffroy (1971) and Meers (2003) confirm that crocodylians have a ‘teres major’. Romer (1944) also states that there is a ‘teres major’ in crocodylians, and that this muscle is absent in Sphenodon and Aves, but, contrary to Dilkes (2000), he argues that the ‘teres major’ is also missing in the whole of Squamata. In our dissections, we were unable to find a distinct ‘teres major’ in ‘lizards’ such as Timon. Walker (1973) and Wyneken (2001) state that turtles often have a ‘teres major’, although this structure is often indistinct from the latissimus dorsi. Howell (1937b) defends the definition that only mammals have a ‘true’ teres major, thus suggesting that the ‘teres major’ of reptiles, such as crocodylians and turtles is not homologous to the mammalian teres major. However, in view of our dissections, comparisons and review of the literature, we see no reasons to discard the hypothesis that the ‘teres major’ of reptiles such as crocodylians and turtles is homologous to the teres major of mammals. In fact, the ‘teres major’ muscles of these three latter groups have similar configurations and attachments, running mainly in a lateral direction from its proximal origin on the scapula (and adjacent structures in some cases, e.g. also from the carapace in turtles) to its tendinous distal insertion on the proximal humerus (e.g. Wyneken, 2001; Meers, 2003; this work). If future studies reveal that a ‘teres major’ is present in at least some lepidosaurs, as stated by Dilkes (2000), it would be phylogenetically more parsimonious to assume that the LCA of amniotes had a teres major and that this muscle was secondarily lost in the branch leading to Aves (two evolutionary steps), than to assume that it was independently acquired in lepidosaurs, turtles, crocodylians, and mammals (four evolutionary steps). We plan to address this subject in a future work, by carefully dissecting more lepidosaurs and comparing the dorsal pectoral muscles found in the dissected taxa to those muscles found in the other extant reptiles and in extant mammals.
The latissimus dorsi is a dorsal muscle of the pectoral girdle and the triceps brachii is mainly an extensor of the forearm. Both these muscles are consistently present in urodeles, anurans, turtles, lepidosaurs, crocodylians and birds (Fig. 2; Tables 1 and 2). In all these taxa the latissimus dorsi connects the axial skeleton to the humerus, being mainly associated with the retraction of the arm. This muscle is mainly undivided in crocodylians, lepidosaurs and turtles, but is often divided into a ‘pars cranialis’ and a ‘pars caudalis’ in birds (e.g. Holmes, 1977; Dilkes, 2000; Meers, 2003; this work). The triceps brachii (Figs 2 and 3) usually connects the scapula and humerus to the proximal region of the forearm and is often divided into various bundles. In urodeles this muscle usually includes coracoideus (‘coracotriceps’), scapularis medialis (‘dorsitriceps’), humeralis lateralis (‘humerotriceps lateralis’) and humeralis medialis (‘humerotriceps lateralis’) sections, which correspond respectively to the ‘anconeus coracoideus’, ‘anconeus scapularis medialis’, ‘anconeus humeralis lateralis’ and ‘anconeus humeralis medialis’sensuWalthall & Ashley-Ross (2006), Diogo & Abdala (2007) and Diogo (2007). Howell (1935, 1937b) seem to suggest that the ‘coracotriceps’ of urodeles such as Necturus might correspond to the triceps coracoideus of reptiles such as Iguana and thus to the dorso-epitrochlearis of mammals (see also Diogo et al. 2009a). He also stated that in anurans such as Rana, the ‘coracotriceps’ is not present as a distinct structure, but that in these amphibians the ‘dorsitriceps’ (or ‘anconeus scapularis’) is present and the ‘humerotriceps’ is divided into three divisions comprising ‘laterale’, ‘mediale’ and ‘profundum’, the latter division being merely a separable part of the ‘mediale’ division. In turtles the triceps brachii usually has a ‘scapular’ head and a ‘humeral’ head (which are designated as ‘long lateral head and short lateral head’ by Holmes (1977), but in some taxa, such as Dermochelys, only one head (the ‘humeral’ head according to Wyneken, 2001) is present (e.g. Walker, 1973; Holmes, 1977; Dilkes, 2000; Wineken 2001; this work). There are usually four heads of the triceps (‘scapular’, ‘coracoid’, ‘lateral humeral’, and ‘medial humeral’) in lepidosaurs, including Sphenodon (e.g. Holmes, 1977; Dilkes, 2000). According to Dilkes (2000) crocodylians usually have five (‘scapular’, ‘coracoid’, ‘lateral humeral’, ‘medial humeral’, and an ‘extra humeral’ head known as the ‘posticum’). Holmes (1977) had suggested that crocodylians usually only have four heads, but Meers (2003) also described five heads (which he designated as ‘triceps longus lateralis’, ‘triceps longus caudalis’, ‘triceps brevis cranialis’, ‘triceps brevis intermedius’ and ‘triceps brevis caudalis’), thus corroborating the observations of Dilkes (2000). Dilkes (2000) also stated that the number of heads of the triceps brachii is usually two or three (‘scapulotriceps’, ‘humerotriceps’, and occasionally a greatly reduced ‘coracotriceps’) in Aves. Authors such as Grim (1971) and Haninec et al. (2009) state that Aves, such as chickens, have a ‘dorsoepitrochlearis’, which is usually named ‘metapatagial latissimus dorsi’, and which would correspond to the triceps coracoideus sensu the present work and thus to the ‘coracotriceps’sensuDilkes (2000). However, Sullivan (1962, 1967) only describes a ‘humerotriceps’ and a ‘scapulotriceps’ in chickens. Holmes (1977) argued that having four heads is the plesiomorphic condition for reptiles. Diogo et al. (2009a) supported the idea that this is also the plesiomorphic condition for amniotes and for living tetrapods as a whole. Extant amphibians such as salamanders and reptiles such as lepidosaurs often have four heads of the triceps (see above), and mammals usually have three heads of the triceps plus a dorsoepitrochlearis, which derives from/corresponds to the coracoid head of the triceps of other tetrapods. This hypothesis is effectively the most parsimonious under the phylogenetic scenario followed in the present work, because it only requires three or four evolutionary steps, three evolutionary steps only if future studies will confirm that birds often have three heads of the triceps brachii plus a ‘dorsoepitrochlearis’, i.e. four heads in total. In this case, the LCA of tetrapods had four heads, and the main changes occurred in the lineage leading to anurans, crocodylians and turtles, which seem to commonly have three, five and two heads, respectively. Considering that the LCA of tetrapods had any other number of heads, e.g. two, three or five heads, would require a greater number of evolutionary steps.
The humeroantebrachialis of urodeles such as Ambystoma very likely corresponds to the brachialis and to part (the long head) of the biceps brachii of amniotes; in many anuran amphibians (and in at least some urodeles, such as Triturus: see Smith, 1926) there is also a coracoradialis, which probably corresponds to the short head of the biceps brachii of amniotes, although it is possible that this short head derives instead/also from the coracobrachialis (e.g. Romer, 1944; Kardong, 2002; Diogo et al. 2009a; Tables 1 and 2). Our dissections of anurans confirmed that, contrary to urodeles such as Ambystoma, which only have a humeroantebrachialis (usually running from the humerus to the forearm and commonly flexing this forearm) and a coracobrachialis (usually running from the coracoid to the humerus and commonly retracting the arm), anurans such as Rhinella have a humeroantebrachialis, a coracobrachialis, and a coracoradialis (this latter muscle usually connecting the omosternum and/or the epicoracoid to the forearm, and often promoting the flexion of this forearm: e.g. Duellman & Trueb, 1986; this work; Tables 1 and 2). Walker (1973) stated that turtles often have a ‘superficial’ head and a ‘deep’ head of the biceps brachii, which usually originate from the coracoid. He suggested that in testudinines and sea turtles the biceps brachii is mainly undivided or only partially divided, but Wyneken (2001) argued that in most sea turtles the biceps brachii is actually clearly divided into ‘superficial’ and ‘deep’ heads. Holmes (1977) and Dilkes (2000) state that apart from some birds, in which there is usually an origin from the humerus and the coracoid, the biceps brachii of reptiles normally originates from the coracoid only; as described by these authors, the biceps brachii commonly has more than one belly in some lepidosaurs. According to Meers (2003), a poorly developed ‘short head’ of the biceps, originated from the shoulder joint capsule, is occasionally found in crocodylians. The coracobrachialis medius/proprius and coracobrachialis profundus/brevis seem to be missing in urodeles such as Taricha (Walthall & Ashley-Ross, 2006), but are present in various other urodeles according to authors such as Howell (1935), Romer (1944) and Jollie (1962). In turtles, ‘lizards’ and Sphenodon the coracobrachialis has a ‘caput longum’ and a ‘caput brevis’ (e.g. Jollie, 1962; Walker, 1973; Holmes, 1977; Dilkes, 2000; Russell & Bauer, 2008; this work). Romer (1944) suggested that the ‘coracobrachialis externus’ plus ‘coracobrachialis internus’ of birds correspond to the coracobrachialis brevis of crocodylians, and, thus, that the coracobrachialis longus is absent in birds. However, as explained by authors such as Jollie (1962), Holmes (1977) and Dilkes (2000), and corroborated by our dissections, birds do seem to have both a coracobrachialis longus and a coracobrachialis brevis sensu the present work, which are often designated, in these reptiles, as ‘coracobrachialis cranialis’ (or ‘anterior’) and ‘coracobrachialis caudalis’ (or ‘posterior’), respectively. According to Sullivan (1962, 1967), the muscles that he designates as ‘coracobrachialis anterior’ and ‘coracobrachialis’ in birds correspond to the muscles that are often named as ‘deltoideus minor’ and ‘coracobrachialis anterior’ by other authors, respectively. Also according to him, the avian muscle that he designates as ‘coracobrachialis posterior’ has no separate homologue in other, non-avian reptiles. Romer (1944), Holmes (1977) and Dilkes (2000) suggested that crocodylians have only a ‘coracobrachialis brevis’, but Meers (2003) reported a ‘coracobrachialis brevis ventralis’ and a ‘coracobrachilais brevis dorsalis’ in these reptiles. As the coracobrachialis brevis/proprius and corocabrachialis longus/profundus are consistently found in at least urodeles, turtles and lepidosaurs, and also in mammals (see Diogo et al. 2009a), one can conclude that these two heads of the coracobrachialis were present in the LCA of tetrapods (see Fig. 1). Before passing to the next section, we would like to note that, interestingly, despite having a carapace and a highly modified skeletal anatomy, i) the orientation and attachments of the pectoral muscles are very similar across the adult members of different testudine taxa (the main differences being related to aspects such as the size of some muscles, as stressed by authors such as Bojanus, 1819; Walther, 1922 and Walker, 1973); and ii) the pectoral muscles of adult testudines are basically the same muscles found in other reptiles (Table 2).
Appendicular muscles of the forearm and hand
The muscles of the forearm and hand of tetrapods may be divided into three main groups: the ventral muscles of the forearm (usually flexors of the hand/digits and/or pronators of the forearm), the muscles of the hand, and the dorsal muscles of the forearm (usually extensors of the hand/digits and/or supinators of the forearm). In both amphibian and reptiles, the extensor (dorsal) and (ventral) layers of the forearm have the same basic structure, both being composed superficially of three muscular complexes: the ‘ulnar extensors/flexors’, the ‘radial extensors/flexors’ and the ‘extensor/flexor digitorum communis/longus’. These muscular complexes usually arise from the humerus and insert onto the distal portion of the radius, the distal portion of the ulna, and the hand (carpal, metacarpal and/or phalangeal) bones, respectively. These six muscular complexes are present in all major extant clades of limbed amphibians and reptiles. We prefer to use the name muscular complexes because all these six complexes actually include more than one muscle in at least one of these clades (see Tables 1 and 3, and also below) (Figs 2–11).
Fig. 11.
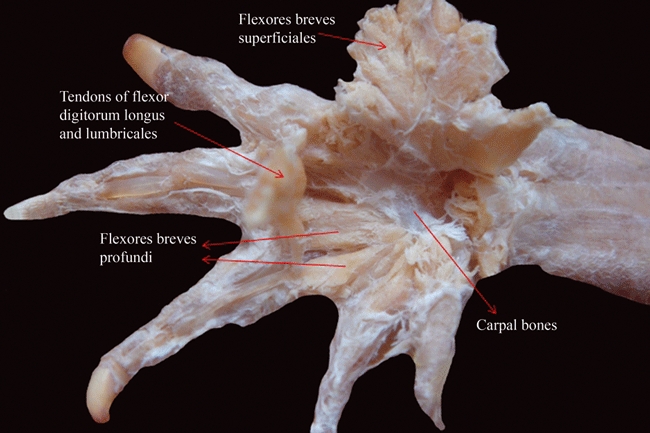
Caiman latirostris (Reptilia, Crocodylia): ventral view of the deep (dorsal) musculature of the palm of the hand after resection of the more superficial (ventral, or palmar) layer.
The ‘flexor digitorum communis/longus’ muscular complex usually arises tendinously from the distal portion of the humerus, and inserts onto the terminal phalanx of the hand digits, its main function being to flex the digits. As explained by Diogo et al. (2009a), the flexor accessorius lateralis and the flexor accessorius medialis of urodeles are fused with the flexor digitorum communis to form the flexor digitorum longus of reptiles and monotremes, which in therian mammals is usually divided into a flexor digitorum profundus and a flexor digitorum superficialis. The tendons of the flexor digitorum superficialis of therian mammals are usually bifurcated to surround the insertion tendons of the flexor digitorum profundus. These bifurcated tendons of therian mammals correspond to the bifurcated tendons of the flexores breves superficiales of non-mammalian tetrapods (Tables 1 and 3; Diogo et al. 2009a). According to Holmes (1977) and Dilkes (2000), in reptiles such as ‘lizards’, Sphenodon and crocodylians the flexor digitorum longus usually has ‘superficial’, ‘deep ulnar’ and ‘deep humeral’ heads, the latter being very likely fused with the structure that Holmes (1977) designates as ‘pronator radii teres’(see Tables 1 and 3, and below). Therefore, it is possible that the ‘superficial’ head corresponds to the amphibian flexor digitorum communis (Fig. 4), and that the two reptilian ‘deep’ heads correspond to the amphibian flexor accessorius lateralis and flexor accessorius medialis. In non-archosaur amniotes (i.e. in mammals, turtles and lepidosaurs) the flexor digitorum longus/flexor digitorum profundus usually inserts onto all five digits, the flexor digitorum longus being often divided into a superficial bundle and a deep bundle in turtles (e.g. Ribbing, 1907; Abdala et al. 2008) and the flexor digitorum profundus differentiated into various heads in mammals (e.g. Jouffroy, 1971). In birds and crocodylians the flexor digitorum longus muscle usually inserts onto only some digits (e.g. Ribbing, 1938; Holmes, 1977; Dilkes, 2000; Meers, 2003; this work). According to Meers (2003), in crocodylians the flexor digitorum longus has humeral, ulnar and carpal heads (the humeral head clearly corresponds to the superficial head sensuHolmes, 1977, whereas the two other heads seem to correspond to the deep humeral and deep ulnar heads sensuHolmes, 1977) and inserts onto the penultimate phalanx of digits 1, 2 and 3 (and not to digits 2, 3 and 4 as stated by Dilkes, 2000). But Holmes (1977) and Dilkes (2000) state that in lepidosaurs, turtles and crocodylians the flexor digitorum longus usually inserts onto the distal phalanges of the digits instead, and in the crocodylian specimen shown in Fig. 16 of Meers (2003) this muscle does seem to insert onto the distal phalanges. In the crocodylian specimens dissected by us, the muscle also seems to insert onto the distal phalanges. According to authors such as Sullivan (1962), Shellswell & Wolpert (1977), Meyers (1996) and Dilkes (2000), birds usually have a superficial head and a deep head of the flexor digitorum longus, which, in neognath birds, are usually inserted onto the two phalanges of digit 2 (i.e. digit 3 according to embryology) and onto the distal phalanx of the same digit, respectively. Also according to Dilkes (2000), the kiwi Apteryx lacks the superficial head and has a mostly tendinous deep head that inserts on the terminal phalanx of digit 2. Considering the phylogenetic framework followed in the present work, one can conclude that in the LCA of living reptiles the flexor digitorum longus insertw onto digits 1–5, the configuration found in crocodylians and birds thus being derived.
Fig. 4.
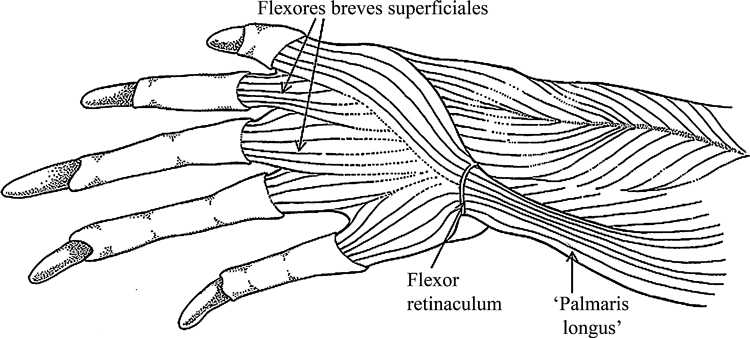
Tupinambis meriane (Reptilia, Lepidosauria): ventral view of the superficial musculature of the forelimb, showing the continuous layer between the flexores digitorum breves and the ‘palmaris longus’.
Authors such as Ribbing (1938) described a ‘flexor digitorum sublimis’ in birds but, considering its topology, this structure clearly seems to correspond to the ‘superficial’ head of the flexor digitorum longus sensuHolmes (1977) and Dilkes (2000), and not to the flexor digitorum superficialis (often called ‘sublimis’) of marsupial and placental mammals (Diogo et al. 2009a). McMurrich (1903a) and Ribbing (1907) argued that the flexor accessorius lateralis and medialis of urodeles correspond to part of the flexor digitorum longus of reptiles. Although those authors state that the contrahentium caput longum of urodeles may also correspond to part of the flexor digitorum longus of reptiles, they consider that it is more likely, based on topology and innervation, that the contrahentium caput longum is completely missing in amniotes, an idea recently corroborated by Diogo et al. (2009a). The flexor accessorius of anurans such as Rhinella topologically corresponds to the flexor accessorius medialis plus flexor accessorius lateralis of urodeles such as Ambystoma (e.g. Ribbing, 1907; see Tables 1 and 3).
One muscle that also is part of the flexor muscles of the forearm is the ‘palmaris longus’ (Fig. 4), which is variable among tetrapods and is often the most superficial ventral forearm muscle. As explained by Diogo et al. (2009a), it is possible that some of the structures that are designated as ‘palmaris longus’ in different tetrapod groups are probably not homologous to each other. For instance, Gaupp (1896), described a ‘palmaris longus’ in anurans. However, Howell (1935, 1936a,b) and Straus (1942) stated that a ‘true palmaris longus’ is only seen as a variant in some reptiles such as Iguana, and is only consistently present in mammals. In fact, it should be noted that the flexor digitorum communis of amphibians is often designated, in the old literature, as ‘palmaris communis’ and/or as ‘flexor digitorum longus’. Therefore, it would actually not be surprising if Gaupp (1896) would have simply combined these names and used the name ‘palmaris longus’ to designate the flexor digitorum communis sensu the present work (Tables 1 and 3). Regarding reptiles, there is no ‘palmaris longus’ in Timon (Tables 1 and 3), but there is a ‘palmaris longus’ in other ‘lizards’(Fig. 5), as well as in other reptiles such as turtles (e.g. Howell, 1936a,b; Haines, 1939, 1950; Walker, 1973; Abdala et al. 2008; Russell & Bauer, 2008; this work). As described by authors such as Walker (1973) and Abdala et al. (2008), turtles often have a broad muscle ‘palmaris longus’. According to Howell (1936b) the ‘palmaris longus’ found in some reptiles is probably derived from part of the flexor carpi radialis, although he states that some reptiles may have a ‘palmaris longus’ derived from the flexor carpi ulnaris, thus supporting the idea that at least some of these ‘palmaris longus’ are not homologous. In a recent review, Russell & Bauer (2008) consider the ‘palmaris longus’ of ‘lizards’ an additional ‘humeral’ head of the flexor digitorum longus. According to our dissection, the ‘palmaris longus’ is a muscle occasionally present in ‘lizards’ such as Tupinambis (Fig. 5), Teyus, Ameiva and varanids, but absent in iguanids (but see above). In the ‘lizards’ that we have dissected in which the ‘palmaris longus’ is present, it tends to have a more ulnar topology (Fig. 5) (but see also above). The lepidosaurian ‘palmaris longus’ usually originates from the humerus and inserts superficially onto the distal end of the common tendon of the flexor digitorum longus and/or onto the palmar aponeurosis, being the only ventral forearm muscle that has some connection with the most superficial muscles of the hand. As can be seen in Fig. 4, the ‘palmaris longus’ often forms a continuum with the layer of the flexores breves superficiales. Taking this into consideration, we agree with the statements of Howell (1935, 1936a,b) and Straus (1942) that anurans lack a ‘palmaris longus’, because the only muscle that connects the forearm to the most superficial layer of the hand muscles in the anurans dissected by us is the flexor digitorum communis (Fig. 5). A ‘palmaris longus’ with the same overall configuration as that found in lepidosaurs is present in turtles such as Trachemys and Chelonoidis (e.g. Walker, 1973; Abdala et al. 2008; this work; Tables 1 and 3). Haines (1950) and Lewis (1989) state that the ‘palmaris longus’ might have been part of the muscular equipment of the LCA of amniotes. As our dissections and review of the literature indicate that the ‘palmaris longus’ is present in at least some turtles and lepidosaurs, it is likely that this muscle is homologous to the palmaris longus of mammals and, thus, that it was effectively present in the LCA of amniotes and then secondarily lost, within reptiles, in the archosaurs (see Fig. 1). However the monotremes, which are the most plesiomorphic extant mammals, also do not have a palmaris longus. Therefore, following the phylogenetic framework used in the present work, it is cladistically as parsimonious to infer that the ‘palmaris longus’ was independently acquired in turtles, lepidosaurs and non-monotreme mammals, as to infer that the ‘palmaris longus’ was present in the LCA of amniotes and then secondarily lost in archosaurs and monotremes (three evolutionary steps). We also plan to address this subject in future work but, for the moment, following this phylogenetic framework, we prefer to prudently write ‘palmaris longus’ in Tables 1 and 3 (in the columns concerning lepidosaurs and turtles), to indicate that the ‘palmaris longus’ of turtles might actually not be homologous to the ‘palmaris longus’ of lepidosaurs and to the palmaris longus of therian mammals.
Fig. 5.
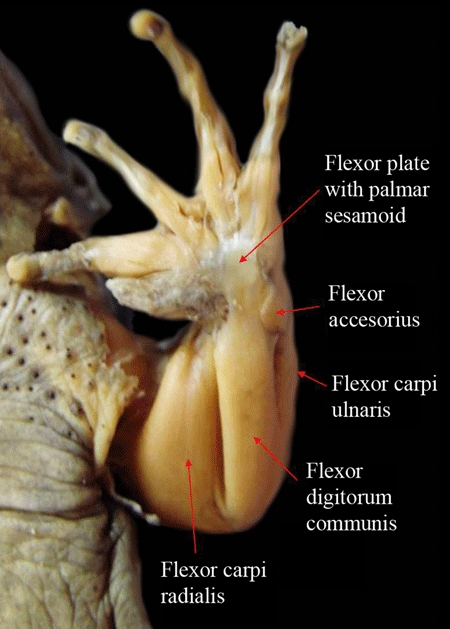
Telmatobius laticeps (Amphibia, Anura): ventral view of the superficial musculature of the forelimb and hand showing the flexor plate with the embedded sesamoid.
Regarding the ulnar ventral (flexor) muscular complex of the forearm, in amphibians, reptiles and mammals this usually includes a flexor carpi ulnaris (Figs 2 and 5) and an epitrochleoanconeus (Tables 1 and 3). This latter muscle, which is often designated as ‘flexor antebrachii ulnaris’, usually runs from the medial epicondyle of the humerus to the proximal portion of the ulna, being often very thin proximally and being very easily missed or confused with the flexor carpi ulnaris in dissections of the forearm. According to Walthall & Ashley-Ross (2006), there is a ‘flexor antebrachii et carpi ulnaris’ in the urodele Taricha. However, our dissections indicate that a distinct flexor carpi ulnaris and a distinct epitrochleoanconeus may be present in at least some members of the genus Ambystoma, and McMurrich (1903a,b);, Ribbing (1907) and Straus (1942) confirm that these two muscles are differentiated in at least some urodeles. The epitrochleoanconeus is commonly present in reptiles (Tables 1 and 3). Authors such as Walker (1973) and Abdala et al. (2008) did not recognize a distinct epitrochleoanconeus in the turtle Trachemys, but Holmes (1977) stated that he found this muscle in a specimen of this genus. Straus (1942) and Meers (2003) suggest that this muscle is absent in crocodylians, but we found it in one specimen of Caiman latirostris dissected by us (Tables 1 and 3). Ribbing (1938) described a flexor carpi ulnaris and a epitrochleoanconeus (‘flexor antebrachii ulnaris’) in birds. Holmes (1977) wrote that the epitrochleoanconeus is differentiated in lepidosaurs, including Sphenodon, and argued that this muscle was probably present in the LCA of reptiles, an hypothesis that is corroborated in the present work (see Tables 1 and 3).
Regarding the radial ventral (flexor) muscular complex, Macalister (1869) stated that in most amphibians, including urodeles, the flexor antebrachii et carpi radialis is usually differentiated into a ‘flexor antebrachii radialis’ (pronator teres sensu the present work) and a flexor carpi radialis (Figs 2 and 5). The main difference between these two muscles concerns their insertion onto the radius (the insertion of the pronator teres is usually proximal to that of the flexor carpi radialis), because in amphibians and reptiles both commonly arise tendinously from the distal portion of the humerus. According to authors such as McMurrich (1903a,b); and Straus (1942), these structures are usually not present as separate, distinct muscles in urodeles, and this seems to be the case in the Ambystoma specimens dissected by us (Tables 1 and 3), which apparently have a single muscular insertion onto the whole length of the radius (see also Walthall & Ashley-Ross, 2006). Ribbing (1907) also supports this idea, stating that the flexor carpi radialis and the pronator teres are present as distinct muscles in anurans, but not in urodeles (Tables 1 and 3). According to Walthall & Ashley-Ross (2006), the flexor antebrachii et carpi radialis of urodeles such as Taricha flexes, but also helps to pronate, the hand, that is, it does the function of the flexor carpi radialis and of the pronator teres of other tetrapods. There is some confusion regarding the identity of the flexor carpi radialis and of the pronator teres in reptiles. The ‘two heads of the flexor carpi radialis’sensu authors such as Holmes (1977) and Dilkes (2000) and of the ‘pronator teres’sensuMeers (2003) (which are present in lepidosaurs such as Sphenodon, Iguana and Timon, in some crocodylians, in turtles, and in birds, corresponding to the ‘pronator superficialis’ and ‘pronator profundus’ of these latter reptiles), correspond topologically to the flexor carpi radialis and pronator teres of mammals. However, the structure that McMurrich (1903a) and Holmes (1977) describe as ‘pronator radii teres’ in taxa such as Sphenodon and ‘lizards’ seems to derive from the flexor digitorum longus, as recognized by these two authors. That is, this ‘pronator radii teres’ probably does not correspond to the pronator teres sensu the present work, which derives from the flexor antebrachii et carpi radialis (Tables 1 and 3). Our dissections indicate that the pronator teres and the flexor carpi radialis are differentiated in Caiman, corresponding to the ‘two heads of the flexor carpi radialis’sensu authors such as Holmes (1977) and Dilkes (2000). According to the phylogenetic framework shown in Fig. 1, it is as parsimonious to consider that the pronator teres was present in the LCA of tetrapods and then secondarily lost in urodeles as it is to consider that it was independently acquired in anurans and in amniotes (two evolutionary steps).
The remaining ventral muscles of the forearm are the pronator quadratus and pronator accessorius (Tables 1 and 3). The pronator quadratus is usually present in urodeles, anurans, turtles, lepidosaurs, crocodylians, birds and mammals, corresponding to the pronator profundus sensuDiogo & Abdala (2007), but it is missing in some turtles (e.g. Walker, 1973; Holmes, 1977); we prefer to use the name pronator quadratus in the present paper because this name is used by most researchers working with both non-mammalian and mammalian tetrapods: see Jouffroy, 1971; Jouffroy & Lessertisseur, 1971; Diogo et al. 2009a). This muscle usually runs from the medial side of the ulna to the radial side of the wrist and/or of the distal portion of the forearm. As explained above, the structure that is often designated as ‘pronator profundus’ in birds corresponds topologically to the pronator teres, and not to the pronator quadratus, sensu the present work (see also Table 1 and Fig. 3). However, birds have a ventral forearm muscle, which is often designated as ‘ulnimetacarpalis ventralis’ (e.g. Sullivan, 1962; Shellswell & Wolpert, 1977; Meyers, 1996) and usually connects the distal portion of the ulna to the metacarpal region. This muscle probably corresponds to the pronator quadratus and/or possibly (less likely) to the pronator accessorius sensu the present work (see Tables 1 and 3). This idea is supported by authors such as Straus (1942) and Holmes (1977), who state that the pronator quadratus is present as a distinct muscle in all major extant groups of reptiles. Our results indicate that the quadratus was probably present in the LCA of all living tetrapods (see Tables 1 and 3, and Fig. 1). As explained by authors such as Straus (1942) and Diogo et al. (2009a), the pronator accessorius is a peculiar reptilian muscle that very likely corresponds to part of the pronator quadratus of tetrapods such as amphibians. As noted in our recent reviews (e.g. Diogo & Abdala, 2007; Abdala et al. 2008), the pronator accessorius is commonly present in turtles and lepidosaurs. Straus (1942) stated that the only major group of living reptiles where the pronator accessorius is missing is the Crocodylia, thus suggesting that this muscle is present in at least some birds. Meers (2003) did confirm that the pronator accessorius is missing in crocodylians, and this muscle did seem to be missing in the crocodylians dissected by us. However, in the chickens we dissected the pronator accessorius also did not seem to be present as a distinct structure, and this muscle was not described in the chickens and the other birds analyzed by authors such as Meyers (1996), Shellswell & Wolpert (1977) and Maxwell & Larsson (2007). If further studies confirm that the flexor accessorius is effectively present in at least some birds, as suggested by Straus (1942), this would provide further evidence that this muscle was effectively present in the LCA of living reptiles. If not, it is as parsimonious to assume that it was present in this LCA and then secondarily lost in archosaurs, as it is to assume that it was independently acquired in lepidosaurs and in turtles: two evolutionary steps. We plan to address this issue in future work by dissecting several specimens from all the major groups of birds.
Regarding the dorsal muscles of the forearm, one issue that has been the subject of much confusion in the literature concerns the homologies of the extensor antebrachii et carpi radialis (Figs 3 and 6) and its derivatives in tetrapods. This subject was recently discussed in detail by Diogo et al. (2009a). The dissections, comparisons and review of the literature that we did for the present work mainly supported the hypotheses of homology proposed by Diogo et al. (2009a). The radial dorsal complex comprises muscles that usually originate from the lateral epicondyle of the humerus and insert onto the radius and/or radiale, and that mainly extend the carpus and the forearm. Authors such as Howell (1936b) and Meers (2003) describe an ‘extensor carpi radialis longus’ and an ‘extensor carpi radialis brevis’ in reptiles such as Iguana and crocodylians, respectively. However, most authors argue that reptiles have a single ‘extensor carpi radialis’, which corresponds to the extensor antebrachii et carpi radialis sensu the present work and is usually subdivided into three bundles in amphibians, such as urodeles, and reptiles, such as turtles, crocodylians and lepidosaurs, i.e. ‘superficialis’, ‘profundus’, and ‘supinator’sensuRussell (1988) or ‘superficialis’, ‘profundus’, and ‘intermedius’ (see Humphry, 1872a,b; Walker, 1973; Holmes, 1977; Lewis, 1989; Dilkes, 2000; Abdala et al. 2008; see also Tables 1 and 3). It should, however, be noted that in most ‘lizards’, except Varanus exanthematicus, Varanus griseus and Varanus niloticus, the ‘superficialis’ bundle is lost (e.g. Russell 1988). Apart from those three bundles of the extensor antebrachii et carpi radialis, reptiles usually also have a muscle ‘supinator longus’/’tractor radii’sensuHolmes (1977) (see also Russell & Bauer, 2008), which is actually the probable homologue of the mammalian brachioradialis (see Tables 1 and 3, and below). Therefore, the muscle mass formed by the extensor antebrachii et carpi radialis of reptiles such as crocodylians and turtles seems to correspond topologically to the structure that has given rise to the mammalian extensor carpi radialis longus, extensor carpi radialis brevis and supinator, but not to the mammalian brachioradialis. It is, however, possible that the structure that has been often designated as the ‘intermedius’ head of the extensor antebrachii et carpi radialis in other non-mammalian tetrapods such as urodeles actually corresponds to the structure that has given rise to the mammalian brachioradialis, as suggested by authors such as Humphry (1872a,b); and Lewis (1989). That is, it is possible that the ‘intermedius’ head of taxa such as urodeles is not homologous to the ‘intermedius’ head of reptiles such as crocodylians and turtles. In crocodylians, the extensor antebrachii et carpi radialis sensu the present work seems to include the ‘extensor carpi radialis longus’, the ‘extensor carpi radialis brevis’, and the ‘abductor radialis’sensuMeers (2003), although part of the ‘extensor carpi radialis longus’sensuMeers (2003) might actually correspond to the abductor pollicis longus sensu the present work (see Tables 1 and 3, and below). This is because in the specimens dissected by us the tendon of the ‘extensor carpi radialis longus’sensuMeers (2003) surrounds the first digit, inserting onto the base of the metacarpal I. The crocodylian ‘extensor carpi radialis longus’, ‘extensor carpi radialis brevis’ (or at least its ‘pars radialis’), and the ‘abductor radialis’sensuMeers (2003) might therefore correspond to the ‘pars superficialis, pars intermedia and pars profunda of the extensor carpi radialis’sensuHolmes (1977), and, thus, to the structures that have differentiated, in mammals, to give rise to the distinct extensor carpi radialis longus, extensor carpi radialis brevis and supinator muscles, respectively. This is because the two former crocodylian structures insert onto hand bones, as usually do the mammalian extensor carpi radialis longus and extensor carpi radialis brevis, whereas the latest, third structure does not reach the hand bones, inserting distally onto the forearm bones only, as usually does the mammalian supinator. This hypothesis is supported by the fact that some authors have designated the ‘extensor carpi radialis profundus’sensuHolmes (1977) as ‘supinator’ or ‘supinator brevis’ (see, e.g. Walker, 1973; see also Tables 1 and 3, and below).
Fig. 6.
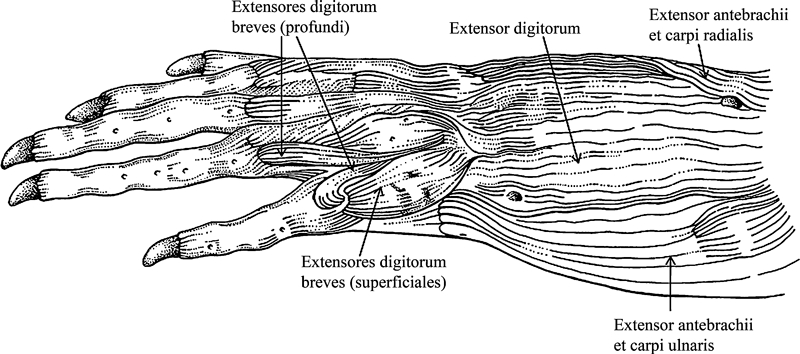
Tupinambis meriane (Reptilia, Lepidosauria): dorsal view of the dorsal (extensor) muscles of the forearm and hand.
The extensor carpi radialis longus and brevis are not present as independent muscles in Ambystoma. However, according to Howell (1936b) these two muscles may be found in at least some other amphibians such as Necturus. Haines (1939) described three heads of the extensor antebrachii et carpi radialis in urodeles such as Salamandra: ‘superficialis’, ‘intermedius’ and ‘profundus’ (see above). According to Haines (1939), the extensor antebrachii et carpi radialis sensu the present work is divided into five divisions in anurans such as Rana, which he designated as ‘extensor radialis profundus’ (‘flexor antebrachii lateralis profundus’sensuGaupp, 1896), ‘extensor radialis intermedius’, or ‘brachioradialis’ (‘flexor antebrachii lateralis superficialis, caput inferius’sensuGaupp, 1896), ‘extensor radialis superficialis’ (‘extensor carpi radialis, caput inferius’sensuGaupp, 1896), and two ‘small accessory slips’ (‘extensor carpi radialis caput superius’ and ‘flexor antebrachii lateralis superficialis caput superius’sensuGaupp, 1896).
The ‘supinator longus’ (‘tractor radii’) sensuHolmes (1977), which in reptiles such as turtles is innervated by the ‘inferior brachial nerve’ and the radial nerve (e.g. Haines, 1939), seems to correspond clearly to the brachioradialis of mammals, because its origin on the humerus is more lateral and more proximal than that of the other derivatives of the ‘extensor antebrachii et carpi radialis anlage’ (see Fig. 19 of Holmes, 1977). This idea is supported by the fact that in the old literature the mammalian brachioradialis was often designated as ‘supinator longus’ and the reptilian ‘tractor radii’ as brachioradialis (see Walker, 1973; Diogo et al. 2009a). Jollie (1962) suggested that the ‘humeroradialis’ is present in crocodylians and Sphenodon and corresponds to the ‘tensor patagii’ of birds and to the brachioradialis of mammals. Meers (2003) stated that the ‘humeroradialis’ of crocodylians is homologous to the ‘tensor propatagialis’ of birds, but that this muscle is missing in other living reptiles. It is important to note that the overall configuration and function of the ‘humeroradialis’sensuMeers (2003) are in fact somewhat similar to those of the mammalian brachioradialis, because the ‘humeroradialis’ is derived ontogenetically from the dorsal (extensor) anlage but acts mainly as a flexor of the antebrachium (see Meers, 2003; and Table 1). However, regarding its innervation, the ‘humeroradialis’ is innervated by the axillary nerve in crocodylians (Meers, 2003), and thus it does not seem to be homologous to the mammalian brachioradialis, which is innervated by the radial nerve (Straus, 1942). Moreover, the ‘supinator’sensuMeers (2003) also has an overall configuration and function that are similar to those of the mammalian brachioradialis (i.e. it is part of the extensor musculature but also acts mainly as a flexor of the antebrachium) and, contrary to the humeroradialis, is mainly innervated by the radial nerve, as is the mammalian brachioradialis (see Meers, 2003). Therefore, the mammalian brachioradialis seems to be homologous to the ‘supinator’, and not to the ‘humeroradialis’ of crocodylians (Tables 1 and 3). Haines (1939) stated that the ‘tractor radii’ is not present as a separate muscle in amphibians such as Salamandra, but, at the same time, he designated the ‘intermedius’ head of the extensor antebrachii et carpi radialis of Salamandra as a ‘brachioradialis’. This seems to support the hypothesis, proposed above, that the structure that is often designated as the ‘intermedius’ head of the extensor antebrachii et carpi radialis in taxa such as urodeles is actually not directly homologous to the ‘intermedius’ head of reptiles such as turtles and crocodylians. None of the muscles described in chickens and other birds by authors such as Sullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996) seems to correspond to the brachioradialis sensu the present work, unless the ‘humeroradialis’/‘tensor propatagii’ of birds does correspond to the brachioradialis sensu the present work (see above). Our dissections indicate that Timon also does not have a distinct, separate brachioradialis muscle such as that found in mammals, but Haines (1939) stated that the ‘supinator longus’/‘tractor radii’ is present as a distinct muscle in Sphenodon. This statement supports Holmes’ (1977) observation that the ‘supinator longus’ (brachioradialis) is commonly present in extant reptiles and that this muscle was probably present in the LCA of extant reptiles. The taxonomic distribution of this character indicates that the brachioradialis was probably present in the LCA of amniotes as a whole, because this muscle is apparently present in at least turtles, lepidosaurs such as Sphenodon, crocodylians, and most mammals, including monotremes (its absence in Rattus being an exception within mammals: Table 3; see Fig. 1).
Regarding the ulnar dorsal (extensor) muscular complex, this usually originates from the distal portion of the humerus and inserts onto the ulna and/or ulnar side of the carpal/metacarpal region. The anconeus (often designated as ‘extensor antebrachii ulnaris’) and the extensor carpi ulnaris do not appear to be present as independent muscles in Ambystoma and Timon (see Tables 1 and 3 and also Diogo et al. 2009a). But authors such as Haines (1939), Sullivan (1962), Jouffroy (1971), Jouffroy & Lessertisseur (1971), Holmes (1977) and Shellswell & Wolpert (1977) do describe an anconeus in amphibians such as Salamandra and various reptiles such as Sphenodon and some birds. However, it should be noted that we did not find a separate anconeus in the numerous ‘lizards’ dissected by us. Howell (1936a,b); also did not report an anconeus in urodeles such as Necturus and ‘lizards’ such as Iguana, nor did Meers (2003) in crocodylians. The flexor ulnaris sensuMeers (2003) clearly corresponds to the extensor antebrachii et carpi ulnaris sensu the present work; as described by authors such as Holmes (1977) and Dilkes (2000) and corroborated by our dissections, in crocodylians this muscle seems to be mainly related to the extension of the antebrachium, and not to its flexion, as proposed by Meers (2003). Haines (1939), who described an anconeus in Salamandra, Triton and Rana, argued that, excepting these few genera, the anconeus is rarely present as a separate, distinct muscle in urodeles or apparently in anurans, suggesting that the anconeus of reptiles, the anconeus of amphibians, and the anconeus of mammals were acquired independently in the evolution of these clades, i.e. that they are not homologous to each other. However, Ribbing (1907) stated that, contrary to urodeles, in anurans the ‘extensor carpi ulnaris’ and ‘extensor antebrachii carpi ulnaris’ (anconeus sensu the present work) are actually often present as distinct muscles. Also, as described by Ribbing (1907), Walker (1973) and Abdala et al. (2008), in Trachemys the extensor antebrachii et carpi ulnaris is mainly undivided, but in some other turtles such as Testudo, Pelomedusa, Chelodina and Emys, this structure is divided into an ‘extensor carpi ulnaris’, connecting the humerus and ulna, and an ‘extensor carpi ulnaris accessorius’, connecting the ulna and carpus, the former probably corresponding to the anconeus sensu the present work. Moreover, Sullivan (1962), Shellswell & Wolpert (1977), Meyers (1996), and Maxwell & Larsson (2007) describe a distinct muscle anconeus (‘ectepicondylo-ulnaris’) in birds such as chickens which connects the distal dorsal margin of the humerus to the proximal dorsal margin and derives ontogenetically from the extensor antebrachii et carpi ulnaris sensu the present work, as does the anconeus of mammals. As the anconeus that authors such as Haines (1939), Sullivan (1962), Jouffroy (1971), Jouffroy & Lessertisseur (1971), Holmes (1977) and Shellswell & Wolpert (1977) describe in some amphibians and reptiles and the anconeus of mammals have a similar overall configuration (usually running from the distal portion of the humerus to the proximal portion of the ulna), a similar innervation (radial nerve), and derive from the same anlage (i.e. derive from the extensor antebrachii et carpi ulnaris), it is likely that this muscle is homologous across these tetrapod groups. Actually, these similarities, together with the fact that the anconeus is present in at least some anurans and birds, in mammals, and seemingly also in at least some lepidosaurs (such as the phylogenetically plesiomorphic genus Sphenodon: see above and Table 1), turtles (e.g. Testudo, Pelomedusa, Chelodina and Emys) and urodeles (e.g. Salamandra; see above and Tables 1 and 3), indicate that this muscle was present in the LCA of tetrapods and then secondarily lost in the lineage leading to crocodylians. If further studies confirm that this muscle is really always missing in these reptiles, this hypothesis requires two evolutionary steps, whereas the second more parsimonious hypotheses, i.e. that the muscle was independently acquired in amphibians and in amniotes and then secondarily lost in crocodylians, or, alternatively, that the muscle was independently acquired in the LCA of tetrapods, then secondarily lost in archosaurs, and then acquired again in the lineage leading to birds, require three evolutionary steps (see Fig. 1).
The remaining muscles of the dorsal (extensor) layer of the forearm and hand are the extensor digitorum, the extensores digitorum breves (often designated as ‘short extensors of the digits’, running mainly from the carpal region and/or, sometimes, from the ulna, to the digits), and the abductor pollicis longus (usually running from the ulna to the metacarpal 1, the radial portion of the carpus, and/or sometimes to the distal part of the radius: see below). In urodeles such as Ambystoma and Taricha the latter muscle is possibly fused with the short extensor of digit 1, forming the abductor et extensor digit 1 (see Tables 1 and 3). Within crocodylians, Meers (2003) includes, in his ‘intrinsic extensors of the manus’: five ‘extensores digitorum superficiales’ that often attach to the distal phalanges of digits 1, 2, 3, 4 and 5; six ‘extensores digitorum profundi’ that often attach to the distal phalanges of these five digits; one ‘extensor pollicis superficialis et indicus proprius’ attaching to the distal portions of digits 1 and 2; one ‘extensor metacarpi I’ attaching to metacarpal I; and one ‘extensor metacarpi IV’ attaching to metacarpal IV. All these 14 muscles seem to partially correspond to the extensores digitorum breves sensu the present work. The ‘extensor metacarpi I’ or possibly the ‘extensor digiti I superficialis’, innervated by the radial nerve (Meers, 2003), might correspond to the abductor pollicis longus sensu the present work, which is also innervated by the radial nerve (see Tables 1 and 3). The ‘extensor digiti I superficialis’ could correspond to the abductor pollicis longus because, as often the case with this latter muscle, it is the largest and most lateral dorsal (extensor) muscle of the hand (compare Fig. 13 of Meers, 2003 with Fig. 2 of Moro & Abdala 2004). However, this ‘extensor digiti I superficialis’ inserts onto the distal phalanx of digit 1, and not onto the metacarpal I and/or the radial part of the carpus, as often does the abductor pollicis longus of other reptiles (see below). This might indicate that, if the abductor pollicis longus sensu the present work is present in crocodylians, it might correspond to the ‘extensor metacarpi I’sensuMeers (2003), because this latter structure does insert onto metacarpal I, and not onto the distal phalanx of digit I. However, the most likely hypothesis, in view of our dissections, comparisons, and review of the literature, is that all these 14 muscles described by Meers (2003) are actually part of the extensores digitorum breves sensu the present work. It is therefore possible that the abductor pollicis longus sensu the present work actually corresponds to the ‘extensor carpi radialis brevis pars ulnaris’sensuMeers (2003), because this latter structure is well-developed, is innervated by the radial nerve, and runs from the ulna to the carpal/metacarpal region (onto the radiale bone according to Meers, 2003), as usually does the abductor pollicis longus of other reptiles. In fact, Holmes (1977) stated that all major groups of living reptiles have an abductor pollicis longus and that this muscle usually runs from the distal end of the ulna to the carpal/metacarpal region in crocodylians, turtles and Sphenodon, and also to the distal end of the radius in ‘lizards’.
The extensores digitorum breves exist as a muscular complex in most tetrapod taxa (see, e.g. Fig. 6), although this is not the case in mammals, which only conserve some parts of this complex as individual muscles (Tables 1 and 3). We found an origin of this muscular complex from the ulna/ulnare in various lepidosaurs and turtles, while in other taxa dissected by us the origin often also comprised the radiale (e.g. urodeles, birds, crocodylians). Urodeles such as Ambystoma often have three extensores digitorum breves, going to digits 2, 3 and 4 (e.g. Diogo et al. 2009a). Haines (1939) argued that, apart from the abductor pollicis longus, there is also a distinct, short extensor to digit 1 in urodeles such as Salamandra. If this is the case, this latter extensor is thus directly homologous to the extensor pollicis longus of mammals. Also according to Haines (1939), anurans such as Rana usually have eight extensores digitorum breves, i.e. these amphibians have two muscles inserting onto each of the four digits. Walker (1973) and Abdala et al. (2008) reported that, in turtles such as Trachemys, there are five extensores digitorum breves, each going to each of the five digits; Walker (1973) stated that the insertion of these muscles is onto the penultimate phalanges of the digits, whereas Abdala et al. (2008) stated it is onto the ‘first phalanx’ of the digits. Holmes (1977) reported that in Sphenodon and ‘lizards’ the extensores digitorum breves insert onto the distal phalanges of the digits, and suggested that the plesiomorphic condition for reptiles is that in which there are five extensores digitorum breves, one for each digit, as is commonly the case in turtles and in lepidosaurs such as Sphenodon and numerous ‘lizards’. It should be noted that Russell & Bauer (2008) describe, in lepidosaurs, a ‘superficial extensores digitores brevis’ complex and an ‘interossei dorsales’ complex, the former complex being subdivided into superficial and deep components (see Fig. 6). According to our dissections, observations and review of the literature, we consider that the dorsometacarpales sensu the present work (see Tables 1 and 3) correspond to their ‘extensores digitores brevis profundus’. In chickens the extensores digitorum breves include the ‘extensor indicis brevis’sensuSullivan (1962) and Shellswell & Wolpert (1977) (this latter structure corresponds to the ‘extensor brevis alulae’sensuMeyers, 1996; and goes to digit 1, i.e. to digit 2 according to embryology), and also the ‘extensor medius brevis’sensuSullivan (1962) and Shellswell & Wolpert (1977) (which goes to digit 2, i.e. to digit 3 according to embryology). The ‘ulnimetacarpalis dorsalis’sensuSullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996) might correspond to a reduced short extensor (‘extensor digiti brevis’) of digit 3 (i.e. of digit 4 according to embryology), although one cannot discard the hypothesis that it actually corresponds to a reduced abductor digiti minimi sensu the present work (see, e.g. fig. 1 of Shellswell & Wolpert, 1977, and Table 1).
The extensor digitorum is consistently present in all the major tetrapod groups (Figs 3 and 6, Tables 1 and 3). As explained by Howell (1936a,b);, Haines (1939), Straus (1941a,b);, Holmes (1977) and Dilkes (2000), in extant reptiles this muscle is usually inserted onto the metacarpals but, in taxa such as birds, it often extends distally to insert onto the phalanges of the digits. In turtles such as Trachemys the extensor digitorum has eight tendons attaching onto the ulnar and radial sides of the distal end of each metacarpal, except digits 1 and 5, which lack tendons to their radial and ulnar sides, respectively (e.g. Walker, 1973; Abdala et al. 2008; this work). As reported by Holmes (1977) and Dilkes (2000), in crocodylians the extensorum digitorum (‘extensor carpi ulnaris longus’sensuMeers, 2003) usually originates on the distal portion of the humerus, and inserts variably onto the metacarpals of digits 2, 3 and/or 4. The avian extensor digitorum probably includes the ‘extensor digitorum communis’sensuSullivan (1962), Shellswell & Wolpert (1977), and Meyers (1996), which goes to digits 1 and 2 (i.e. 2 and 3 according to embryology), but usually does not extend distally to the proximal phalanges of these digits. In chickens, the ‘extensor metacarpi longus digiti majoris’sensuMeyers (1996) (‘extensor medius longus’sensuSullivan, 1962 and Shellswell & Wolpert, 1977) often goes from the proximal portion of the radius and/or ulna to the distal phalanx of digit 2 (i.e. digit 3 according to embryology). According to Sullivan (1962) and Shellswell & Wolpert (1977) the structure that they designated as ‘extensor indicis longus’ corresponds to part of the long extensors of the hand, i.e. of the extensor digitorum sensu the present work. Their ‘extensor medius brevis’ connects the metacarpal region to digit 2 (i.e. digit 3 according to embryology), and, thus, seems to correspond to part of the extensores digitorum breves sensu the present work. Before describing the hand muscles, it is worth noting that, contrary to most other non-mammalian tetrapods, crocodylians have a more distal insertion of the ‘radial extensors/flexors’ and the ‘ulnar extensors/flexors’. For instance, the ‘pars superficialis’ and ‘pars intermedia’ (sensuHolmes, 1977) of the extensor antebrachii et carpi radialis of crocodylians insert onto the radiale bone (i.e. a carpal bone), and not onto the radius (see above). In birds this tendency is still more acute; for instance, part of the extensor antebrachii et carpi radialis extends distally to insert onto the proximal end of metacarpal I (e.g. Hudson et al. 1972; this work) (Fig. 3). In mammals, the insertion of the muscles of the forearm onto hand bones is common (see Jouffroy, 1971; Diogo et al. 2009a). Interestingly, a similar trend is also found in some anurans (e.g. Phyllomedusa: Manzano, 1996; this work; see Discussion below).
The homologies of the hand muscles of tetrapods have been the subject of numerous discussions, and recently were reviewed in detail by Diogo et al. (2009a). Examples of amphibian and reptilian hand muscles include: (i) the flexores breves superficiales, which are ventral (palmar, superficial) to the other muscles; (ii) the abductor pollicis brevis and abductor digiti minimi, which usually lie on the ventrolateral (radial) and ventromesial (ulnar) surface of the hand and abduct the most lateral (radial) and most medial (ulnar) digits, respectively; (iii) the lumbricales, which are deeper and are usually associated with the tendons of the flexor digitorum communis/longus, being often related to the extension and/or flexion of different parts of the digits; (iv) the contrahentes digitorum, which are deep to the lumbricales; (v) the flexores breves profundi, which usually are deep to the contrahentes digitorum and which usually insert onto both the radial and ulnar sides of the digits (note that each of the ‘biccipital muscles’ that are often described in the literature as going to both these sides of a same digit are considered to be two distinct flexores breves profundi muscles, according to Diogo et al. 2009a, and to the present work); (vi) the intermetacarpales, which are the deepest (most dorsal) muscles of the ventral (palmar) layer; and (vii) the dorsometacarpales, which are part of the dorsal layer of the hand and thus are the most dorsal intrinsic muscles of the hand (the dorsometacarpales are not present as distinct muscles in mammals) (Tables 1 and 3).
The flexores breves superficiales are consistently present in limbed amphibians and reptiles, forming a muscular complex that often originates from the flexor retinaculum and/or carpal bones and inserts onto the distal phalanges (see Figs 4, 7–11; Tables 1 and 3). In amphibians the flexores breves superficiales have a particular conformation because they are often markedly reduced and mainly associated to the structure that is often designated as ‘palmar aponeurosis’ in the literature (e.g. Ecker, 1889; Walthall & Ashley-Ross, 2006). It should be taken into account, however, that the name ‘palmar aponeurosis’ is misleading, as this structure is actually not an aponeurosis, but a strong tendon with a palmar sesamoid embedded in it. We found this structure in anurans such as Rhinella and Telmatobius and called it the flexor plate (Fig. 5). However, in some anurans this flexor plate might be very small (e.g. Pseudis minutus) or even completely missing (e.g. Pseudis paradoxa) (Manzano, 1996). Some reptiles do have a ‘true palmar aponeurosis’, that is, a superficial (ventral) structure that has a typical aponeurotic configuration, and that is often related to the flexores breves superficiales (Haines, 1950; Meers, 2003; Abdala et al. 2008).
Fig. 7.
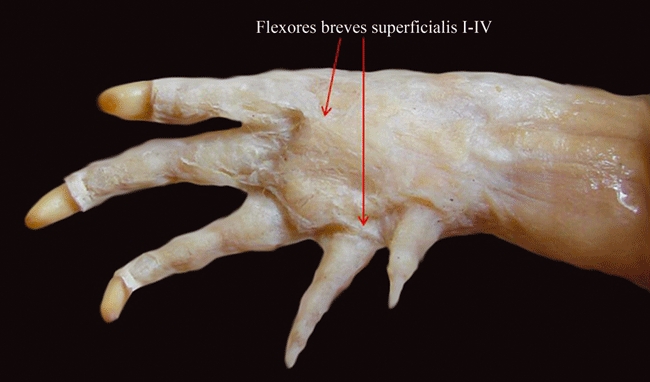
Caiman latirostris (Reptilia, Crocodylia): ventral view of the most superficial (ventral, or palmar) layer of the hand muscles.
As described by authors such as McMurrich (1903a,b);, amphibians such as Ambystoma usually have four flexores breves superficiales, each inserting onto each of the four digits. In turtles, including Trachemys, there are five flexores breves superficiales, one to each digit (each of the muscles to the three middle digits often having two slips, and each of the muscles going to digits 1 and 5 often having a single slip; e.g. Walker, 1973; Abdala et al. 2008; this work). According to Walker (1973), the specific insertions of these muscles are variable across different testudine taxa, i.e. they may be onto the proximal phalanges (as is the case in Trachemys; this is corroborated by Abdala et al. 2008 and by the present work), onto the sheaths of the flexor digitorum longus, or onto the penultimate phalanges. Lepidosaurs such as ‘lizards’ often have five flexores breves superficiales, inserting onto digits 1, 2, 3, 4 and 5 (e.g. McMurrich, 1903a,b; for recent reviews, see Diogo & Abdala, 2007 and Diogo et al. 2009a). There is some confusion in the literature about the presence of these muscles in birds. Holmes (1977) suggested that the flexores breves superficiales are present in all major extant groups of reptiles. However, Ribbing (1938) reported that the flexores breves superficiales are not present as a group in birds, and we could not identify, in the birds dissected by us, muscles that clearly correspond to the flexores breves superficiales of other extant reptiles. But it is possible, and even likely, that the ‘flexor indicis’sensuSullivan (1962) and Shellswell & Wolpert (1977) and/or the ‘flexor digiti quarti’sensuSullivan (1962) and Shellswell & Wolpert (1977) are part of the flexores breves superficiales sensu the present work (see Fig. 2). The ‘flexor indicis’ goes to digit 1, i.e. to digit 2 according to most embryologists, and corresponds to the ‘flexor alulae’ or ‘flexor pollicis’ or ‘flexor digiti II’ or ‘flexor digiti secundi manus’ or ‘adductor indicis’sensuMeyers, 1996. The ‘flexor digiti quarti’ goes to digit 3, i.e. digit 4 according to most embryologists, and corresponds to the ‘flexor digiti minoris’ or ‘flexor minimi digiti’ or ‘flexor minimi digiti + flexor minimi digiti brevis’ or ‘flexor digiti III’ or ‘flexor digiti IV’ or ‘flexor digiti quarti brevis + abductor digiti quarti proprius’ or ‘flexor digiti quarti manus longus’ or ‘flexor longus muscle of the fourth digit’sensuMeyers, 1996. However, we cannot completely discard the hypothesis that at least some of these latter muscles correspond, instead, to part of the flexores breves profundi, if the ‘interossei ventralis’ and ‘interossei dorsalis’sensuSullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996) actually correspond to the intermetacarpales and dorsometacarpales sensu the present work, respectively (see Tables 1 and 3).
The lumbricales are small muscles that often run from the tendons of the flexors of the forearm to the distal phalanges of the digits, and which are usually present in anurans, turtles, lepidosaurs and crocodylians, but absent in most urodeles and seemingly also in birds. In the Ambystoma ordinarium specimens dissected by us, the lumbricales were seemingly not present as distinct, separate muscles, and these muscles were also not described in urodeles such as Taricha (see Walthall & Ashley-Ross, 2006). However, McMurrich (1903a) shows an Ambystoma tigrinum specimen where the lumbricales are present as distinct muscles. Anurans often have lumbricales and, in at least some cases, including the Phyllomedusa bicolor specimens dissected by us, these muscles are differentiated into ‘lumbricales breves’ and ‘lumbricales longi’ (e.g. Gaupp, 1896; Manzano, 1996; this work). In turtles, including Trachemys, there are five flexores breves superficiales, one to each digit (each of the muscles to the three middle digits often having two slips, and each of the muscles going to digits 1 and 5 often having a single slip; e.g. Walker, 1973; Abdala et al. 2008; this work). According to authors such as McMurrich (1903a,b);, ‘lizards’ usually also have five lumbricales inserting onto digits 1, 2, 3, 4 and 5, although some ‘lizards’ have fewer lumbricales (note that Russell & Bauer, 2008 designate the flexores breves profundi sensu the present work as ‘lumbricales’). As reported by Meers (2003), crocodylians often have five lumbricales, the first attaching to digit 2, the second to digit 2, the third to digit 3, the fourth to digit 3, and the fifth to digit 5. Authors such as Sullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996) have not used the name ‘lumbricales’ to describe any hand muscles of chickens and other groups of Aves, and our dissections indicate that birds such as chickens effectively do not seem to have distinct lumbricales such as those seen in other tetrapods. However, these authors do describe a muscle (‘abductor medius’sensuSullivan, 1962 and Shellswell & Wolpert, 1977; which goes to digit 2, i.e. digit 3 according to most embryologists, and corresponds to the ‘abductor digiti majoris’sensuMeyers, 1996) that is ‘applied to’ the tendons of the flexor pollicis longus according to Sullivan (1962) and Shellswell & Wolpert (1977) and ‘covered by’ these tendons according to Meyers (1996), and that could thus correspond to part of the lumbricales sensu the present work. If this muscle is actually not part of the lumbricales, it would probably correspond to part of the intermetacarpales sensu the present work, because it seems mainly to abduct digit 2, i.e. digit 3 according to most embryologists. As the lumbricales are present in, and have a similar overall configuration, similar attachments, and a similar function across at least some urodeles, in testudines, lepidosaurs, crocodylians and mammals, these muscles were very likely present in the LCA of tetrapods (see Fig. 1 and Table 3).
The contrahentes digitorum usually run from carpal bones, metacarpal bones and/or the contrahens fascia, to the bases of the proximal phalanges of the digits. As described by authors such as McMurrich (1903a,b);, urodeles such as Ambystoma often have four contrahentes digitorum, each inserting onto each of the four digits. According to Ribbing (1907), anurans such as Discoglossus also have four contrahentes digitorum sensu the present work, which probably include the ‘flexor teres indicis’, the ‘caput volare des m. flexor teres digiti V’ and the ‘adductor proprius digiti V’sensuGaupp (1896). Holmes (1977) seems to suggest that the contrahentes digitorum are present in all major extant groups of reptiles. The ‘adductor indicis’ reported by Sullivan (1962) and Shellswell & Wolpert (1977) in birds, which goes to digit 1 (i.e. digit 2 according to embryology) and corresponds to the ‘adductor alulae’sensuMeyers (1996), is possibly part of the contrahentes digitorum sensu the present work. Walker (1973) describes a single contrahens in turtles such as Trachemys, which he designates as ‘adductor digiti minimi’, that goes to digit 5. According to him, some turtles have contrahentes digitorum to digits 4 and 5, whereas other turtles completely lack contrahentes. Abdala et al. (2008) stated that turtles such as Trachemys have ‘contrahentes’ to the proximal phalanx of each digit, but they stated that these ‘contrahentes’ are the deepest ventral (palmar) muscles of the hand, so these ‘contrahentes’ probably do not correspond to the contrahentes digitorum sensu the present work, which are usually deep (dorsal) to the flexores breves superficiales, but superficial (ventral) to the flexores breves profundi and to the intermetacarpales (see Diogo et al. 2009a). As stressed by Lewis (1989), the ‘flexores digitorum intermedii’sensu authors such as Holmes (1977) and also sensuMeers (2003), or ‘flexores digitorum breves medii’sensu authors such as McMurrich (1903a,b);, clearly seem to correspond to the contrahentes digitorum sensu the present work. This is because, as indicated by the names used by these latter authors, these muscles are dorsal to the flexores breves superficiales and ventral to the flexores breves profundi. This idea is also supported by authors such as Howell (1936a,b);, who explicitly designate the ‘flexores digitorum breves intermedii/medii’ of reptiles as contrahentes digitorum. According to Meers (2003), crocodylians usually have a ‘flexor digitorum intermedius digiti IV et V’ (that is, a contrahens sensu the present work), which is commonly inserted onto the distal end of the proximal phalanx of digit 4 and, sometimes, also onto the distal metacarpal of digit 5. Meers (2003) describes an additional muscle in Alligator mississippiensis, the ‘flexor digitorum intermedius digiti V’, which was absent in all the other crocodylian species examined by him and which, according to him, possibly derives from the flexores breves profundi, and not from the contrahentes layer.
As reported by McMurrich (1903a,b);, urodeles such as Ambystoma usually have eight flexores breves profundi sensu the present work, inserting onto the ulnar and radial sides of each of the four digits (note that the muscles that insert onto the ulnar and radial side of each digit are often considered ‘heads’ of a single, ‘biccipital’ muscle, so authors such as McMurrich actually often refer to four ‘biccipital’ muscles, which thus correspond to the eight flexores breves profundi sensuDiogo et al. 2009a and sensu the present work). According to Ribbing (1907) there are eight flexores breves profundi sensu the present work (also often described as ‘four biccipital muscles’) in anurans such as Rana, which include the ‘opponens indicis’, ‘flexor ossis metacarpi III’, ‘flexor ossis metacarpi IV’ and ‘opponens digiti V’ and possibly the ‘abductor secundus digiti V’sensuGaupp (1896). The anuran ‘flexores digitorum minimi’sensuRibbing (1907) are flexors of the digits and probably correspond to, or are derived from, muscles such as the flexores breves profundi sensu the present work. The anuran ‘flexores digitorum minimi’ are often, but not always, superficial (ventral) to the intermetacarpales according to Burton (1998), and correspond to the ‘flexor teres digitorum III, IV, and V’sensuGaupp (1896), and to the ‘flexores teretes I, II, III and IV’sensuBurton (1998). They also correspond to the ‘interphalangei’sensuRibbing (1907), which correspond to the ‘interphalangeus digiti IV and interphalangeus digiti V’sensuGaupp (1896). According to Ribbing (1907) and Burton (1998), these two groups of muscles (i.e. the ‘flexores digitorum minimi’ and ‘interphalangei’) are also present in at least some urodeles. Regarding the testudines, the flexores breves profundi sensu the present work possibly correspond to part or the totality of the ‘interossei volares’sensuWalker (1973) and/or of the ‘flexores digiti brevis profundus’sensuAbdala et al. (2008). Note that the ‘interossei dorsales’sensuWalker (1973) possibly correspond to the intermetacarpales + dorsometacarpales sensu the present work (Table 1). However, Walker (1973) stated that the ‘interossei volaris’ insert onto the proximal phalanges in Trachemys, whereas Abdala et al. (2008) reported that, in the members of this genus, the ‘flexores digiti brevis profundi’ insert onto the metacarpals. As described by authors such as McMurrich (1903a,b);, ‘lizards’ usually have 10 flexores breves profundi sensu the present work, two for each of the five digitis (these 10 muscles are often described as ‘five biccipital muscles’). Meers (2003) described five ‘flexores breves profundi’ (or six, if the muscle that he named as ‘flexor digitorum intermedius digiti V’ is also part of the deep flexor layer) in crocodylians. Thus, these reptiles clearly seem to have the full series of deep flexors, i.e. 10 flexores breves profundi sensu the present work. Each digit receives two of these muscles, i.e. each of the five ‘muscles’ described by Meers (2003) corresponds to two of the flexores breves profundi sensu the present work. According to authors such as Ribbing (1938) and Holmes (1977) birds do have flexores breves profundi. It is possible that the ‘flexor indicis’ and/or ‘flexor digiti quarti’ reported in birds by Sullivan (1962) and Shellswell & Wolpert (1977) correspond to part of the flexores breves profundi sensu the present work, although they might actually correspond to the flexores breves superficiales. In this latter case, the flexores breves profundi sensu the present work might instead correspond to part/the totality of the interossei ventralis sensuSullivan (1962) and Shellswell & Wolpert (1977) (see flexores breves superficiales above).
As their name indicates, the intermetacarpales usually connect two adjacent metacarpals. As reported by authors such as McMurrich (1903a,b);, urodeles such as Ambystoma usually have three intermetacarpales, connecting the metacarpales of the four digits. A similar configuration is usually found in anurans (e.g. Ribbing, 1907; Burton, 1998; this work). Abdala et al. (2008) stated that turtles such as Trachemys have four intermetacarpales connecting the metacarpals of the five digits. Walker (1973) did not describe intermetacarpales in turtles, but it is possible that the muscles that he described under the name ‘interossei dorsales’ include the intermetacarpales sensu the present work (see Table 1). ‘Lizards’ often have four ‘intermetacarpales I’ connecting the metacarpals of the five digits and four ‘intermetacarpales II’, also connecting the metacarpals of these digits (e.g. Abdala & Moro, 2006; this work). Meers (2003) reported various ‘dorsal interossei’ and various ‘ventral interossei’ in crocodylians, but these muscles are not homologous to the dorsal and ventral interossei of mammals such as humans because these latter muscles were not present in the LCA of mammals. The ‘dorsal interossei’ and ‘ventral interossei’sensuMeers (2003) clearly seem to correspond, instead, to the intermetacarpales and the dorsometacarpales of other reptiles (see Diogo et al. 2009a). The intermetacarpales sensu the present work probably correspond to part of the ‘interossei dorsales’ and/or ‘interossei ventrales’ that were described in birds by Sullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996), although they might also/instead include the ‘abductor medius’sensuSullivan (1962) and Shellswell & Wolpert (1977), which corresponds to the ‘abductor digiti majoris’sensuMeyers, 1996 (see lumbricales above).
The dorsometacarpales usually run from the bases to the distal portion of the metacarpals. They were seemingly not present as distinct muscles in the Ambystoma specimens dissected by us, and were also not described in urodeles such as Taricha (e.g. Walthall & Ashley-Ross, 2006). However, they were described in other urodeles. For instance, Straus (1941a,b); stated that Salamandra and Cryptobranchus do have dorsometacarpales (see his Table 1). He also illustrated a Necturus specimen with ‘dorsometacarpales’ in his Fig. 1, although he explained that, in this specific case, the ‘dorsometacarpales’ of Necturus probably correspond to the extensores digitorum breves sensu the present work. Haines (1939) argues that anurans such as Rana and urodeles such as Salamandra clearly have both extensores digitorum breves and dorsometacarpales, so at least some urodeles and anurans do seem to have dorsometacarpales sensu the present work (see Tables 1 and 3). Actually, according to Haines (1939) the dorsometacarpales (‘extensores breves profundi’sensuGaupp, 1896) are highly developed in anurans such as Rana. Holmes (1977) stated that the dorsometacarpales are usually found in all the major extant groups of reptiles. Our dissections indicate that ‘lizards’ usually have five dorsometacarpales inserting onto digits 1, 2, 3, 4 and 5. Turtles such as Trachemys have five dorsometacarpales, each covering the dorsal surface of each of the five digits, and sending a tendon that attaches from the second phalanx to the ungual phalanx of each digit (e.g. Abdala et al. 2008; this work). In birds, the dorsometacarpales sensu the present work correspond, very likely, to part, or the totality, of the ‘interossei dorsalis’sensu authors such as Sullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996) (see text about intermetacarpales above).
Lastly, the abductor pollicis brevis and abductor digiti minimi are consistently present in most major extant clades of limbed amphibians and reptiles, the exceptions being urodeles, which lack an abductor pollicis brevis, and birds, which seemingly lack an abductor digiti minimi, as will be discussed in the Discussion below (see Tables 1 and 3).
Discussion
Our dissections, comparisons and review of the literature indicate that the pectoral and forelimb musculature of limbed amphibians and reptiles conforms to a general pattern that seems to have been acquired very early in the evolutionary history of tetrapods, and is highly conserved in its anatomy and function. Regarding the total number of pectoral and forelimb muscles, there is not a great difference between the condition found in amphibians such as urodeles and in amniotes such as ‘lizards’ (see also Diogo et al. 2009a). Therefore, although some muscles may be reduced or missing in some amphibian and reptilian clades, and a clear departure of this general pattern is obviously present in birds, the same general muscular configuration is easily distinguishable in all major extant clades of limbed amphibians and reptiles. This idea has also been stressed by authors such as Holmes (1977; pp. 101, 130), who stated that ‘the evolution of the forearm musculature has been quite conservative’ in tetrapods and that the pectoral girdle and limb ‘musculature of living reptiles of such divergent types of Sphenodon, Iguana, Pseudemys, and Crocodylus shows many features in common, suggesting a similar pattern for primitive reptiles as well’.
Among the most notable anatomical differences between groups, one that seems to have relevant evolutionary and functional implications concerns the distal insertion points of part of the forearm musculature. Plesiomorphically, in tetrapods the muscles of the radial and ulnar complexes of the forearm are mainly inserted onto the radius/ulna and/or onto the more proximal carpal bones, but in mammals some of these muscles insert more distally onto bones such as the metacarpals (e.g. the extensor carpi radialis longus, the extensor carpi radialis brevis, the flexor carpi radialis and the flexor carpi ulnaris). Interestingly, a similar trend (towards a more distal insertion onto hand bones) is also found in some anurans with peculiarly subtle digital movement abilities, such as Phyllomedusa (see Manzano et al. 2008; Diogo et al. 2009a). One can thus argue that the complexity of the ‘extrinsic’ musculature of the hand (i.e. of the forearm musculature), as well as the more distal insertion of at least some of its components, evolved in a few, derived tetrapod taxa in correlation with the acquisition of these more subtle digital movement abilities. This hypothesis could seem to be counterintuitive, because one tends to think that these abilities are necessarily related with a greater number and/or a more complex configuration of the intrinsic hand muscles. However, it is strongly supported by the configuration found in taxa such as humans, which have the capacity to make and manipulate complex tools using a remarkably wide range of digital movements, particularly with the help of the thumb. In humans, the number of intrinsic muscles of the hand is actually smaller than that found in chimpanzees and numerous other primates, as well as in other tetrapods such as ‘lizards’ and urodeles; what is actually peculiar in humans is the great number of forearm muscles that attach directly on the digits, including muscles that are not differentiated in most other tetrapods and even in most other primates, such as the extensor pollicis brevis and the flexor pollicis longus (e.g. Diogo & Wood, 2009; Diogo et al. 2009a).
Regarding the similarities of the general configuration of the pectoral and forelimb muscles of the major extant groups of limbed tetrapods, it is interesting to note that in at least some cases even the reduction of the number of digits in some groups has provoked no profound modification in the corresponding musculature, indicating that muscles probably form and insert where needed to be capable of moving the most extreme (i.e. radial and ulnar) digits. For instance, the anuran muscle that is commonly accepted (see Haines, 1939) to be the homologue of the abductor pollicis longus of reptiles is often designated in the literature as ‘abductor indicis longus’ (see Gaupp, 1896; see also Tables 1 and 3). This is because it is commonly accepted that the most radial digit of adult anurans corresponds to digit 2 of tetrapods with five digits, i.e. in anurans the probable homologue of the abductor pollicis longus goes to digit 2, and not to digit 1, as is often the case in other tetrapods. So, interestingly, in this specific case, what seems to be important for the formation and attachments of the abductor pollicis longus is mainly the position, and not the ‘specific identity’, of the digit to which the muscle attaches (i.e. the muscle does not insert onto digit 1, as is the case in most tetrapods, because this digit is lacking in adult anurans, but instead inserts onto digit 2, which is the most radial digit of adult anurans). This idea is also supported by some other examples. For instance, in anurans the probable homologue of the abductor pollicis brevis (see Table 1) also attaches onto digit 2 of adults, and not onto digit 1, as is the case in most tetrapods. Also, in urodeles such as Taricha and Ambystoma, the probable homologue of the abductor digiti minimi of other tetrapods (i.e. the ‘extensor lateralis digiti IV’sensu authors such as Walthall & Ashley-Ross, 2006) goes to digit 4 and not to digit 5 (which is commonly accepted to be missing in adult urodeles such as Ambystoma, i.e. these adult urodeles have only digits 1, 2, 3 and 4). This contrasts with the patterning and development of the head muscles in tetrapods and other vertebrates, in which there is a highly constrained pattern of cranial skeletomuscular connectivity; each rhombomeric neural crest population remains coherent throughout ontogeny, forming both the connective tissues of specific muscles and their respective attachment sites onto the neuro- and viscerocranium (e.g. Köntges & Lumsden, 1996; Noden & Francis-West, 2006). That is, in the head there is a strong link between the insertions of the muscles and the identity of the specific neural crest population that forms the skeletal elements to which they attach. For instance, Köntges & Lumsden (1996) have shown that in tetrapods such as birds the posterior region of the mandible in which the depressor mandibulae attaches comprises neural crest derivatives of the hyoid arch, and not of the mandibular arch. So, the attachment of the depressor mandibulae is not primarily linked to the position (back of the mandible) but rather to the identity (neural crest derivatives of the hyoid arch) of the portion of the skull to which it attaches.
However, within the pectoral and forelimb muscles analyzed in the present paper, there are some cases in which the attachments of the muscles also seem to be primarily related to the identity, and not the position, of the skeletal elements to which they attach. For instance, authors such as Sullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996) do not describe an abductor of the most medial/ulnar digit of birds such as chickens, so the abductor digiti minimi seems to be lacking in these reptiles. We were also unable to find a distinct abductor digiti minimi in the chickens dissected by us (Tables 1 and 3). The evidence available strongly indicates that digit 5 is missing in adult chickens, i.e. the most ulnar digit of adult chickens is digit 3 according to most studies of fossils and hox genes and digit 4 according to most embryological studies (see Materials and methods). So, in this case, it seems that the ‘specific identity’ of the digit is actually important, that is, there is no abductor digiti minimi to digit 3 (i.e. 4 according to most embryologists), even if this is the most ulnar digit of adult chickens. However, it should be noted that some authors have designated the ‘ulnimetacarpalis dorsalis’sensuSullivan (1962), Shellswell & Wolpert (1977) and Meyers (1996) as ‘flexor and abductor of the fourth digit’, thus suggesting that this muscle, which in chickens usually goes from the distal portion of the ulna to the ulnar/medial portion of the carpometacarpal region, might actually correspond to a reduced abductor digiti minimi sensu the present work. Another plausible hypothesis is that this ‘ulnimetacarpalis dorsalis’ corresponds instead to a reduced short extensor (i.e. part of the extensores digitorum breves) to digit 3, that is, to digit 4 according to most embryologists (see Fig. 1 of Shellswell & Wolpert, 1977; see also Table 1). In our opinion, it would thus be interesting to address, in future developmental and/or genetic studies, this puzzling issue of the relationship between the formation and attachments of a muscle and the position vs. the ‘specific identity’ of the digit(s) to which it attaches. As stressed above, future work is also needed to address some other crucial questions that need to be clarified. For instance, further studies, ideally including a detailed analysis of the innervation and development of the ‘rhomboideus’ and the ‘palmaris longus’ of a broader sampling of amphibian and reptilian taxa, are needed to investigate whether these structures are homologous to the mammalian rhomboideus and palmaris longus, respectively. We hope that the present work will stimulate future research into these issues.
Acknowledgments
We greatly appreciate M. J. Tulli (Fundación Miguel Lillo – CONICET), A. Dupuy (Fundacion Miguel Lillo) and Ricardo Montero (Fundación Miguel Lillo – CONICET – UNT) for helping with the production of the figures. V.A. greatly appreciates the enthusiastic support of her sister and friends C. Braks, C. Abdala and A.G. Padilla. We would also like to thank M. Carleton, D. Schmidt and L. Gordon (National Museum of Natural History), R. Etheridge (San Diego State University), J. Daza (University of Puerto Rico), B. Sacham and Y. Werner (The Hebrew University of Jerusalem), D. Baldo, E. Lavilla, S. Ktretschmar, A. Echavarria, M. Cánepa, J. C. Stazonelli and G. Scrocchi (Fundación Miguel Lillo), A. Manzano (CCyTTP, Diamante), R. Montero (Universidad Nacional de Tucumán), J. Joss (Macquarie University), A. Gosztonyi (Centro Nacional Patagónico), Gregory Watkins-Colwell (Peabody Museum of Natural History, Yale University), and J. Fernández and I. Doadrio (Museo Nacional de Ciencias Naturales de Madrid) for kindly providing a large part of the specimens analyzed for this study. Anthony Herrel (Muséum National d’Histoire Naturelle, Paris) generously permitted the dissection of many specimens from his personal collection. Andrea Arcucci (Universidad de San Luis) and Juan Martin Leardi (UBA – CONICET) kindly provided part of our consulted literature. Dr. Mason Meers contribution to improve our manuscript is very much appreciated. R.D. was supported by a George Washington University Presidential Merit Fellowship. V.A. was supported by the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina PIP 6347 and 112-200801-00225.
References
- Abdala V, Moro S. Comparative myology of the forelimb of Liolaemus sand lizards (Liolaemidae) Acta Zool. 2006;87:1–12. [Google Scholar]
- Abdala V, Manzano AS, Herrel A. The distal forelimb musculature in aquatic and terrestrial turtles: phylogeny or environmental constraints? J Anat. 2008;213:159–172. doi: 10.1111/j.1469-7580.2008.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnarsson I, Coddington JA. Quantitative test of primary homology. Cladistics. 2007;23:1–11. [Google Scholar]
- Benton MJ. Classification and phylogeny of the diapsid reptiles. Zool J Linn Soc. 1985;84:97–164. [Google Scholar]
- Bojanus LH. Anatome testudinis europaeae. Facsimile reprints in Herpetology. 1819;26:178. [Google Scholar]
- Brooks HSJ. Morphology of the muscles on the extensor aspect of the middle and distal segments of the limbs: with an account of the various paths which are adopted by the nerve-trunks in these segments. Stud Mus Zool Univ Coll Dundee. 1889;1:1–17. [Google Scholar]
- Bunnell S. Surgery of the intrinsic muscles of the hand other than those producing opposition of the thumb. J Bone Joint Surg Am. 1942;24:1–31. [Google Scholar]
- Burton TC. Pointing the way: the distribution and evolution of some characters of the finger muscles of frogs. Am Mus Nov. 1998;3229:1–13. [Google Scholar]
- Cao Y, Sorenson MD, Kumazawa Y, et al. Phylogenetic position of turtles among amniotes: evidence from mitochondrial and nuclear genes. Gene. 2000;259:139–148. doi: 10.1016/s0378-1119(00)00425-x. [DOI] [PubMed] [Google Scholar]
- Carroll RL. The Paleozoic ancestry of salamanders, frogs and caecilians. Zool J Linn Soc. 2007;150:1–140. [Google Scholar]
- Conrad JL. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull Am Mus Nat Hist. 2008;310:1–182. [Google Scholar]
- Dawkins R. The Ancestor's Tale, A Pilgrimage to the Dawn of Life. Boston: Houghton Mifflin; 2004. [Google Scholar]
- De Braga M, Rieppel O. Reptile phylogeny and the interrelationships of turtles. Zool J Linn Soc. 1997;120:281–354. [Google Scholar]
- Dilkes DW. Appendicular myology of the hadrosaurian dinosaur Maiasaura peeblesorum from the Late Cretaceous (Campanian) of Montana. Trans R Soc Edinburgh Earth Sci. 2000;90:87–125. [Google Scholar]
- Diogo R. Morphological evolution, aptations, homoplasies, constraints, and evolutionary trends: catfishes as a case study on general phylogeny and macroevolution. Enfield: Science Publishers; 2004a. [Google Scholar]
- Diogo R. Muscles versus bones: catfishes as a case study for an analysis on the contribution of myological and osteological structures in phylogenetic reconstructions. Anim Biol. 2004b;54:373–391. [Google Scholar]
- Diogo R. On the origin and evolution of higher-clades: osteology, myology, phylogeny and macroevolution of bony fishes and the rise of tetrapods. Enfield: Science Publishers; 2007. [Google Scholar]
- Diogo R. Comparative anatomy, homologies and evolution of the mandibular, hyoid and hypobranchial muscles of bony fish and tetrapods: a new insight. Anim Biol. 2008;58:123–172. [Google Scholar]
- Diogo R. The head musculature of the Philippine colugo (Dermoptera: Cynocephalus volans), with a comparison to tree-shrews, primates and other mammals. J Morphol. 2009;270:14–51. doi: 10.1002/jmor.10666. [DOI] [PubMed] [Google Scholar]
- Diogo R, Abdala V. Comparative anatomy, homologies and evolution of the pectoral muscles of bony fish and tetrapods: a new insight. J Morphol. 2007;268:504–517. doi: 10.1002/jmor.10531. [DOI] [PubMed] [Google Scholar]
- Diogo R, Wood BA. Comparative anatomy and evolution of the pectoral and forelimb musculature of primates: a new insight. Am J Phys Anthropol, Meeting Suppl. 2009;48:119. [Google Scholar]
- Diogo R, Hinits Y, Hughes SM. Development of mandibular, hyoid and hypobranchial muscles in the zebrafish: homologies and evolution of these muscles within bony fishes and tetrapods. BMC Dev Biol. 2008a;8:24–46. doi: 10.1186/1471-213X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R, Abdala V, Lonergan N, et al. From fish to modern humans – comparative anatomy, homologies and evolution of the head and neck musculature. J Anat. 2008b;213:391–424. doi: 10.1111/j.1469-7580.2008.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R, Abdala V, Lonergan N, et al. From fish to modern humans – comparative anatomy, homologies and evolution of the pectoral and forelimb musculature. J Anat. 2009a;214:694–716. doi: 10.1111/j.1469-7580.2009.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R, Wood BA, Aziz MA, et al. On the origin, homologies and evolution of primate facial muscles, with a particular focus on hominoids and a suggested unifying nomenclature for the facial muscles of the Mammalia. J Anat. 2009b;215:300–319. doi: 10.1111/j.1469-7580.2009.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duellman WE, Trueb L. The Biology of Amphibians. New York: McGraw-Hill Book Company; 1986. [Google Scholar]
- Ecker A. Anatomy of the Frog. Oxford: Clarendon Press; 1889. [Google Scholar]
- Edgeworth FH. The cranial muscles of vertebrates. Cambridge: Cambridge University Press; 1935. [Google Scholar]
- Fürbringer M. Zur vergleichenden Anatomie der Schultermuskeln – 3 teil, capitel IV, Saurier und Crocodile. Gegenbaurs Morphol Jahrb. 1876;1:636–816. [Google Scholar]
- Galis F, Kundrát M, Sinervo B. An old controversy solved: bird embryos have five fingers. Trends Ecol Evol. 2003;18:7–9. [Google Scholar]
- Galis F, Kundrát M, Metz JAJ. Hox genes, digiti identities and the theropod/bird transition. J Exp Zool B Mol Dev Evol. 2005;304:198–205. doi: 10.1002/jez.b.21042. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Kluge AG, Rowe T. Amniote phylogeny and the importance of fossils. Cladistics. 1988;4:105–209. doi: 10.1111/j.1096-0031.1988.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Gaupp E. A. Ecker's und R. Wiedersheim's Anatomie des Frosches, part 1. Braunschweig: Friedrich Vieweg und Sohn; 1896. [Google Scholar]
- Ghetie V, Chitescu ST, Cotofan V, et al. Atlas de Anatomía de las Aves Domésticas. Madrid: Editorial Acribia; 1981. [Google Scholar]
- Grim M. Development of the primordia of the latissimus dorsi muscle of chicken. Folia Morphol (Prague) 1971;19:252–258. [PubMed] [Google Scholar]
- Haines RW. A revision of the extensor muscles of the forearm in tetrapods. J Anat. 1939;73:211–233. [PMC free article] [PubMed] [Google Scholar]
- Haines RW. The flexor muscles of the forearm and hand in lizards and mammals. J Anat. 1950;84:13–29. [PMC free article] [PubMed] [Google Scholar]
- Haninec P, Tomás R, Kaiser R, et al. Development and clinical significance of the musculus dorsoepitrochlearis in men. Clin Anat. 2009;22:481–488. doi: 10.1002/ca.20799. [DOI] [PubMed] [Google Scholar]
- Hedges SB, Poling LL. A molecular phylogeny of reptiles. Science. 1999;283:998–1001. doi: 10.1126/science.283.5404.998. [DOI] [PubMed] [Google Scholar]
- Hetherington TE, Tugaoen JR. Histochemical studies on the amphibian opercularis muscle (Amphibia) Zoomorphology. 1990;109:273–279. [Google Scholar]
- Hill RV. Integration of morphological data sets for phylogenetic analysis of Amniota: the importance of the integumentary characters and increased taxonomic sampling. Sys Bio. 2005;54:530–547. doi: 10.1080/10635150590950326. [DOI] [PubMed] [Google Scholar]
- Holliday CM. New insights into dinosaur jaw muscle anatomy. Anat Rec. 2009;292:1246–1265. doi: 10.1002/ar.20982. [DOI] [PubMed] [Google Scholar]
- Holliday CM, Witmer LM. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J Morphol. 2007;268:457–484. doi: 10.1002/jmor.10524. [DOI] [PubMed] [Google Scholar]
- Holmes R. The osteology and musculature of the pectoral limb of small captorhinids. J Morphol. 1977;152:101–140. doi: 10.1002/jmor.1051520107. [DOI] [PubMed] [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part III – Amphibia. Q Rev Biol. 1935;10:397–431. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part IV – Reptilia. Q Rev Biol. 1936a;11:183–208. [Google Scholar]
- Howell AB. Phylogeny of the distal musculature of the pectoral appendage. J Morphol. 1936b;60:287–315. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part V – Monotremata. Q Rev Biol. 1937a;12:191–205. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture, Part IV – Therian Mammalia. Q Rev Biol. 1937b;12:440–463. [Google Scholar]
- Hudson GE, Schreiweis DO, Wang SYC. A numerical study of the wing and leg muscles of Tinamous (Tinamidae) Northwest Sci. 1972;46:207–255. [Google Scholar]
- Humphry GM. Observations in Myology – the Myology of Cryptobranch, Lepidosiren, Dog-fish, Ceratodus, and Pseudopus palasii, with the Nerves of Cryptobranch and Lepidorsiren and the Disposition of Muscles in Vertebrate Animals. Cambridge: Macmillan & Co; 1872a. [Google Scholar]
- Humphry GM. The disposition of muscles in Vertebrate animals. J Anat Physiol. 1872b;6:293–376. [PMC free article] [PubMed] [Google Scholar]
- Jollie M. Chordate Morphology. New York: Reinhold; 1962. [Google Scholar]
- Jouffroy FK. Musculature des membres. In: Grassé PP, editor. Traité de Zoologie XVI, 3. Paris: Masson et Cie; 1971. pp. 1–475. [Google Scholar]
- Jouffroy FK, Lessertisseur J. Particularités musculaires des Monotrémes – musculature post-craniénne. In: Grassé PP, editor. Traité de Zoologie XVI, 3. Paris: Masson et Cie; 1971. pp. 733–836. [Google Scholar]
- Kardong KV. Vertebrates: Comparative Anatomy, Function, Evolution. 3rd edn. New York: McGraw-Hill; 2002. [Google Scholar]
- Kardong KV, Zalisko EJ. Comparative Vertebrate Anatomy – A Laboratory Dissection Guide. New York: McGraw-Hill; 1998. [Google Scholar]
- Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kundrát M. Primary chondrification foci in the wing basipodium of Struthio camelus with comments on interpretation of autopodial elements in Crocodilia and Aves. J Exp Zool B Mol Dev Evol. 2009;312:30–41. doi: 10.1002/jez.b.21240. [DOI] [PubMed] [Google Scholar]
- Lewis OJ. Functional Morphology of the Evolving Hand and Foot. Oxford: Clarendon Press; 1989. [Google Scholar]
- Macalister A. On the arrangement of the pronator muscles in the limbs of vertebrate animals. J Anat Physiol. 1869;3:335–340. [PMC free article] [PubMed] [Google Scholar]
- Mannen H, Li SL. Molecular evidence for a clade of turtles. Mol Phylogenet Evol. 1999;13:144–148. doi: 10.1006/mpev.1999.0640. [DOI] [PubMed] [Google Scholar]
- Manzano AS. 1996. Análisis de la musculatura de la familia Pseudidae (Amphibia: Anura) Unpublished PhD Thesis Universidad Nacional de Tucumán, Argentina. [Google Scholar]
- Manzano AS, Abdala V, Herrel A. Morphology and function of the forelimb in arboreal frogs: specializations for grasping ability? J Anat. 2008b;213:296–307. doi: 10.1111/j.1469-7580.2008.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell EE, Larsson HCE. Osteology and myology of the wing of the Emu (Dromarius novaehollandiae), and its bearing on the evolution of vestigial structures. J Morphol. 2007;268:423–441. doi: 10.1002/jmor.10527. [DOI] [PubMed] [Google Scholar]
- McMurrich JP. The phylogeny of the forearm flexors. Am J Anat. 1903a;2:177–209. [Google Scholar]
- McMurrich JP. The phylogeny of the palmar musculature. Am J Anat. 1903b;2:463–500. [Google Scholar]
- Meers MB. Crocodylian forelimb musculature and its relevance to Archosauria. Anat Rec. 2003;274A:891–916. doi: 10.1002/ar.a.10097. [DOI] [PubMed] [Google Scholar]
- Meyers RA. Morphology of the antebrachial musculature in the American Kestrel, Falco sparverius (Aves) Ann Anat. 1996;178:49–60. doi: 10.1016/S0940-9602(96)80012-4. [DOI] [PubMed] [Google Scholar]
- Mivart SG. Notes on the myology of Menopoma alleghaniense. Proc Zool Soc Lond. 1869;1869:254–261. [Google Scholar]
- Modesto SP, Anderson JS. The phylogenetic definition of Reptilia. Sys Bio. 2004;53:815–821. doi: 10.1080/10635150490503026. [DOI] [PubMed] [Google Scholar]
- Moro S, Abdala V. Análisis descriptivo de la miología flexor y extensora del miembro anterior de Polychrus acutirostris (Squamata, Polychrotidae) Pap Avulsos Zool (São Paulo) 2004;44:81–89. [Google Scholar]
- Müller J. Early loss and multiple return of the lower temporal arcade in diapsid reptiles. Naturwissenschaften. 2003;90:473–476. doi: 10.1007/s00114-003-0461-0. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Patterson C. Homology in classical and molecular biology. Mol Biol Evol. 1988;5:603–625. doi: 10.1093/oxfordjournals.molbev.a040523. [DOI] [PubMed] [Google Scholar]
- de Pinna MC. Concepts and test of homology in the cladistic paradigm. Cladistics. 1991;7:367–394. [Google Scholar]
- Piatt J. Morphogenesis of the cranial muscles of Amblystoma punctatum. J Morphol. 1938;63:531–587. [Google Scholar]
- Piekarski N, Olsson L. Muscular derivatives of the cranialmost somites revealed by long-term fate mapping in the Mexican axolotl (Ambystoma mexicanum) Evol Dev. 2007;9:566–578. doi: 10.1111/j.1525-142X.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Ribbing L. Die distale Armmuskulatur der Amphibien, Reptilien und Säugetiere. Zool Jahrb. 1907;23:587–680. [Google Scholar]
- Ribbing L. Die Muskeln und Nerven der Extremitäten. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere Urban & Schwarzenberg. Amsterdam: Asher & Co; 1938. pp. 543–656. [Google Scholar]
- Rieppel O. Osteology of Simosaurus gaillardoti and the relationships of stem-group sauropterygia. Field Geol. 1994;1462:1–85. [Google Scholar]
- Rieppel O. Turtles as diapsid reptiles. Nature. 2000;384:453–455. [Google Scholar]
- Rieppel O, Reisz RR. The origin and early evolution of turtles. Ann Rev Ecol Sys. 1999;30:1–22. [Google Scholar]
- Romer AS. The locomotor apparatus of certain primitive and mammal-like reptiles. Bull Am Mus Nat Hist. 1922;46:517–606. [Google Scholar]
- Romer AS. Pectoral limb musculature and shoulder-girdle structure in fish and tetrapods. Anat Rec. 1924;27:119–143. [Google Scholar]
- Romer AS. The development of tetrapod limb musculature – the shoulder region of Lacerta. J Morphol. 1944;74:1–41. [Google Scholar]
- Russell AP. Limb muscles in relation to lizard systematics: a reappraisal. In: Estes R, Pregill G, editors. Phylogenetic Relationships of Lizard Families: Essays Commemorating Charles L. Camp. Stanford: Stanford University Press; 1988. pp. 119–281. [Google Scholar]
- Russell AP, Bauer AM. The appendicular locomotor apparatus of Sphenodon and normal-limbed squamates. In: Gans C, Gaunt AS, Adler K, editors. Biology of Reptilia. Vol. 21. New York: Society for the Study of Amphibians and Reptilian; 2008. pp. 1–465. [Google Scholar]
- Shellswell GB, Wolpert L. The pattern of muscle and tendon development in the chick wing. In: Ede D, Hinchcliffe R, Balls M, editors. Vertebrate Limb and Somite Morphogenesis. Cambridge: Cambridge University Press; 1977. pp. 71–86. [Google Scholar]
- Smith GM. 1926. The detailed anatomy of Triturus torosus. Unpublished Masters Thesis University of British Columbia. [Google Scholar]
- Straus WL. The phylogeny of the human forearm extensors. Hum Biol. 1941a;13:23–50. [Google Scholar]
- Straus WL. The phylogeny of the human forearm extensors (concluded) Hum Biol. 1941b;13:203–238. [Google Scholar]
- Straus WL. The homologies of the forearm flexors: urodeles, lizards, mammals. Am J Anat. 1942;70:281–316. [Google Scholar]
- Sullivan GE. Anatomy and embryology of the wing musculature of the domestic fowl (Gallus) Aust J Zool. 1962;10:458–518. [Google Scholar]
- Sullivan GE. Abnormalities of the muscular anatomy in the shoulder region of paralysed chick embryos. Aust J Zool. 1967;15:911–940. [Google Scholar]
- Tsuihiji T. Homologies of the longissimus, iliocostalis, and hypaxial muscles in the anterior presacral region of extant diapsida. J Morphol. 2007;268:986–1020. doi: 10.1002/jmor.10565. [DOI] [PubMed] [Google Scholar]
- Vargas AO, Fallon JF. Birds have dinosaur wings, the molecular evidence. J Exp Zool B Mol Dev Evol. 2005a;304:86–90. doi: 10.1002/jez.b.21023. [DOI] [PubMed] [Google Scholar]
- Vargas AO, Fallon JF. The digits of the wing of birds are 1, 2, and 3, a review. J Exp Zool B Mol Dev Evol. 2005b;304:206–219. doi: 10.1002/jez.b.21051. [DOI] [PubMed] [Google Scholar]
- Vargas AO, Kohlsdorf T, Fallon JF, et al. The evolution of HoxD-11 expression in the bird wing: insights from Alligator mississippiensis. PLoS ONE. 2008;3:e3325. doi: 10.1371/journal.pone.0003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WF. The locomotor apparatus of testudines. In: Gans C, Parsons TS, editors. Biology of the Reptilia. Vol. 14. New York: Academic Press; 1973. pp. 1–100. [Google Scholar]
- Walthall JC, Ashley-Ross MA. Postcranial myology of the California newt, Taricha torosa. Anat Rec A. 2006;288:46–57. doi: 10.1002/ar.a.20279. [DOI] [PubMed] [Google Scholar]
- Walther WG. 1922. Die Neu-Guinea-Schieldkröte Carettochelys insculpta Ramsay. Unpublished PhD Thesis University of Leiden. [Google Scholar]
- Wyneken J. The Anatomy of Sea Turtles. Miami: U.S. Department of Commerce NOAA Technical Memorandum NMFS-SEFSC-470; 2001. [Google Scholar]


