Abstract
To identify the forces that may exist in the parabronchus of the avian lung and that which may explain the reported strengths of the terminal respiratory units, the air capillaries and the blood capillaries, the arrangement of the parabronchial collagen fibers (CF) of the lung of the domestic fowl, Gallus gallus variant domesticus was investigated by discriminatory staining, selective alkali digestion, and vascular casting followed by alkali digestion. On the luminal circumference, the atrial and the infundibular CF are directly connected to the smooth muscle fibers and the elastic tissue fibers. The CF in this part of the parabronchus form the internal column (the axial scaffold), whereas the CF in the interparabronchial septa and those associated with the walls of the interparabronchial blood vessels form the external, i.e. the peripheral, parabronchial CF scaffold. Thin CF penetrate the exchange tissue directly from the interparabronchial septa and indirectly by accompanying the intraparabronchial blood vessels. Forming a dense network that supports the air and blood capillaries, the CF weave through the exchange tissue. The exchange tissue, specifically the air and blood capillaries, is effectively suspended between CF pillars by an intricate system of thin CF, elastic and smooth muscle fibers. The CF course through the basement membranes of the walls of the blood and air capillaries. Based on the architecture of the smooth muscle fibers, the CF, the elastic muscle fibers, and structures like the interparabronchial septa and their associated blood vessels, it is envisaged that dynamic tensional, resistive, and compressive forces exist in the parabronchus, forming a tensegrity (tension integrity) system that gives the lung rigidity while strengthening the air and blood capillaries.
Keywords: air and blood capillaries, bird, collagen fibers, lung, parabronchus, tensegrity
Introduction
‘Although the materials found in biology are often very different from those used in engineering, the geometries of the structures in which materials can be employed to carry loads are generally much the same. Nature is frequently more clever than engineers at developing the potential of a given structural concept’ (Gordon, 1988).
One of the most confounding properties of the functional design of the avian respiratory system concerns the remarkable strengths of the minuscule terminal respiratory units, the air capillaries (ACs) and the blood capillaries (BCs) of the lung. About three decades ago, Macklem et al. (1979) reported that the ACs remained open when the lung was subjected to a positive pressure of 20 cm H2O (≈2 kPa). They remarked that ‘unknown factors serve to confer a remarkable stability on these fine structures’. A little later, Powell et al. (1985) showed that in contrast to the mammalian lung, where the pulmonary vascular resistance (PVR) decreases as cardiac output increases, owing mainly to the distension and recruitment of the BCs (Borst et al. 1956; Glazier et al. 1969), in the avian lung, PVR doubled after ligation (occlusion) of the left pulmonary artery, an experimental procedure that doubled the flow of blood to the right lung: the investigators concluded that ‘the BCs are noncompliant’. The exceptional strength of the ACs and the BCs has now been corroborated by other investigators (Wideman, 2001; West et al. 2006, 2007a; Wideman et al. 2007; Watson et al. 2008). West et al. (2007a) and Watson et al. (2008) observed that ‘the avian pulmonary BCs behave like rigid tubes that defy either expansion or compression’. Although lined by a less efficient surfactant with paucity of palmitoylmyristoylphosphatidylcholine and one lacking surfactant proteins (SP)-A and SP-C (Bernhard et al. 2001), the surface tension prevailing at the air–water interface of the ACs that range in diameter from 3 to 20 μm (Duncker, 1972; Maina & Nathaniel, 2001; Woodward & Maina, 2008) should in all likelihood, as opined by Scheuermann et al. (1997) and Watson et al. (2008), be much greater than that in an alveolus of equivalent diameter. That even under such conditions the ACs are not only stable but also very strong is most perplexing.
Although it is insightful that the strengths of the ACs and the BCs should have structural underpinnings (reviewed by Maina, 2008), the particular components and/or mechanism(s) that grant the exceptional robusticity remain unclear and controversial. Scheuermann et al. (1997) suggested that presence of pairs of epithelial cells processes, that they termed retinacula, and that of the proteinaceous trilaminar substance provide additional support to the ACs; Klika et al. (1997) considered the presence of the trilaminar substance in the cytoplasm of the squamous epithelial cells to provide intercapillary anastomosing skeletal support; West et al. (2006) suggested that the ‘close-packing’ (the ‘honeycomb-like’ arrangement) of the ACs and the BCs grants strength; West et al. (2007a,b);, Watson et al. (2007, 2008), and West (2009) speculated that the mechanical support of the BCs arises from the epithelial cell struts (differently called ‘cross-braces’, ‘cross-bridges’, and ‘epithelial ‘plates’ by various investigators) that separate the ACs while connecting the BCs; Maina (2007a,b); made a case for the existence of an interdependent, tightly coupled infrastructure of a network of tension and compression structural elements in the avian lung that forms a tensegrity system; recently, West et al. (2010) observed that junctions of the epithelial bridges with the BC walls have thickening of epithelial cells and accumulation of extracellular matrix which may modulate hoop stress in the capillary walls.
The strengths of the ACs and the BCs are particularly bewildering. In contrast to the mammalian lung, where the interalveolar septum comprises a conspicuously thick (supporting) side that is plentifully endowed with collagen fibers (Weibel, 1973; Maina, 2002) and a thin (gas exchange) side that comprises epithelial and endothelial cells that share a thin basement membrane (Weibel, 1973, 1984; Crouch et al. 1997), the blood-gas barrier of the avian lung is relatively uniform in thickness, much thinner, and connective tissue elements are lacking or very scarce (Maina & King, 1982; Maina et al. 1989; Klika et al. 1997; Scheuermann et al. 1997; Watson et al. 2007). These structural attributes foremost informed Scheuermann et al. (1997) to conclude that the ‘avian air capillaries are delicate structures compared to the mammalian pulmonary alveolus’. Fuller, (1961) defined tensegrity as ‘an engineering principle of continuous-tension and discontinuous-compression’ that imparts great stability upon composite structures. In biology, tensegrets have been reported in structures ranging from molecular cellular, tissue, organ, and organismal levels (Chen & Ingber, 1999; Yamada et al. 2000; Wang et al. 2001; Frantsevich & Gorb, 2002; Ingber, 2003, 2004; Zannoti & Guerra, 2003). In such assemblages, shape and strength are presumed to be granted by an infinite number of continuous tensional adjustments between closely interconnected structural components and by discontinuous local compressions of the rigid parts.
The primary goal of this study was to determine the arrangement of the CFs in the parabronchus of the lung of the domestic fowl (chicken), Gallus gallus variant domesticus. The arrangement of the elastic and the smooth muscle connective tissue elements was also examined. Based on published observations and our own, an implicit integrative mechanistic model of the forces that may exist in the parabronchus is constructed. Existence of a tensegrity system is proposed. The terminology of names of the avian species and the anatomical structures of the lung conforms to the recommendation made in the Nomina Anatomica Avium (King, 1979).
Materials and methods
Specimens and preparation of lungs
With the experimental procedure having received approval by the University of the Witwatersrand's Animal Ethics Committee (clearance number 2007/53/01), healthy, adult, free range, mixed breed of domestic chickens, G. gallus variant domesticus, were injected with 2.5 cm3 heparin (1000 IU) through the left branchial vein. The birds were left for 10 min for the anticoagulant to circulate properly through the body. They were subsequently killed by injection of the 5 cm3 of Euthanse® (200 mg cm3 thiopentone sodium) into the right branchial vein. With the birds lying in a supine position, the heart was exposed after removing the sternum by incising through the associated muscles (the pectorals and the supracoracoideus), ribs, and the coracoid bones. The pulmonary trunk was exposed and cannulated by passing a suitably sized cannula through an incision made across the right ventricle. For the outflow of the perfusate, the heart was cut close to the apex, a cannula of appropriate size introduced into the left ventricle and pushed past the atrio-ventricular valve into the left atrium. The outflow tube was placed at the level of the heart. After ligating the cannula around the pulmonary trunk, the lungs were perfused with phosphate-buffered saline (PBS) at a pressure of 12 cm H2O (1.2 kPa) until practically all the blood was removed from the lung. This was assessed by appearance of clear out-flow and breaching of the lung. For selective staining and digestion for collagen fibers, perfusion with PBS was immediately followed by perfusion with 2.5% glutaraldehyde buffered in phosphate and pH adjusted to 7.4 for about 20 min. The lungs were left in place for 4 h and then carefully removed from their vertebral and costal attachments. Some of the samples were processed for scanning electron microscopy (SEM) and others for transmission electron microscopy (TEM). These preparations served as ‘controls’, i.e. they were used to confirm the observations made on the collagen-stained and alkali-digested preparations. The pictures from such preparations are mostly given as inserts to the figures.
Staining for collagen fibers by van Gieson's method
Lung tissue samples measuring about 3 × 3 × 6 mm were obtained and processed for light microscopy by the standard laboratory methods and embedded in paraffin wax (Bancroft & Steven, 1996). Sections were cut on a Jung Biocut 2035 rotary microtome at 5 μm thickness. After deparaffinization, the sections were placed in Weigert's iron hematoxylin solution for 5 min and washed briefly in running tap water before rinsing in two changes of distilled water. Subsequently, the sections were placed in Van Gieson's solution for 3 min. This was followed by dehydration in three changes of 95% alcohol and three changes of absolute alcohol. After clearing, the sections were put through three changes of xylene and a coverslip mounted with Entellen®. The staining showed sharp contrast between the collagen fibers (red), cell nuclei (blue-black), and other tissues (yellow).
Alkali-digestion of lung tissues for collagen fibers
Tissues samples measuring about 2 × 3 × 5 mm were taken from the lung and digested to remove all tissue components except collagen according to the methods described by Ohtani (1987, 1992) and Tochima et al. (2004). Briefly, the tissues were placed in a 10 m solution of sodium hydroxide (NaOH) for 4 days at room temperature (20 °C). This was followed by washing in distilled water for a further 4 days. At the end of this time, when the tissues had turned whitish and semi-translucent, they were treated with 1% tannic acid solution overnight. After rinsing in distilled water for 4 h, the pieces were post-fixed in 1% aqueous solution of osmium tetroxide (OsO4) for 2 h. The specimens were then dehydrated in a series of graded concentration of ethanol. After critical-point drying with liquid carbon dioxide, the samples were freeze-cracked in liquid nitrogen, mounted on aluminium stubs, and observed on a JEOL 840 SEM under an accelerating voltage of 15 kV.
Casting and digestion of the lung for collagen fibers
To more effectively show the normal, i.e. in-life (in vivo), arrangement of CFs around the BCs, after the lung was thoroughly perfused with PBS, the vasculature was cast by latex rubber. Once the latex set, it provided a scaffold that preserved the natural spatial arrangement of the CFs: in uncast preparations, during digestion, CFs folded, curled, and collapsed once the supporting lung tissue was removed. The procedure of combining casting and digestion was successfully used by Gonçalves et al. (1995) to study the organization of the elastic fibers in the rat lung. Here, a 10-cm3 syringe was used to inject latex rubber through the pulmonary trunk until moderate tension developed in the lung. At that point, the blood vessel was ligated to maintain the intrapulmonary pressure. The body was put in a cold room (4 °C) and setting of the latex rubber was continually assessed for 1 week. When this was satisfactory, the lung was carefully removed from the body and samples taken for digestion in 10 m solution of sodium hydroxide. After digestion, the tissues were left in running tap water for about 6 h before they were processed and viewed on a JEOL 840 SEM.
Results
The parabronchi of the lung of the domestic fowl are separated by a conspicuous band of connective tissue, the interparabronchial septum (Figs 1 and 2). In cross-section, the parabronchi are more-or-less hexagonal in shape (Figs 1 and 2). Evaginating from the parabronchial lumen, atria are well developed (Fig. 3). Prominent atrial septa separate the atria (Figs 3–6). The smooth muscles of the atria are interconnected by collagen fibers that run through the interparabronchial septa (Figs 5 and 6). The same fibers extend outwards to form the infundibular collagen fibers. In three dimensions, the atrial collagen fibers form an intricate system of longitudinal, transverse, and oblique fibers (Fig. 7) which connect to the elastic fibers (Fig. 8). A continuum is formed by the atrial, infundibular, and gas exchange systems of collagen fibers (Figs 9 and 10). The interparabronchial blood vessels, arteries and veins, which arise from the principal branches of the pulmonary artery and vein, are located in the interparabronchial septum (Figs 1–3). The arteries (Fig. 11) give rise to intraparabronchial arteries (Figs 12 and 13) that enter the gas exchange tissue to originate arterioles that terminate in blood capillaries (Figs 12–20). Collagen fibers are associated with the tunica adventitia of the walls of the interparabronchial blood vessels (Figs 2, 3, and 11) and occur in abundance in the interparabronchial septum (Figs 2 and 3). From the interparabronchial septa, the fibers enter the parenchyma, the gas exchange tissue, directly from the interparabronchial arteries (Fig. 11) or indirectly by accompanying the intraparabronchial arteries (Figs 12 and 13). In the gas exchange tissue (Figs 12–18), collagen those fibers run in the blood–gas barrier (i.e. the sites that separate air from blood, Figs 15–22), in the sites where blood capillaries lie next to each other (Figs 15 and 16) and sites where air capillaries lie side-by-side (Figs 14 and 15).
Figs 1–9.
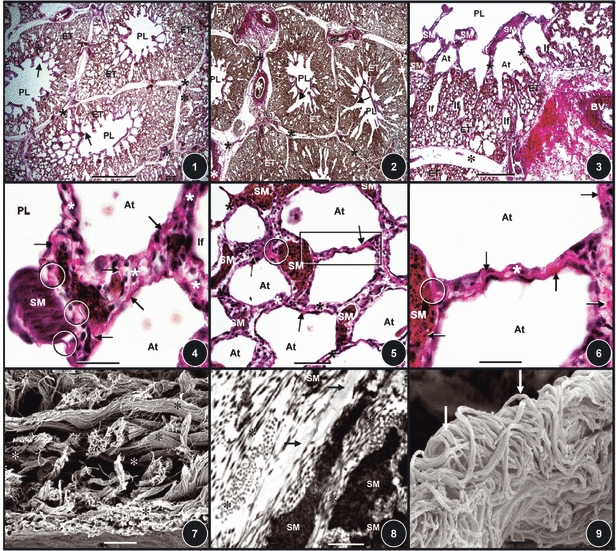
(Figs 1, 2) Lung of the domestic fowl stained for collagen fibers. The parabronchi comprise a lumen (PL) surrounded by exchange tissue (ET). The parabronchi are separated by interparabronchial septa (stars). Asterisks, interparabronchial blood vessels. Arrows, atria. Scale bars: (Fig. 1) 0.2 mm; (Fig. 2) 0.2 mm. (Fig. 3) Cross-section of a parabronchus stained to show collagen fibers (red). Asterisk, interparabronchial septum; BV, interparabronchial blood vessel; stars, interatrial septa; SM, atrial smooth muscle; PL, parabronchial lumen; If, infundibula; ET, exchange tissue; At, atria. Scale bar: 0.1 mm. (Figs 4–6) Collagen fibers (arrows) and smooth muscle fibers (SM) in the interatrial septa (stars). At, atria; circles, areas where collagen fibers attach onto smooth muscle fibers. Scale bars: (Fig. 4) 20 μm; (Fig. 5) 30 μm; (Fig. 6) 20 μm. (Fig. 7) The complex arrangement of collagen fibers (stars) in the interatrial septa. Scale bar: 0.5 μm. (Fig. 8) The connection between the collagen fibers (stars), the smooth muscle fibers (SM), and the elastic tissue fibers (arrows) in the interatrial septum. Scale bar: 0.3 μm. (Fig. 9) Collagen fibers (arrows) in an interatrial septum. Scale bar: 0.5 μm.
Figs 10–18.
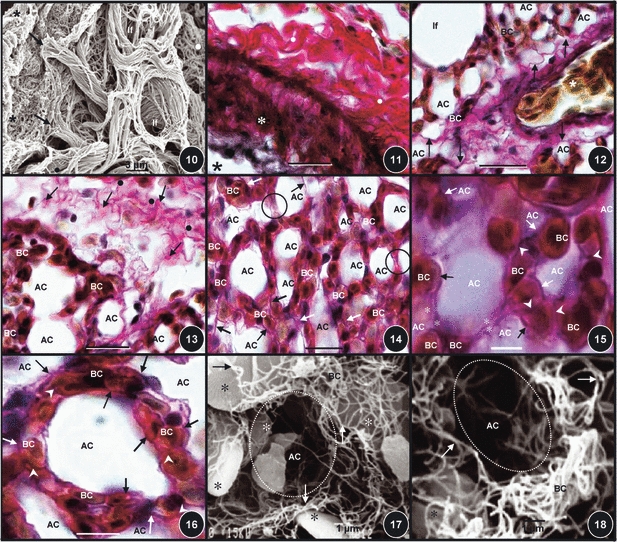
(Fig. 10) Collagen fibers of the atrial septa (star) connecting to the infundibula ones (arrows) which in turn connect to the very thin collagen fibers of the exchange tissue (dot). Scale bar: 20 mm. (Fig. 11) The wall of an interparabronchial blood vessel (asterisk) surrounded by a thick layer of collagen fibers in the tunica adventitia (dots). Star, lumen of the blood vessel. Scale bar: 20 μm. (Fig. 12) An intraparabronchial artery (star) entering the exchange tissue accompanied by collagen fibers (arrows). AC, air capillaries; BC, blood capillaries. If, infundibulum. Scale bar: 20 μm. (Fig. 13) Collagen fibers running from the interparabronchial septum (dots) into the exchange tissue (arrows). BC, blood capillaries; AC, air capillaries. Scale bar: 15 μm. (Fig. 14) Diffuse organization of collagen fibers (arrows) in the exchange tissue. BC, blood capillaries; AC, air capillaries. Circled areas, sites where air capillaries lie adjacent to each other and where collagen fibers accompany the epithelial cell extensions. Scale bar: 10 μm. (Fig. 15) Collagen fibers that are located in the blood–gas barrier (arrows) and in the epithelial cell extensions (stars) [areas that separate the air capillaries (AC) while connecting the blood capillaries (BC)], and also in the areas where BCs lie next to each other (arrowheads). Scale bar: 8 μm. (Fig. 16) Collagen fibers that are associated with air capillaries. Arrows, collagen fibers in the blood–gas barrier; arrowheads, collagen fibers in sites where blood capillaries lie adjacent to each other; BC, blood capillaries; AC, air capillaries. Scale bar: 5 μm. (Figs 17, 18) Collagen fiber network in the exchange tissue (arrows). The red blood cells which were spared during the digestion process are shown by stars. AC and circled areas, air capillaries; BC, blood capillaries (BC). Scale bars: (Fig. 17) 10 μm, (Fig, 18) 10 μm.
Figs 19–26.
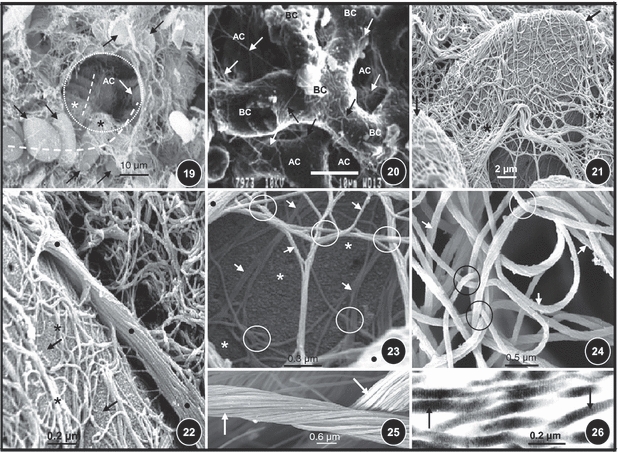
(Fig. 19) Red blood cells (arrows) and rows of red blood cells in blood capillaries (dashed lines) passing across the wall of the an air capillary (AC, circled area). The collagen fibers in the blood-gas barrier are shown by stars. (Fig. 20) Latex cast digested lung tissue showing collagen fibers (arrows) encircling the blood capillaries (BC) as they run in the blood–gas barrier. AC, air capillaries. (Fig. 21) Red blood cells (arrows) that had resisted digestion, covered by a network of collagen fibers (stars) that runs in the basement membrane of the blood–gas barrier. (Fig. 22) Digested lung tissue with a preserved red blood cell (arrows) showing a thick band of collagen fibers (dots) giving rise to thinner branches (asterisks) that cover the red blood cells as they run in the blood–gas barrier. (Fig. 23) Digested lung tissue showing thick (dots) and thin (arrows) collagen fibers that are intricately and tightly intertwined (circled areas). Star, undigested red blood cell. (Fig. 24) Intertwining (circled areas) collagen fibers (arrows) in the exchange tissue of the avian lung. (Fig. 25) Collagen cables (arrows). (Fig. 26) A transmission electron micrograph showing the banding of collagen fibers (arrows).
Latex rubber vascular cast and digested lung preparations showed a network of collagen fibers in the blood–gas barrier in their more natural form and orientation (Fig. 20). Despite lack of the supporting framework which in life is granted by the cellular and other structural components of the parenchyma, in the digested preparations, unmistakable morphologies of the air and blood capillaries together with the associated collagen fiber scaffolding were clearly seen (Figs 15–21). This was particularly conspicuous in the rare cases where the red blood cells had been spared during the digestion treatment of the lung tissues (Figs 17–19, 21–23). In some areas, collagen fibers were tightly entwined with each other (Figs 23 and 24), whereas in others they were arranged in thick bundles (Figs 25 and 26).
Schematically, Figs 27 and 28 show that the parabronchial collagen fibers, elastic tissue, and smooth muscle fibers are compartmentalized and intricately interconnected. Next to the parabronchial lumen, a circumferential band of atrial collagen fibers is coupled to smooth muscle and elastic tissue fibers with the triad forming the internal (axial) parabronchial supporting column. The infundibular collagen fibers interconnect the atrial ones and those of the exchange tissue. The collagen fibers in the interparabronchial septum and those associated with the walls of the interparabronchial blood vessels form the external (peripheral) supporting column: the exchange tissue appears to be effectively ‘suspended’ between two columns (pillars).
Fig. 27.
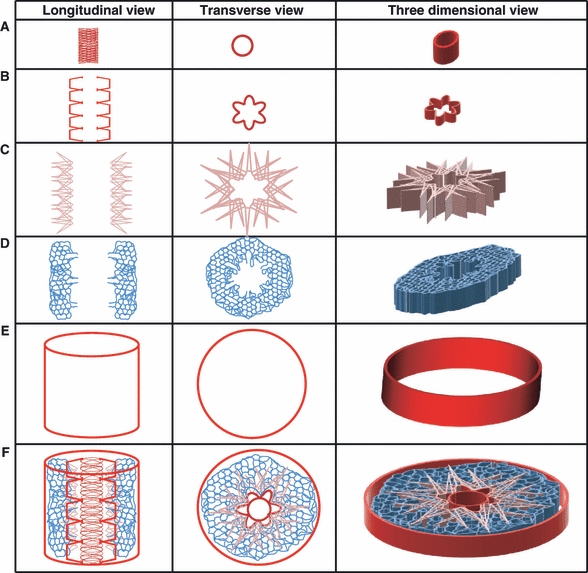
Schematic illustration of the components of the collagen fiber system in the parabronchus of the lung of the domestic fowl shown in longitudinal, transverse, and three-dimensional views. From the inside out, the internal (axial/central) column mainly consists of the collagen fibers that occur in the rims of the atria (A); the interatrial septal collagen band (B) is intercalated between the collagen fibers that form the rims of the atria (A) and those that form the interinfundibular band (C). A diffuse network of collagen fibers runs in the basement membranes of the air and blood capillaries that form the exchange tissue (D). The external (peripheral) pillar (E) is formed both by the collagen fibers in the interparabronchial septum and by those associated with the interparabronchial blood vessels. The complete assemblage is shown in (F). The exchange tissue, i.e. the area containing the air and the blood capillaries, is literally ‘suspended’ between the axial (A) and peripheral (E) pillars by a dense network of collagen fibers.
Fig. 28.
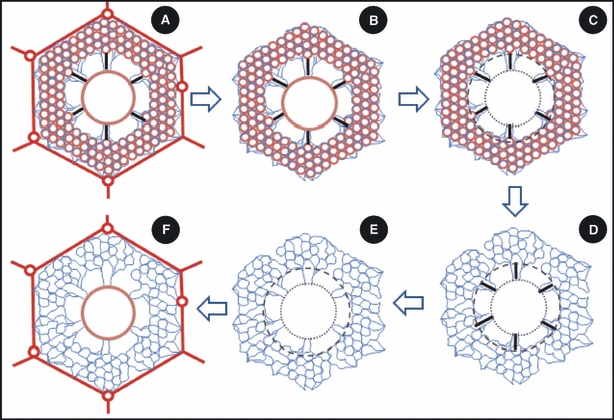
Sequential schematic cross-sectional illustrations of the arrangement of the structural and connective tissue components of the parabronchus of the lung of the domestic fowl. (A) The exchange tissue of the air and the blood capillaries (many open red circles) is located between the peripheral (outer hexagonal red outline) and the axial (central circle) columns (pillars) of collagen fibers. The wavy blue lines that run from the periphery to terminate on the axial pillar are collagen fibers that support the air and blood capillaries. The thick black lines that run outwards to end on the inner border of the exchange tissue show the elastic tissue fibers running from the axial pillar to the inner limit of the exchange tissue. (B) The external pillar has been removed, leaving collagen fibers (blue wavy lines) which support the air- and blood capillaries in the exchange tissue. The elastic tissue fibers (thick black lines) connect the central pillar to the inner limit of the exchange tissue. (C) The central pillar (circle with close dashes) has been removed. The circle with longer dashes shows the inner limit of the exchange tissue. (D) The exchange tissue has been removed leaving the elastic tissue fibers (thick black lines) and the collagen fibers of the exchange tissue. (E) The elastic tissue fibers have been removed, leaving the collagen fibers in the exchange tissue. (F) The external pillar consisting of collagen fibers in the interparabronchial septa (thick lines) and those surrounding the interparabronchial blood vessels (circles in the outline of the interparabronchial septa) and the central pillar (middle circle) literally suspend the collagen fibers that support the exchange tissue (wavy blue lines).
Discussion
Morphologically, the avian respiratory system differs remarkably from the mammalian one. Briefly, the lung is deeply inserted into the ribs and the vertebrae on the dorsal lateral aspect and is ventrally attached to the horizontal septum, a tough membranous connective band that peripherally firmly attaches onto the vertebral ribs (King & McLelland, 1984; McLelland, 1989; Maina, 2005). As much as one-fifth to one-third of its volume is sandwiched between the ribs (King & Molony, 1971). These attachments have rendered the lung practically rigid. During a respiratory cycle, the avian lung changes in volume by a mere 1.4% (Jones et al. 1985). Its rigidity means that surface tension is not a severely limiting factor to the degree of internal subdivision of the gas-exchange tissue (parenchyma). The air capillaries (ACs) range in diameter from 3 to 20 μm (Duncker, 1972; Maina & Nathaniel, 2001; Woodward & Maina, 2008). Contained in the thoracic cavity and surrounded by a pleural space, in the mammalian lung, the smallest alveoli have been reported to measure about 35 μm in diameter (Tenney & Remmers, 1963). Whereas in mammals the compliance of the respiratory system (excepting the thoracic walls) is determined by the terminal parts of the respiratory tree (mainly the alveolar spaces), in avians, compliance has been relegated to the air sacs (Piiper & Scheid, 1989), which act as mechanical ventilators and play no direct role in gas exchange (Magnussen et al. 1976). In the domestic fowl, the maximum compliance of the respiratory system was reported to be 9.6 mL cm–1 H2O (Scheid & Piiper, 1969), in the duck 30 mL cm–1 H2O (Gillespie et al. 1982), and in the anesthetized pigeon, 2.8 mL cm–1 H2O (Kampe & Crawford, 1973).
Although accounts on the remarkable strengths of the ACs and the blood capillaries (BCs) of the avian lung have been in the scientific domain for about three decades (Macklem et al. 1979; Powell et al. 1985), until recently, only cursory references and speculations existed on this interesting property, which has now been sufficiently corroborated by, for example, Wideman (2001), West et al. (2007a), and Watson et al. (2008). The particular structures and/or mechanisms that can explain this property have remained obscure and contentious. Klika et al. (1997), Scheuermann et al. (1997), West et al. (2006, 2007a), and Watson et al. (2007, 2008) ascribed the strength to the presence of what they termed ‘retinacula’, ‘cross-braces’, ‘struts’, ‘plates’, ‘extensions’, and ‘cross-bridges’, pairs of thin parallel epithelial cell processes that separate the ACs while connecting the BCs; Klika et al. (1997) associated it with the lipoproteinaceous trilaminar substance that is found in the cytoplasm of the squamous respiratory cells and also secreted onto the surface of the atria and infundibula. Based on the hexagonal (geodesic) shape of the parabronchi, Klika et al. (1998) observed that ‘the rigid compartmentalization of the parabronchial respiratory units constitutes the skeletal support of the delicate exchange mantle’. They also considered the rigid atrial part of the parabronchial units to form the ‘skeletal support of the delicate gas exchange tissue’. West et al. (2006) and Watson et al. (2007) related the strength to the ‘close-packing’ (what they termed ‘honeycomb’ arrangement) of the ACs and the BCs, whereas, based on the topographical locations and arrangement of the structural components and their known physical and mechanical properties and behavior, Maina (2007a,b); made a case for the existence of a tensegrity system in the avian lung.
Collagen and elastic tissue fibers, proteoglycans, and other glycoproteins are the main structural macromolecules of the connective tissue elements of mammalian lung (Hance & Crystal, 1975; Hopkins et al. 1986; Crouch et al. 1997; Tochima et al. 2004). More recent studies report that collagen fibers fail at strains between 6 and 22% (Liao & Belkoff, 1999). A collagen fiber of a diameter of 1 mm can support a 0.5-kg weight before breaking (Elden, 1968). In contrast, elastic tissue fibers have much lower tensile strength but high extensibility: they can stretch by as much as ∼150% of their original (relaxed) length before they break (Gosline, 1976; Gosline & French, 1979; Robins, 1988; Alter, 2004). Elastic tissue fibers produce what has been termed ‘reverse elasticity’, i.e. the capacity of a stretched material to return to its resting (former) state when released, whereas collagen grants the rigid constraint that checks inordinate deformations of the elastic fibers. Collagen is thus directly responsible for properties such as tensile strength and relative inextensibility of the constitutive parts. With collagen forming 6–20% of the matrix proteins of the dry lung weight (Bradley et al. 1980), together with elastin, the two kinds of connective tissue fibers form a framework that supports and maintains the normal tissue architecture required for efficient gas exchange, while preserving airway, alveolar, and vascular elasticity and tensile strength that ensures normal mechanical behavior (Mead, 1961; Hance & Crystal, 1975; Hopkins et al. 1986; Gadek et al. 1984; Crouch et al. 1997; Willet et al. 1999; Thibeault et al. 2000; Cavalcante et al. 2005). Arising from the need to exploit and integrate their distinctly different mechanical properties, in animal tissues, elastic tissue fibers are almost constantly found in close topographical relationship to the collagenous fibers (Elden, 1968; Gosline, 1976; Gosline & French, 1979; Alter, 2004). In the compliant mammalian lung, elastic tissue occurs abundantly and diffusely (Gonçalves et al. 1995; Tochima et al. 2004). Carton et al. (1964) observed that more elastic tissue was present in the lungs of two mammalian species than was theoretically expected. In the human lung, collagen and elastic tissue fibers occur in a higher ratio than in the visceral organs (2.5 : 1 vs. 10 : 1, respectively) (Weibel, 1984). According to Gosline (1976), Gosline & French (1979) and Robins (1988), elastic tissue fibers perform various functions including dissipation of stress that originates at various points, promoting coordination of rhythmic motions of the constitutive parts. Within physiological limits, at all lung volumes, collagen limits lung expansion while elastin brings about its recoil (Mead, 1961; Senior et al. 1975).
The alveoli of the mammalian lung have a dedicated collagen cable that tracks the ‘supporting side’ of interalveolar septum (Weibel, 1973, 1984), forming a connective tissue scaffold that constitutes part of the ‘fibroskeleton’ of the lung (Wang & Ying, 1977; Gil & Martinez-Hernandez, 1984; Matsuda et al. 1987; Amenta et al. 1988; Mercer & Crapo, 1990 1992; Mercer et al. 1994; Ohtani & Nakatani, 1994; Gonçalves et al. 1995; Maina, 2002; Tochima et al. 2004). The corresponding structures of the avian lung have not been investigated in detail. Ogawa (1920) reported that reticular fibers exist in the exchange tissue of the avian, whereas elastic tissue fibers were seen to be very scarce or totally lacking (Fischer, 1905;Ogawa, 1920;Groebbels, 1922;King, 1966;King et al. 1967;King & McLelland, 1984). King & Cowie (1969), Drescher & Welsch (1985), and Klika et al. (1998) investigated the arrangement of the smooth muscle fibers, particularly in the parabronchus. King (1966) observed that elastic tissue was lacking or very scarce in the gas-exchange tissue of the avian lung and concluded that the lung is less elastic than the mammalian one. King & Cowie (1969), King & Molony (1971), Duncker (1972), and McLelland (1989) remarked that whereas movements occur in the atrial muscular rings and the atrial septa, the gas-exchange tissue is a relatively immobile part of the parabronchus. In this study, it was observed that in a parabronchus, the exchange tissue of the ACs and the BCs is literally ‘suspended’ between internal and external columns of collagen fibers (Figs 27 and 28): the stresses and strains are dynamically regulated by contraction of the smooth muscle fibers and transmission to and storage of energy/forces in the elastic tissue fibers. The ACs and the BCs appear to be buffered from extreme tensions that arise from the luminal and peripheral aspects of the parabronchus. Contraction of the smooth muscle fibers on the luminal aspect is counterbalanced partly by the resistance offered by the peripherally located inextensible column of collagen fibers and by the surface tension force in the ACs. Theoretically, without the surface tension force being countered by the tone of the atrial smooth muscles aided by the elastic tissue fiber recoil (Fig. 29), the ACs would collapse by pulling the atrial-infundibular structural column outwards, i.e. in the direction of the interparabronchial septum. This peripheral force should, however, normally be lessened by the presence of the surfactant on the respiratory surface of the ACs (Bernhard et al. 2001). King et al. (1967) noted that the interatrial septa had abundant elastic tissue fibers which run to the atrial floor, abruptly terminating in the infundibula. Free afferent nerve endings which signaled in response to the movements of the atria were reported by King & Cowie (1969) in the parabronchus of the domestic fowl, particularly in the atrial muscles: they also observed spontaneous atrial muscular rhythmicity in parabronchial tissue preparations. In this study, it was noted that in the atrial septa, direct connection between the surface membrane of the smooth muscle fibers and the elastic tissue fibers occurred. From their topographical relationships, the three structural components, i.e. the smooth muscle, elastic, and collagen fibers appear to function as an integrated unit. The elastic tissue fibers may act as energy-storing elements in opposition to the intrinsic tone of the smooth muscle fibers, and the collagen fibers may limit the stretchability of the contractile components. McLelland (1989) described the orientation of the atrial septa as that of a ‘shallow-pitched helix, and King & Cowie (1969), Gerisch & Schwartz (1972), and West et al. (1977) observed that the larger atrial muscles form an angle of 60–70°, whereas the smaller ones form an angle of ∼45° to the long axis of the parabronchus; the strut-like oriented atrial muscles may provide linear stiffening and efficient dissipation of compressional and tensional forces. The presence of smooth muscle and elastic tissue fibers in the atrial region and their absence peripherally suggests that the luminal aspect of the parabronchus is the more movable one. King & Cowie (1969) showed that samples of bronchial rings contracted on exposure to cholinergic drugs and relaxed after immersion in the adrenergic ones; in addition, vagal stimulation constricted the atria. Compared to the alveoli of the mammalian lung (Gehr et al. 1981), the ACs have relatively thinner blood–gas barriers (Maina, 1989; Maina et al. 1989) and the basement membrane lacks or has a paucity of collagen fibers, the principal supporting connective tissue element (Maina & King, 1982; Watson et al. 2007). Because of this, the ACs are deceptively delicate.
Fig. 29.
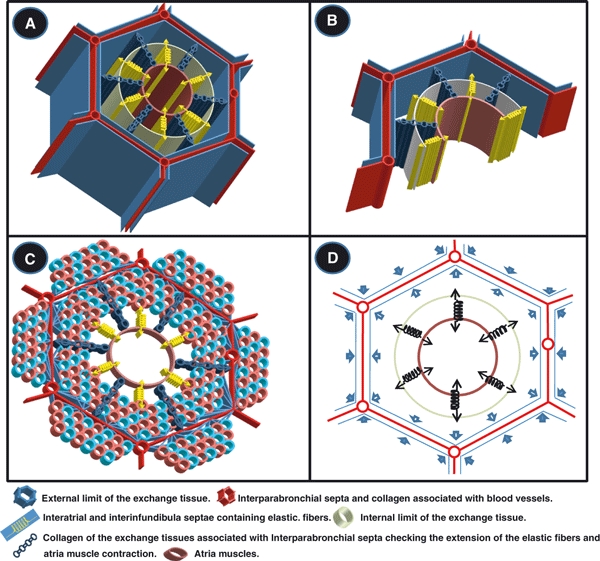
Three-dimensional schematic diagrams showing some of the structural and connective tissue components of the parabronchus of the lung of the domestic fowl and the foremost forces that act within and around the parabronchus. (A–C) Collagen fibers in the interparabronchial septa (thick red lines) and those surrounding the interprarbronchial blood vessels (tubular structures connecting the red lines) delimit the parabronchus forming the peripheral pillar. (C) The many cyan and red circles show the exchange tissue in the parabronchus and in adjacent ones: the exchange tissue of the air and the blood capillaries. (A,B) Outer limit of the exchange tissue is shown by the blue hexagonal boundary, and the internal one by the cream cylindrical sketch. (C) Blue lines between the exchange tissue and the interparabronchial septum (IPS) are the collagen fibers the run from the IPS and the interparabronchial blood vessels. The parabronchial lumen (brown circle) is bordered by the atria muscles. Contraction of the atrial smooth muscles exerts a pulling force directed towards the center of the parabronchial lumen (the arrows of the springs projecting into the parabronchial lumen). The pull is transmitted to the elastic fibers (shown by the springs) in the interatrial and the interinfundibula septae. The elastic fibers stretch along the direction of the pull. The collagen fibers (chains), which are not elastic, regulate the extension of the elastic fibers and the atrial muscles. The potential energy stored in the elastic fibers after the contraction of the smooth muscle fibers is converted to kinetic energy when the muscle fibers relax, causing recoil (arrows of the springs pointing towards exchange tissue). (D) The elastic tissue fibers (springs) shown in the interatrial and interinfundibular septa balance the inward (centripetal) force produced by the contraction of the atrial smooth muscle (arrows of the springs pointing towards and projecting into the parabronchial lumen). The outward (centrifugal) pull (arrows of the springs pointing towards exchange tissue) is produced by the surface tension force that is generated in the air capillaries. This surface tension force is counterbalanced by the elastic recoil of the elastic tissue fibers and the nonflexible collagen fibers (not shown). The surface tension forces in one parabronchus (thick open arrows) meet those of the adjacent parabronchi (thick closed arrows) at the interparabronchial septa and at the apices of the hexagonal interparabronchial septa. In the later, three forces converge. The convergences of the forces make the peripheral part of the parabronchus particularly rigid.
On account of the fact that the indisputable strengths of the ACs and the BCs cannot be sufficiently explained by their structural features, although direct measurements of forces are lacking mainly due to the challenging technical problems of determining them, it was considered plausible by Maina (2007a,b); that ‘mechanisms’ and/or ‘processes’ may instruct the strengths of the avian lung and its structural components. The conclusion was based on the location of the lung (firmly attached to the ribs and vertebrae) and the presence, the abundance, the layout, and the known physical properties and mechanical behaviors of the lung and its structural elements. Here, focusing on the parabronchus, similar conclusions were reached based on its architecture and that of the placements of the collagen, elastic tissue, and smooth muscle fibers (Figs 27 and 28). Neurogenically controlled (King & Cowie, 1969), contraction of the smooth muscle fibers tenses the elastic tissue fibers, which store energy that is converted to kinetic energy on relaxation; the inflexible collagen fibers determine the extent to which the smooth muscle fibers can contract and to which the elastic tissue fibers can extend. The counterbalance of the forces between the structures that produce them (e.g. the smooth muscles), those that resist it, e.g. the central and peripheral collagenous pillars, and those that may exert outward force, e.g. the interparabronchial arteries (from the prevailing intramural pressure), suggests that the parabronchus exists in a dynamically tensed state (Fig. 29). The morphology of the parabronchus and its constitutive parts fit every definition of a tensegrity structure, a tensegret (see Ingber, 2003 for a past list of such biological structures). Tensegrets are stabilized by balancing of opposing forces of tension and compression (Fuller, 1961). The processes of push and pull that are often assumed to be simple opposites do not in reality oppose each other but rather complement each other to impart stability/strength (Wilken, 2001). In the lungs of those species of birds that lack interparabronchial septa, such as the nongalliform ones (Duncker, 1971; Maina et al. 1982; McLelland, 1989), the tensegrity system may to a greater extent include features like the solidity of the lung, robust structures such as the intrapulmonary primary bronchus, which possesses cartilaginous support, and the thick-walled secondary bronchi and large blood vessels. Phylogenetically, all the contemporary avian taxa including the flightless the ones such as the penguins, the rhea, the kiwi, the ostrich, the cassowaries, the emu, and the domestic fowl arose from flying progenitors (Welty, 1979). The chicken was domesticated from the still extant wild jungle fowl, G. gallus of the South East Asia some 8000 years ago (West & Zhou, 1988). With some largely minor and inconsequential differences (e.g. variations in the sizes and locations of the air sacs, presence and lack interparabronchial septa, and development of the paleopulmonic and the neopulmonic parabronchi), the morphology and function of the avian lung have been highly conserved (King, 1966; Duncker, 1971; Scheid, 1979; McLelland, 1989; Maina, 2005). Regarding the strengths of the ACs and the BCs, the subject of this study, the mechanisms and principles developed here should reasonably apply to all species of birds.
Acknowledgments
We are grateful to Dr. Chris Murphy, Faculty of Medicine, University of Sydney, in whose laboratory some of the work was done. We wish to thank Mrs. P. Sharp, Mrs. C. Lalkhan, and Mr. A. Seema for excellent technical assistance. We are also grateful to Mr. P. N. Selahle of the University of the Witwatersrand's Central Animal Services (CAS) for logistical assistance with the procurement of the animals. This work was funded by the National Research Foundation (NRF).
References
- Alter MJ. Science of Flexibility. 3rd edn. New York: Sheridan Books; 2004. [Google Scholar]
- Amenta PS, Gil J, Martinez-Hernandez A. Connective tissue of rat lung. II. Ultrastructural localization of collagen types III, IV, and VI. J Histochem Cytochem. 1988;36:1167–1173. doi: 10.1177/36.9.3403967. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Steven A. Theory and Practice of Histological Techniques. London: Churchill Livingstone; 1996. [Google Scholar]
- Bernhard W, Gebert A, Vieten G, et al. Pulmonary surfactant in birds: coping with surface tension in a tubular lung. J Appl Physiol. 2001;281:R327–R337. doi: 10.1152/ajpregu.2001.281.1.R327. [DOI] [PubMed] [Google Scholar]
- Borst HG, McGregor M, Whittenberger JL, et al. Influence of pulmonary arterial and left atrial pressures on pulmonary vascular resistance. Circ Res. 1956;4:393–399. doi: 10.1161/01.res.4.4.393. [DOI] [PubMed] [Google Scholar]
- Bradley KH, Kawanami O, Ferrans VJ, et al. The fibroblast of human alveolar structures: a differentiated cell with a major role in lung structure and function. In: Harris CC, Trump BF, Stoner GD, editors. Methods in Cell Biology, vol. 2. New York: Academic Press; 1980. pp. 37–64. [DOI] [PubMed] [Google Scholar]
- Carton RW, Clark JW, Dainauskas J, et al. Estimation of tissue elasticity of the lung. J Appl Physiol. 1964;19:236–242. doi: 10.1152/jappl.1964.19.2.236. [DOI] [PubMed] [Google Scholar]
- Cavalcante FSA, Ito S, Brewer K, et al. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol. 2005;98:672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- Chen CS, Ingber DE. Tensegrity mechanoregulation: from skeleton to cytoskeleton. Osteoarthritis Cartilage. 1999;7:81–94. doi: 10.1053/joca.1998.0164. [DOI] [PubMed] [Google Scholar]
- Crouch EC, Martin GR, Brody JS, et al. Basement membranes. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung: Scientific Foundations, vol. 1. 2nd edn. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 769–791. [Google Scholar]
- Drescher HE, Welsch U. The fine structure of the parabronchi and the gas exchange area of the Adelie penguin lung. Z Mikrosk-Anat Forsch Leipzig. 1985;97:863–879. [PubMed] [Google Scholar]
- Duncker HR. The lung-air sac system of birds. A contribution to the functional anatomy of the respiratory apparatus. Ergeb Anat Entwicklung. 1971;45:1–171. [PubMed] [Google Scholar]
- Duncker HR. Structure of avian lungs. Respir Physiol. 1972;14:44–63. doi: 10.1016/0034-5687(72)90016-3. [DOI] [PubMed] [Google Scholar]
- Elden HR. Physical properties of collagen fibers. Int Rev Connect Tissue Res. 1968;4:283–348. doi: 10.1016/b978-1-4831-6754-1.50013-3. [DOI] [PubMed] [Google Scholar]
- Fischer G. Vergleichende anatomische Untersuchungen über den Bronchialbaum der Vögel. Zool Stuttg. 1905;19:1–46. [Google Scholar]
- Frantsevich L, Gorb S. Arcus as a tensegrity structure in the arlium of wasps (Hymenoptera: Vespidae) Zool. 2002;3:81–94. doi: 10.1078/0944-2006-00067. [DOI] [PubMed] [Google Scholar]
- Fuller B. Tensegrity. Portf Art News Annu. 1961;4:112–127. [Google Scholar]
- Gadek JE, Fells GA, Zimmerman RL, et al. Role of connective tissue proteases in the pathogenesis of chronic inflammatory lung disease. Environ Health Perspect. 1984;55:297–306. doi: 10.1289/ehp.8455297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehr P, Mwangi DK, Amman A, et al. Design of the mammalian respiratory system: V. Scaling morphometric diffusing capacity to body mass: wild and domestic animals. Respir Physiol. 1981;44:61–86. doi: 10.1016/0034-5687(81)90077-3. [DOI] [PubMed] [Google Scholar]
- Gerisch D, Schwartz R. Morphologische Befunde an den Bronchi atriales des Haushuhnes (Gallus gallus domesticus) Dtsch Tierärztl Wochenschr. 1972;79:573–612. [PubMed] [Google Scholar]
- Gil J, Martinez-Hernandez A. The connective tissue of the rat lung: electron immunohistochemical studies. J Histochem Cytochem. 1984;32:230–238. doi: 10.1177/32.2.6363520. [DOI] [PubMed] [Google Scholar]
- Gillespie JR, Sagot JC, Gendner JP, et al. Impedance of the lower respiratory system in ducks under four conditions: pressure breathing, anaesthesia, paralysis or breathing CO2-enriched gas. Respir Physiol. 1982;47:177–191. doi: 10.1016/0034-5687(82)90092-5. [DOI] [PubMed] [Google Scholar]
- Glazier JB, Hughes JMB, Maloney JE, et al. Measurements of capillary dimensions and blood volume in rapidly frozen lungs. J Appl Physiol. 1969;26:65–76. doi: 10.1152/jappl.1969.26.1.65. [DOI] [PubMed] [Google Scholar]
- Gonçalves CA, Figueiredo MH, Bairos VA. Three dimensional organization of the elastic fibers in the rat lung. Anat Rec. 1995;243:63–70. doi: 10.1002/ar.1092430108. [DOI] [PubMed] [Google Scholar]
- Gordon JE. The Science of Structures and Materials. New York: Scientific American Library; 1988. [Google Scholar]
- Gosline JM. The physical properties of elastic tissue. Int Rev Connect Tissue Res. 1976;7:211–249. doi: 10.1016/b978-0-12-363707-9.50011-3. [DOI] [PubMed] [Google Scholar]
- Gosline JM, French CJ. Dynamic mechanical properties of elastin. Biopolymers. 1979;18:2091–2103. doi: 10.1002/bip.1979.360180818. [DOI] [PubMed] [Google Scholar]
- Groebbels F. Der Vögel. Bau, Function, Lebenserscheinung, Einpassung, vol. 1. Berlin: Borntraeger; 1922. [Google Scholar]
- Hance AJ, Crystal RG. The connective tissue of lung. Am Rev Respir Dis. 1975;112:657–711. doi: 10.1164/arrd.1975.112.5.657. [DOI] [PubMed] [Google Scholar]
- Hopkins FG, Stothert JC, Greaves IA, et al. Lung recoil: elastic and rheological properties. In: Fishman AP, editor. Handbook of Physiology: The Respiratory System. Bethesda: American Physiological Society; 1986. pp. 195–215. [Google Scholar]
- Ingber DE. Cellular tensegrity: revisited. I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Ingber DE. The mechanochemical basis of cell and tissue regulation. MCB. 2004;1:53–68. [PubMed] [Google Scholar]
- Jones JH, Effmann EL, Schmidt-Nielsen K. Lung volume changes during respiration in ducks. Respir Physiol. 1985;59:15–25. doi: 10.1016/0034-5687(85)90014-3. [DOI] [PubMed] [Google Scholar]
- Kampe G, Crawford EC. Oscillatory mechanics of the respiratory system of pigeons. Respir Physiol. 1973;18:188–193. doi: 10.1016/0034-5687(73)90049-2. [DOI] [PubMed] [Google Scholar]
- King AS. Structural and functional aspects of the avian lung and its air sacs. Int Rev Gen Exp Zool. 1966;2:171–267. [Google Scholar]
- King AS. Systema respiratorium. In: Baumel JJ, King AS, Lucas AM, Breazile JE, Evans HE, editors. Nomina Anatomica Avium. London: Academic Press; 1979. pp. 227–265. [Google Scholar]
- King AS, Cowie AF. The functional anatomy of the bronchial muscle of the bird. J Anat. 1969;105:323–336. [PMC free article] [PubMed] [Google Scholar]
- King AS, McLelland J. Birds: Their Structure and Function. London: Baillière Tindall; 1984. [Google Scholar]
- King AS, Molony V. The anatomy of respiration. In: Bell DF, Freeman BM, editors. Physiology and Biochemistry of the Domestic Fowl, vol. 1. London: Academic Press; 1971. pp. 347–384. [Google Scholar]
- King AS, Ellis RNW, Watts SMS. Elastic fibers in the avian lung. J Anat. 1967;101:607. [Google Scholar]
- Klika E, Scheuermann DW, De Groodt-Lasseel MHA, et al. Anchoring and support system of pulmonary gas exchange tissue in four bird species. Acta Anat. 1997;159:30–41. doi: 10.1159/000147962. [DOI] [PubMed] [Google Scholar]
- Klika E, Scheuermann DW, De Groodt-Lasseel MHA, et al. An SEM and TEM study of the transition of the bronchus to the parabronchus in quail (Coturnix cortunix) Ann Anat. 1998;180:289–297. doi: 10.1016/S0940-9602(98)80027-7. [DOI] [PubMed] [Google Scholar]
- Liao H, Belkoff SM. A failure model for ligaments. J Biomech. 1999;32:183–188. doi: 10.1016/s0021-9290(98)00169-9. [DOI] [PubMed] [Google Scholar]
- Macklem P, Bouverot P, Scheid P. Measurement of the distensibility of the parabronchi in duck lungs. Respir Physiol. 1979;33:23–35. doi: 10.1016/0034-5687(79)90004-5. [DOI] [PubMed] [Google Scholar]
- Magnussen H, Willmer H, Scheid P. Gas exchange in the air sacs: contribution to respiratory gas exchange in ducks. Respir Physiol. 1976;26:129–146. doi: 10.1016/0034-5687(76)90057-8. [DOI] [PubMed] [Google Scholar]
- Maina JN. Morphometry of the avian lung. In: King AS, McLelland J, editors. Form and Function in Birds, vol. 4. London: Academic Press; 1989. pp. 307–368. [Google Scholar]
- Maina JN. Functional Morphology of the Vertebrate Respiratory Organs. Lebanon, NH: Oxford and IBH Publishing Company; 2002. [Google Scholar]
- Maina JN. The Lung-Air Sac System of Birds: Development, Structure, and Function. Heidelberg: Springer-Verlag; 2005. [Google Scholar]
- Maina JN. Spectacularly robust! Tensegrity principle explains the mechanical strength of the avian lung. Respir Physiol Neurobiol. 2007a;155:1–10. doi: 10.1016/j.resp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Maina JN. Minutialization at its extreme best! The underpinnings of the remarkable strengths of the air- and the blood capillaries of the avian lung: a conundrum. Respir Physiol Neurobiol. 2007b;159:141–145. doi: 10.1016/j.resp.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Maina JN. Structure of the air- and blood capillaries of the avian lung and the debate regarding the basis of their astounding strengths. In: Morris S, Vosloo A, editors. Proceedings of the 4th Comparative Physiology and Biochemistry Conference in Africa. Bologna: Medimond Srl; 2008. pp. 304–313. [Google Scholar]
- Maina JN, King AS. The thickness of the avian blood-gas barrier: qualitative and quantitative observations. J Anat. 1982;134:553–562. [PMC free article] [PubMed] [Google Scholar]
- Maina JN, Nathaniel C. A qualitative and quantitative study of the lung of an ostrich, Struthio camelus. J Exp Biol. 2001;204:2313–2330. doi: 10.1242/jeb.204.13.2313. [DOI] [PubMed] [Google Scholar]
- Maina JN, Abdalla MA, King AS. Light microscopic morphometry of the lungs of 19 avian species. Acta Anat. 1982;112:264–270. doi: 10.1159/000145519. [DOI] [PubMed] [Google Scholar]
- Maina JN, King AS, Settle G. An allometric study of the pulmonary morphometric parameters in birds, with mammalian comparison. Philos Trans R Soc Lond B Biol Sci. 1989;326B:1–57. doi: 10.1098/rstb.1989.0104. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Fung YC, Sobin SS. Collagen and elastin fibers in human pulmonary alveolar mouths and ducts. J Appl Physiol. 1987;63:1185–1194. doi: 10.1152/jappl.1987.63.3.1185. [DOI] [PubMed] [Google Scholar]
- McLelland J. Anatomy of the lungs and air sacs. In: King AS, McLelland J, editors. Form and Function in Birds, vol. 4. London: Academic Press; 1989. pp. 221–279. [Google Scholar]
- Mead J. Mechanical properties of lungs. Physiol Rev. 1961;41:281–328. doi: 10.1152/physrev.1961.41.2.281. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Crapo JD. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol. 1990;69:756–765. doi: 10.1152/jappl.1990.69.2.756. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Crapo JD. Structural changes in elastic fibers after pancreatic elastase administration in hamsters. J Appl Physiol. 1992;72:1473–1479. doi: 10.1152/jappl.1992.72.4.1473. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Russell ML, Crapo JD. Alveolar septal structure in different species. J Appl Physiol. 1994;77:1060–1066. doi: 10.1152/jappl.1994.77.3.1060. [DOI] [PubMed] [Google Scholar]
- Ogawa C. Contributions to the histology of the respiratory spaces of the vertebrate lungs. Am J Anat. 1920;27:333–393. [Google Scholar]
- Ohtani O. Three-dimensional organization of the connective tissue fibers of the human pancreas. A scanning electron microscopic study of NaOH treated tissues. Arch Histol Jpn. 1987;50:557–566. doi: 10.1679/aohc.50.557. [DOI] [PubMed] [Google Scholar]
- Ohtani O. The maceration technique in scanning electron microscopy of collagen fiber frameworks. It's application in the study of human livers. Arch Histol Cytol. 1992;55:225–232. doi: 10.1679/aohc.55.suppl_225. [DOI] [PubMed] [Google Scholar]
- Ohtani O, Nakatani T. Spatial organization of the collagen and elastin fibers of the lung in the Japanese monkeys, Macaca fuscata. Anthropol Sci. 1994;102:181–187. [Google Scholar]
- Piiper J, Scheid P. Respiratory mechanics and air flow in birds. In: King AS, McLelland J, editors. Form and Function in Birds, vol. 4. London: Academic Press; 1989. pp. 369–391. [Google Scholar]
- Powell FL, Hastings RH, Mazzone RW. Pulmonary vascular resistance during unilateral pulmonary arterial occlusion in ducks. Am J Physiol. 1985;249:R39–R43. doi: 10.1152/ajpregu.1985.249.1.R39. [DOI] [PubMed] [Google Scholar]
- Robins SP. Functional properties of collagen and elastin. Baillieres Clin Rheumatol. 1988;2:1–36. doi: 10.1016/s0950-3579(88)80003-7. [DOI] [PubMed] [Google Scholar]
- Scheid P. Mechanisms of gas exchange in bird lungs. Rev Physiol Biochem Pharmacol. 1979;86:137–186. doi: 10.1007/BFb0031533. [DOI] [PubMed] [Google Scholar]
- Scheid P, Piiper J. Volume ventilation and compliance of the respiratory system in the domestic fowl. Respir Physiol. 1969;6:298–308. doi: 10.1016/0034-5687(69)90029-2. [DOI] [PubMed] [Google Scholar]
- Scheuermann DW, Klika E, De Groodt-Lasseel MHA, et al. An electron microscopic study of the parabronchi epithelium in the mature lung of four bird species. Anat Rec. 1997;249:213–225. doi: 10.1002/(SICI)1097-0185(199710)249:2<213::AID-AR8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Senior RM, Bielefeld DR, Abensohn MK. The effects of proteolytic enzymes on the tensile strength of human lung. Am Rev Respir Dis. 1975;111:184–188. doi: 10.1164/arrd.1975.111.2.184. [DOI] [PubMed] [Google Scholar]
- Tenney SM, Remmers JE. Comparative quantitative morphology of the mammalian lung: diffusing area. Nature. 1963;197:54–56. doi: 10.1038/197054a0. [DOI] [PubMed] [Google Scholar]
- Thibeault DW, Mabry SH, Ekekezie II, et al. Lung elastic tissue maturation and during the evolution of chronic lung disease. Pediatrics. 2000;106:1452–1459. doi: 10.1542/peds.106.6.1452. [DOI] [PubMed] [Google Scholar]
- Tochima M, Ohtani Y, Ohtani O. Three-dimensional architecture of elastin and collagen fiber networks in the human and rat lung. Arch Histol Jpn. 2004;67:31–40. doi: 10.1679/aohc.67.31. [DOI] [PubMed] [Google Scholar]
- Wang NS, Ying WL. A scanning electron microscopic study of alkali-digested human and rabbit alveoli. Am Rev Respir Dis. 1977;115:165–173. doi: 10.1164/arrd.1977.115.3.449. [DOI] [PubMed] [Google Scholar]
- Wang N, Naruse K, Stamenovic D, et al. Mechanical behaviour in living cells consistent with the tensegrity model. Proc Natl Acad Sci U S A. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RR, Fu Z, West JB. Morphometry of the extremely thin pulmonary blood–gas barrier in the chicken lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L769–L777. doi: 10.1152/ajplung.00355.2006. [DOI] [PubMed] [Google Scholar]
- Watson RR, Fu Z, West JB. Minimal distensibility of pulmonary capillaries in avian lungs compared with mammalian lungs. Respir Physiol Neurobiol. 2008;160:208–214. doi: 10.1016/j.resp.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER. Morphological basis of the alveolar-capillary gas exchange. Physiol Rev. 1973;53:419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- Weibel ER. The Pathways for Oxygen: Structure and Function in the Mammalian Respiratory System. Harvard: Harvard University Press; 1984. [Google Scholar]
- Welty JC. The Life of Birds. 2nd edn. Philadelphia: Saunders; 1979. [Google Scholar]
- West JB. Comparative physiology of the pulmonary blood-gas barrier: the unique avian solution. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1625–R1634. doi: 10.1152/ajpregu.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West B, Zhou BW. Did chickens go North? New evidence for domestication. J Archaeol Sci. 1988;15:515–533. [Google Scholar]
- West NH, Bamford OS, Jones DR. A scanning electron microscope study of the microvasculature of the avian lung. Cell Tissue Res. 1977;176:553–564. doi: 10.1007/BF00231407. [DOI] [PubMed] [Google Scholar]
- West JB, Watson RR, Fu Z. The honeycomb-like structure of the bird lung allows a uniquely thin blood-gas barrier. Respir Physiol Neurobiol. 2006;152:115–118. doi: 10.1016/j.resp.2005.12.009. [DOI] [PubMed] [Google Scholar]
- West JB, Watson RR, Fu Z. Major differences in the pulmonary circulation between birds and mammals. Respir Physiol Neurobiol. 2007a;157:382–390. doi: 10.1016/j.resp.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB, Watson RR, Fu Z. The human lung: did evolution get it wrong? Eur Respir J. 2007b;29:11–17. doi: 10.1183/09031936.00133306. [DOI] [PubMed] [Google Scholar]
- West JB, Fu Z, Deerinck TJ, et al. Structure-function studies of blood- and air capillaries in chicken lung using 3-D electron microscopy. Respir Physiol Neurobiol. 2010;170:202–209. doi: 10.1016/j.resp.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman RF. Pathophysiology of heart/lung disorders: pulmonary hypertension syndrome in broiler chickens. World's Poult Sci J. 2001;57:289–307. [Google Scholar]
- Wideman RF, Chapman ME, Hamal KR, et al. An inadequate pulmonary vascular capacity and susceptibility to pulmonary arterial hypertension in broilers. Poult Sci. 2007;86:984–998. doi: 10.1093/ps/86.5.984. [DOI] [PubMed] [Google Scholar]
- Wilken T. A gift tensegrity. 2001. http://www.synearth.net/Restricted-Confidential/Gift/Gift_Tensegrity.html.
- Willet KE, McMenamin P, Pinkerton KE, et al. Lung morphometry and collagen and elastic content: changes during normal development and after prenatal hormone exposure in sheep. Pediatr Res. 1999;45:615–625. doi: 10.1203/00006450-199905010-00002. [DOI] [PubMed] [Google Scholar]
- Woodward JD, Maina JN. Study of the structure of the air- and blood capillaries of the gas exchange tissue of the avian lung by serial section three-dimensional reconstruction. J Microsc. 2008;230:84–93. doi: 10.1111/j.1365-2818.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Yamada T, Richiert D, Tumminia SJ, et al. The tensegrity model applied to the lens: a hypothesis for the presence of the fiber cell ball sockets. Med Hypotheses. 2000;55:36–39. doi: 10.1054/mehy.1999.0994. [DOI] [PubMed] [Google Scholar]
- Zannoti G, Guerra C. Is tensegrity a unifying concept of protein folds. FEBS Lett. 2003;534:7–10. doi: 10.1016/s0014-5793(02)03853-x. [DOI] [PubMed] [Google Scholar]


