Abstract
In spite of numerous investigations it has not been precisely determined whether lymphatic vessels are present in the dental pulp of dogs. Therefore, this study attempted a specific immunohistochemical detection of lymphatic endothelium. The canine teeth of 19 healthy beagle dogs were dissected into three segments (apical, intermediate and occlusal). After decalcification, specimens were embedded in paraffin wax and histologic cross-sections were stained immunohistochemically using a reliable antibody (anti-Prox-1) against the homeobox transcription factor Prox-1, which is located within the nucleus of lymphatic endothelium. Anti-Prox-1 reacted positively with canine control tissues (lymph nodes, gingiva, nasal mucosa), but showed no staining in tissue sections of the dental pulp. The dog dental pulp contained no vascular structures lined with lymphatic endothelium. This suggests that drainage of interstitial fluid makes use of other routes, i.e. extravascular pathways.
Keywords: endodontium, interstitial fluid, lymphatic endothelium, Prox-1, teeth
Introduction
Dogs are frequently used to investigate dental and periodontal diseases. They are also used to test dental and periodontal therapies intended to be performed in humans (Yanpiset & Trope, 2000; da Cunha Pereira et al. 2007; Thibodeau et al. 2007). The results from such studies are transferred from dogs to humans with the implicit understanding that dogs and humans share a very similar dental anatomy. However, especially with regard to the lymphatic system of the dental pulp, the existing literature provides inconsistent data. (The data-related literature is summarized in Tables 1 and 2.) Nevertheless, despite numerous studies, no unanimous opinion about either the presence or absence of lymphatic vessels in the dental pulp of dogs has been achieved (see Table 1).
Table 1.
List of investigations that did or did not detect lymphatic vessels in the dental pulp of dogs
| Investigation | Method | Lymphatic vessels |
|---|---|---|
| Carreras (1894) | Dye tracking | No |
| Schweitzer (1907) | Dye tracking, LM | Yes |
| Fish (1927) | Dye tracking, LM | Yes |
| Noyes (1928) | Dye tracking | Yes |
| MacGregor (1936) | Dye tracking, LM | Yes |
| Zander & Smith (1945) | Dye tracking, LM | No |
| Sulzmann (1955) | LM | Yes |
| Isokawa (1960) | Dye tracking, LM | No |
| Brown et al. (1969) | Calculation of tissue fluid | Yes |
| Bernick & Patek (1969) | LM | Yes |
| Ruben et al. (1971) | Dye tracking, LM | Yes |
| Gängler & Mönch (1980) | LM | No |
| Watts & Paterson (1982) | Dye tracking | Probably |
LM, Light microscopy.
Table 2.
List of investigations that did or did not detect lymphatic vessels in the human dental pulp
| Investigation | Method | Lymphatic vessels |
|---|---|---|
| Magnus (1922) | Dye tracking, LM | Yes |
| Eifinger (1970) | LM, EM | Yes |
| Dahl & Majör (1973) | LM, EM | Yes |
| Bernick (1977a) | LM | Yes |
| Bernick (1977b) | LM | Yes |
| Frank et al. (1977) | Dye tracking | Yes |
| Gängler & Mönch (1980) | LM | No |
| Marchetti et al. (1991) | LM, EM | Yes |
| Matsumoto et al. (1997) | Dye tracking, EHC*, LM, EM | Yes |
| Sawa et al. (1998) | IHC†, LM | Yes |
| Marchetti & Poggi (2002) | LM, EM | Yes |
| Matsumoto et al. (2002) | Dye tracking, EHC*, LM, EM | Yes |
| Oehmke et al. (2003) | Dye tracking, LM, EM | Yes |
| Pimenta et al. (2003) | IHC‡, LM | Yes |
| Rodd & Boissonade (2003) | LM | Yes |
| Gerli et al. (2010) | IHC§, EM | No |
LM, light microscopy; EM, electron microscopy; IHC, immunohistochemistry; EHC, enzyme histochemistry.
5′-nucleotidase-alkaline phosphatase double staining.
Anti-human thoracic duct IgM, anti-human laminin antiserum.
Anti-VEGFR-3, anti-α-smooth muscle actin, anti-CD 31.
Anti-VEGFR-3, anti-LYVE-1, anti-D2-40, anti-Prox-1, anti-CD 31, anti-CD 34, anti-vWF.
The variety of the applied methods is plentiful, including light microscopic, electron microscopic and indirect methods such as radioisotope scanning and calculations of tissue fluid pressure (Table 1). Further efforts are needed, as long as the applied methods do not appear specific enough, to reliably detect lymphatic endothelium. However, immunohistochemical markers, which are exclusively present on lymphatic endothelial cells, have recently become available. Such positive markers are less open to the potential artifacts associated with interpreting negative signals when using negative markers, which are known to be absent from lymphatic endothelial cells (Sleeman et al. 2001). The homeobox gene Prox-1 is a transcription factor located in or beneath the nucleus of lymphatic endothelial cells (Wilting et al. 2002). It is the mammalian homolog of the Drosophila melanogaster gene prospero (Sleeman et al. 2001). Prox-1 is expressed by lymphatic endothelial cell nuclei in normal adult and tumorous tissue (Wilting et al. 2002). An antibody (anti-Prox-1) against this transcription factor has been proven to selectively detect lymphatic endothelial cells in different species including dogs (Wilting et al. 2002; Staszyk et al. 2005; Saito et al. 2006). We hypothesize that the Prox-1 antibody will reliably detect lymphatic endothelial cells in the dental pulp of dogs if present.
Materials and methods
Specimens
The specimens were taken from the canine tooth of the right upper jaw of 19 beagle dogs (aged 136–184 days; 10 females, nine males). The animals were killed for other than dental medical reasons at the University of Veterinary Medicine Hannover. Immediately after killing, the heads of 10 dogs were perfused with Bouin's solution and the remaining nine were perfused with 10% formalin via the common carotid arteries. After perfusion, the jaws were cut in a transverse plane caudal to the second pre-molar tooth and were then separated in the median plane with an oscillating saw. This yielded four divisions (left and right, upper and lower jaw). For this study, specimens from the right upper jaw were used. The tip of the canine tooth was cut off to open the dental cavity for a better post-fixation of the dental pulp (24 h immersion fixation in either Bouin's solution or 10% formalin according to the previous perfusion fixation). After post-fixation, the canine tooth and its surrounding tissue (alveolar bone, periodontal ligament, gingiva and nasal mucosa) were subdivided into three segments with a diamond saw (Fig. 1). The so-called apical segment with the second pre-molar as the orientation landmark contained the apical part of the canine tooth, the so-called intermediate segment (orientation landmark: first pre-molar) contained an intermediate part of the canine tooth, and finally the so-called occlusal segment contained the crown of the canine tooth (Fig. 1A).
Fig. 1.
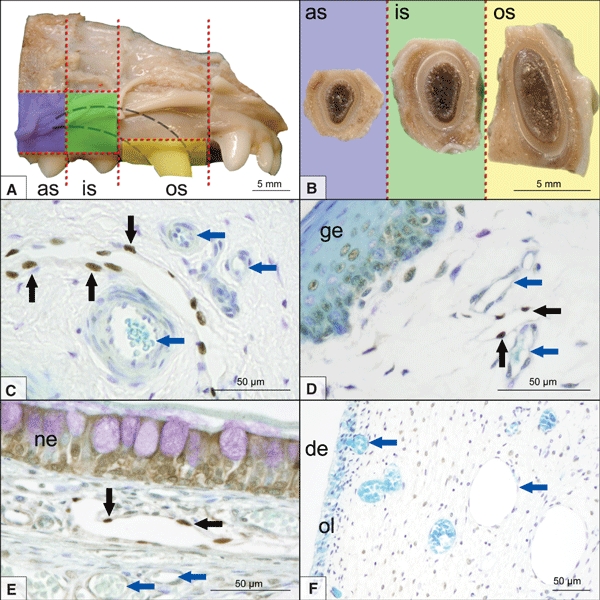
(A) Schematic presentation of the three investigated segments of the right upper jaw of a dog. Blue, apical segment (as); green, intermediate segment (is); yellow, occlusal segment (os). The outline of the canine tooth is indicated by the dashed lines. (B) Sectioned surfaces of the segments. (C) Histomicrograph of Prox-1 [rabbit, anti-human; diaminobenzidine tetrahydrochloride (DAB) immunostaining, toluidine blue counterstain] stained canine lymph node (Ln. mandibularis, hilus region). A lymphatic vessel is lined by positively stained lymphatic endothelial cells (black arrows). Blood vascular endothelium remains unstained (blue arrows). Histomicrographs of Prox-1 (rabbit, anti-human; DAB immunostaining, toluidine blue counterstain) stained sections from the occlusal segment showing positively stained lymphatic endothelial cells (black arrows) in a subgingival position (D) and beneath the nasal epithelium (E). The dental pulp contained an abundant blood vascular system (blue arrows) but never contained Prox-1-positive lymphatic endothelial cells (F). ge, gingival epithelium; ne, nasal epithelium; de, dentin; ol, odontoblast layer.
After complete decalcification (taking 3–4 weeks in 25% EDTA at pH 7.4 and 37 °C), specimens were embedded in paraffin wax (Paraplast Plus, Sherwood Services AG, Schaffhausen, Switzerland), and 3 μm serial sections were prepared. Alternate sections were grouped separately for staining by either Prox-1 immunostaining with toluidine blue counterstain or just toluidine blue. Every 10th section was mounted onto slides (previously covered with 3-aminopropyltriethoxysilane and glutardialdehyde, Merck, Darmstadt, Germany) until 39 sections were available from each of the three tooth segments of each of the 19 dogs (2145 sections in total; the sections of the apical segment of two dogs could not be observed because of artifacts).
Immunohistochemical staining: Prox-1
Sections were dewaxed in xylene and rehydrated in graded ethanol. For antigen retrieval, sections were placed in a glass cuvette containing citric buffer (pH 6.0). The cuvette was heated in a water bath at 100 °C for 20 min. After cooling down to room temperature (approx. 21°C) for 20 min, the sections were rinsed in Tris-buffered saline and placed in pepsin solution (ProTaqs Pepsin Digest, Biocyc, Luckenwalde, Germany) at 37 °C for 10 min. Endogenous peroxidase was quenched by incubation for 10 min with 10% H2O2 in methanol at room temperature. In order to prevent non-specific binding of antibodies, sections were treated with normal goat serum (30 min, room temperature, protein block/normal goat; Biogenex, Hamburg, Germany). Primary antibodies (rabbit IgG, directed against the recombinant human C-terminal part of the homeo domain and prospero domain of Prox-1; ReliaTech, Braunschweig, Germany) were applied at a dilution of 1 : 400 for 16–18 h at 4 °C. Primary antibodies were linked to secondary antibodies (Super Sensitive MultiLink, Biogenex), which were subsequently labeled with streptavidin peroxidase (Super Sensitive Peroxidase Label, Biogenex) and developed in a solution containing 0.05% diaminobenzidine tetrahydrochloride and 0.03% hydrogen peroxide (Liquid DAB Substrate Pack, Biogenex) for 5 min at room temperature. The sections were then counterstained with toluidine blue, mounted and evaluated using a light microscope (Axioskop; Carl Zeiss, Jena, Germany) equipped with a digital imaging system (DP 70, Olympus, Hamburg, Germany).
Sections from canine mandibular lymph nodes served as positive controls to confirm the specificity of staining for anti-human Prox-1 in the canine tissue. Either gingiva or nasal mucosa was present in each of the specimens, these tissues serving as intrasectional positive controls.
The control specimens from the mandibular lymph nodes were either fixed in Bouin's solution or in 10% formalin. All control specimens underwent the immunostaining procedure together with the dental samples. Decalcification with EDTA was performed on some samples and omitted from others in order to determine whether the EDTA influenced the immunostaining of the endothelial cell nuclei.
Negative controls were prepared by substituting the primary antibody with Tris-buffered saline or by incubating the sections with non-immune rabbit IgG (Rabbit Super Sensitive Negative Control, Biogenex).
Results
A dark brown or black nuclear stain was taken as an immunopositive signal; such immunoreaction signals were regularly found in the nasal mucosa and gingiva, as well as in mandibular lymph nodes (i. e. in all positive control tissues) (Fig. 1C–E). These cell nuclei had an oval, flattened shape and projected into the lumen, which was wide open and did not contain any erythrocytes. The outlines of these lymphatic vessels were very thin, as their walls were composed of just endothelial cells. Non-specific, light brown background staining occurred in the basal layers of the gingival epithelium, in epithelial cells of the nasal mucosa and within the collagen fiber component of the dental pulp. Immunostaining results for specimens fixed in Bouin's solution were the same as those for specimens fixed in 10% formalin. Staining results for sections of lymph nodes treated with EDTA were the same as those for sections without EDTA treatment.
The blood vascular endothelial cell nuclei in all tissue samples remained unstained (Fig. 1C–F) and additional cells were sometimes apposed to them to contribute to a two-layer vascular profile, which occasionally contained erythrocytes.
However, no positive nuclear immunoreactions for Prox-1 could be found in any of the 2145 serial sections of the dental pulp from the three segments (apical, intermediate and occlusal) of the canine tooth (Fig. 1F).
Discussion
The antibody anti-Prox-1 has been reported to specifically stain lymphatic endothelial cell nuclei, but not blood vascular endothelial nuclei, in murine, human, equine and canine tissue samples (Mouta Carreira et al. 2001; Staszyk et al. 2005; Saito et al. 2006). The homeobox transcription factor Prox-1 has been identified to play a key role in initiating lymph vascular development and also in maintaining the lymphatic endothelial phenotype (Kim et al. 2010). Therefore, Prox-1 has been regarded as a highly reliable marker for lymph vascular endothelial cells in fetal and adult tissues (Wilting et al. 2002). Accordingly, the absence of Prox-1 suggests the absence of lymph vascular endothelium in the investigated specimens. However, heterogeneity in lymph vascular endothelium, in terms of phenotypic appearance and gene expression profiles, should be considered for further investigations.
In this study, special care was taken to perform all immunoreactions on separate positive-control sections, i.e. specimens of canine lymph nodes, and attention was paid to the intrasectional control tissues, i.e. gingiva and nasal mucosa, to verify the immunostaining. In the controls, positive endothelial cell nuclei of lymphatic capillaries could be clearly distinguished from blood vascular endothelium by means of immunohistochemical and histomorphologic criteria. In none of the sections of the dental pulp (canine tooth), however, were any cell nuclei stained with the antibody anti-Prox-1, even though the number of sections and the chosen topographic heterogeneity were very large (19 dogs, 19 teeth; three segments of each tooth; 39 sections of each segment at 30 μm intervals; 2145 sections in total, the sections of the apical segment of two dogs could not be observed because of artifacts).
With regard to these experimental parameters, the absence of anti-Prox-1 immunoreaction strongly suggests that the dental pulp of the dog's canine tooth is devoid of lymphatic vessels. The dog possesses a heterodont dentition comprising four different functional types of teeth (incisors, canines, pre-molars and molars). The different types of teeth vary in size and shape but follow the same principles of dentinogenesis, share a limited growth characteristic (brachyodont teeth) and show similar histological features of the dental hard substances and dental pulp. Accordingly, it is assumed that the absence of lymphatics in the canine teeth is a general feature of the dog's dentition. In previous studies, different types of brachyodont teeth from one species were used to investigate the pulpal lymph vascular system. Hitherto, no differences with respect to the type of the teeth had been reported (e.g. Bernick & Patek, 1969; Matsumoto et al. 1997; Gerli et al. 2010).
The demonstrated absence of lymphatics in the dental pulp of dogs is in contrast to previous investigations where the existence of lymphatics was documented (see Table 1). However, it agrees with the results of other investigations that denied or questioned the existence of lymphatic vessels in the dental pulp of dogs (see Table 1). It is noteworthy that none of the previous studies in the dog used specific methods, e. g. electron microscopy, enzyme-histochemical procedures or application of immunohistochemical markers, which are accepted as proving the existence of lymphatic vessels (Oehmke et al. 2003). Only recently, immunohistochemical markers for lymphatic endothelial cells (Lyve-1, VEGFR-3, D2-40 and Prox-1) were utilized to investigate the lymph vascular system in the dental pulp of rats and humans (Berggreen et al. 2009; Gerli et al. 2010). Lymphatics had been demonstrated in the rat dental pulp (Berggreen et al. 2009) but no lymphatics were detected in the human dental pulp (Gerli et al. 2010). These findings suggest that the dental pulp of dog and man is equipped with lymph drainage routes other than lymphatics.
As the dental pulp is enclosed in a rigid wall (dentin), it has insufficient space to extend. Without a sufficient route by which lymph-dependent fluids and substances could be removed, the tissue fluid pressure would increase until it exceeded the vascular pressure, causing ischemia, with the risk of necrosis of the pulpal tissue (Heyeraas, 1989). Considering the absence of lymphatic vessels, one may suppose that other pathways for the discharge of fluids and macromolecules from the pulp should be present. It has been hypothesized that pulpal tissue drainage might be provided by either venous blood vessels (Gängler & Merte, 1979) or blood capillaries (Tonder, 1983; Heyeraas et al. 1994; Matthews & Andrew, 1995; Heyeraas & Berggreen, 1999). The absorbance of pulpal tissue fluid by venous blood vessels has been suggested, after transmission electron microscopy provided evidence for the absence of lymphatic vessels in the dental pulp of rat and human teeth (Gängler & Merte, 1979). Blood capillary absorption of interstitial fluid has been proposed to contribute to the equilibration of interstitial fluid pressure in cat dental pulp after an experimental rise in tissue fluid pressure (Heyeraas et al. 1994). It has also been shown that excessive interstitial tissue fluid, which has been filtered into inflamed areas, becomes reabsorbed into nearby capillaries in non-inflamed areas (Tonder, 1983; Matthews & Andrew, 1995; Heyeraas & Berggreen, 1999). In this way, the increased interstitial fluid pressure caused by local inflammation remains restricted to a small tissue area and a generalized rise in tissue fluid pressure with the risk of ischemia and pulpal necrosis is prevented (Heyeraas & Berggreen, 1999).
It must be emphasized that blood capillary absorbance has only been regarded as supplementary and additional to increased lymph flow in the inflamed pulpal areas (Tonder, 1983; Matthews & Andrew, 1995; Heyeraas & Berggreen, 1999). The question of whether the increased lymph flow is facilitated by lymphatic vessels or extravascular fluid pathways remained unsolved. Recently, evidence has been presented for the occurrence of lymphangiogenesis in response to inflammatory stimuli in the pulp of rat molars (Berggreen et al. 2009). As lymphangiogenesis is defined as the sprouting of new lymphatic vessels from pre-existing ones, this mechanism is only applicable to pulp tissue originally containing a lymphatic system (Bruyere & Noel, 2010). This has in fact been demonstrated for the examined dental pulp in the rat molars (Heyeraas & Berggreen, 1999). It has not been clarified whether de-novo formation of lymphatic vessels might occur in the dental pulp, a phenomenon that has already been reported for the inflamed cornea of adult mice (Maruyama et al. 2005).
However, pulpal tissue drainage via blood vessels or lymph capillaries, which are temporarily formed in response to inflammatory stimuli, has been regarded as a possible but physiologically unfavorable option of normal tissue clearance (Oehmke et al. 2003). Instead, a lymph drainage model for human teeth has been supposed, consisting of two different constituents of the lymphatic pathway, i.e. tissue clefts that collect interstitial fluid and macromolecules in the coronal part of the pulp and lymphatic vessels that receive the lymph fluid in the apical area of the pulp (Oehmke et al. 2003). However, as the so-called tissue clefts were lined by flat endothelial-like cells, it remains unclear whether these tissue clefts should be addressed as vascular or extravascular constituents of the lymphatic pathway in the pulp. Further evidence for extravascular pathways for fluid and macromolecule drainage was obtained in experiments using 125I-labeled albumin in feline teeth. The recorded tracer distribution strongly suggested extravascular fluid transport (Heyeraas, 1989).
The results presented in this study also suggest extravascular pathways for lymphatic drainage from the pulpal tissue. It is supposed that the lymph fluid from the pulp is further transported via lymphatic vessels in the apical region of the periodontal ligament. Although detailed information concerning the lymphatic system in the periodontium of dogs is not yet available, results from studies in various other species with brachyodont dentition demonstrated numerous lymphatic vessels in the root region of the periodontal ligament, which were connected with lymphatic vessels coming from the dental pulp (Matsumoto et al. 2002; Berggreen et al. 2009).
Due to the enclosing of the pulp between rigid dentin walls, the pulpal tissue possesses a very low compliance (Heyeraas, 1989). Even small variations of the interstitial fluid volume cause relatively large changes in the interstitial fluid pressure (Heyeraas, 1989; Bishop & Malhotra, 1990). Overall, the pulpal tissue fluid pressure is relatively high compared with other organs and body tissues (Tonder & Kvinnsland, 1983; Matthews & Andrew, 1995). These conditions are reflected by morphologic and functional modifications in the fibrillar component surrounding proposed lymphatic vessels in the dental pulp of human teeth (Marchetti et al. 1992; Matsumoto et al. 2002). In contrast to lymphatic vessels in other tissues, lymphatic vessels in the dental pulp lack elastic fibers in the surrounding connective tissue and anchoring fibers are present only in small numbers (Marchetti et al. 1992). For that reason, pulpal lymphatic vessels are directly connected to the surrounding collagen fibers, ensuring that the lymphatic vessels withstand interstitial pressure and remain open (Marchetti et al. 1992; Oehmke et al. 2003). Accordingly, the existence and proper function of pulpal lymphatic vessels seems to be dependent on the arrangement of the local connective tissue. Distinct connective tissue configurations may either favor the occurrence of lymphatic vessels or the formation of extravascular lymphatic drainage pathways such as tissue clefts. This assumption is supported by the observation that tissue clefts were exclusively found in the loosely structured coronal pulp of human pre-molars and molars, whereas lymphatic vessels were present in the apical region (Oehmke et al. 2003). Other studies that identified lymphatic vessels in the dental pulp also demonstrated heterogeneous distribution patterns with distinct pulpal areas that were devoid of lymphatic vessels (Bishop & Malhotra, 1990; Sawa et al. 1998; Berggreen et al. 2009). To our knowledge, up until now no studies have been undertaken to analyze the existence and distribution of pulpal lymphatic vessels with regard to connective tissue characteristics.
The specimens for this study were taken from dogs aged between 136 and 184 days. Such a developmental stage may be seen as relatively immature with respect to chronobiologic phenomena, presuming that a lymphatic system was not fully developed before the tooth completely erupted. However, the maturation of a tooth is – apart from several other features – characterized by a continuous reduction in dental cavity size due to apposition of secondary dentin. Thus, in 21-month-old dogs, the volume of the dental pulp of all teeth reduced to only 12–5% of the initial volume (Schmidt, 1984). When regarding a hypothesized lymph vascular system in the dental pulp, it appears functionally inappropriate if such a system should initially develop while the volume of its surrounding tissue is reducing more and more.
Conclusion
The presented results provide evidence for the absence of distinct lymphatic vessels in dog dental pulp and suggest extravascular pathways for tissue drainage. Further studies are intended to address open questions concerning the histologic structure of the drainage pathways, age-related changes and lymphatic drainage in the periodontal tissues of dogs.
Recent findings also demonstrated the absence of lymphatic vessels in the human dental pulp (Gerli et al. 2010), thus suggesting fundamental similarities in dog and human endodontic biology.
Acknowledgments
We would like to thank Ms Gudrun Wirth for her highly appreciated technical support.
Author contributions
A.M.: acquisition of data, data interpretation and drafting of the manuscript; H.G.: concept/design, critical revision and approval of the manuscript; C.S.: concept/design, data interpretation, drafting and approval of the manuscript.
Conflict of interest
We affirm that we have no financial affiliation (e.g. employment, direct payment, stock holdings, retainers, consultationships, patent licensing arrangements or honoraria) or involvement with any commercial organization with direct financial interest in the subject or materials discussed in this study, nor have any such arrangements existed in the past 3 years. Any other potential conflict of interest is disclosed.
References
- Berggreen E, Haug SR, Mkonyi LE, et al. Characterization of the dental lymphatic system and identification of cells immunopositive to specific lymphatic markers. Eur J Oral Sci. 2009;117:34–42. doi: 10.1111/j.1600-0722.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- Bernick S. Lymphatic vessels of the human dental pulp. J Dent Res. 1977a;56:70–77. doi: 10.1177/00220345770560011401. [DOI] [PubMed] [Google Scholar]
- Bernick S. Morphological changes in lymphatic vessels in pulpal inflammation. J Dent Res. 1977b;56:841–849. doi: 10.1177/00220345770560072101. [DOI] [PubMed] [Google Scholar]
- Bernick S, Patek PR. Lymphatic vessels of the dental pulp in dogs. J Dent Res. 1969;48:959–964. doi: 10.1177/00220345690480056201. [DOI] [PubMed] [Google Scholar]
- Bishop MA, Malhotra M. An investigation of lymphatic vessels in the feline dental pulp. Am J Anat. 1990;187:247–253. doi: 10.1002/aja.1001870304. [DOI] [PubMed] [Google Scholar]
- Brown AC, Barrow BL, Gadd GN, et al. Tooth pulp transcapillary osmotic pressure in the dog. Arch Oral Biol. 1969;14:491–502. doi: 10.1016/0003-9969(69)90142-3. [DOI] [PubMed] [Google Scholar]
- Bruyere F, Noel A. Lymphangiogenesis: in vitro and in vivo models. FASEB J. 2010;24:8–21. doi: 10.1096/fj.09-132852. [DOI] [PubMed] [Google Scholar]
- Carreras P. Ueber die Absorptionsfähigkeit der Zahnpulpa. Oesterr - Ung Vierteljahrsschr f Zahnheilk. 1894;10:253–264. [Google Scholar]
- da Cunha Pereira C, de Oliveira EP, Gomes MS, et al. Comparative in vivo analysis of the sealing ability of three endodontic sealers in dog teeth after post-space preparation. Aust Endod J. 2007;33:101–106. doi: 10.1111/j.1747-4477.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- Dahl E, Majör IA. The fine structure of the vessels in the human dental pulp. Acta Odontol Scand. 1973;31:223–230. doi: 10.3109/00016357309002508. [DOI] [PubMed] [Google Scholar]
- Eifinger FF. Bonn, Germany: University of Bonn; 1970. Die Mikromorphologie der menschlichen Zahnpulpa. PhD Thesis. [Google Scholar]
- Fish EW. The circulation of lymph in dentin and enamel. J Am Dent Assoc. 1927;14:804–817. [Google Scholar]
- Frank RM, Wiedemann P, Fellinger E. Ultrastructure of lymphatic capillaries in the human dental pulp. Cell Tissue Res. 1977;178:229–238. doi: 10.1007/BF00219050. [DOI] [PubMed] [Google Scholar]
- Gängler P, Merte K. Die vitalmikroskopische Untersuchung der periodontalen Blutzirkulation am Ratteninzisivus. Zahn Mund Kieferheilkd. 1979;67:459–466. [PubMed] [Google Scholar]
- Gängler P, Mönch M. Die morphologische Differenzierung von Kapillaren der Pulpa dentis. Zahn Mund Kieferheilkd. 1980;68:198–206. [PubMed] [Google Scholar]
- Gerli R, Secciani I, Sozio F, et al. Absence of lymphatic vessels in human dental pulp: a morphological study. Eur J Oral Sci. 2010;118:110–117. doi: 10.1111/j.1600-0722.2010.00717.x. [DOI] [PubMed] [Google Scholar]
- Heyeraas KJ. Pulpal hemodynamics and interstitial fluid pressure: balance of transmicrovascular fluid transport. J Endod. 1989;15:468–472. doi: 10.1016/S0099-2399(89)80026-3. [DOI] [PubMed] [Google Scholar]
- Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med. 1999;10:328–336. doi: 10.1177/10454411990100030501. [DOI] [PubMed] [Google Scholar]
- Heyeraas KJ, Kim S, Raab WH, et al. Effect of electrical tooth stimulation on blood flow, interstitial fluid pressure and substance P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc Res. 1994;47:329–343. doi: 10.1006/mvre.1994.1026. [DOI] [PubMed] [Google Scholar]
- Isokawa S. Über das Lymphsystem des Zahnes. Z Zellforsch Mikrosk Anat. 1960;52:140–149. [PubMed] [Google Scholar]
- Kim H, Nguyen VPKH, Petrova TV, et al. Embryonic vascular endothelial cells are malleable to reprogramming via Prox1 to a lymphatic gene signature. BMC Dev Biol. 2010;10:72. doi: 10.1186/1471-213X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor A. An experimental investigation of the lymphatic system of the teeth and jaws. Proc R Soc Med. 1936;29:49–78. [PMC free article] [PubMed] [Google Scholar]
- Magnus G. Über den Nachweis der Lymphgefäße in der Zahnpulpa. Dtsch Mschr Zahnheilk. 1922;40:661–666. [Google Scholar]
- Marchetti C, Poggi P. Lymphatic vessels in the oral cavity: different structures for the same function. Microsc Res Tech. 2002;56:42–49. doi: 10.1002/jemt.10010. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Poggi P, Calligaro A, et al. Lymphatic vessels in the healthy human dental pulp. Acta Anat (Basel) 1991;140:329–334. doi: 10.1159/000147078. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Poggi P, Calligaro A, et al. Lymphatic vessels of the human dental pulp in different conditions. Anat Rec. 1992;234:27–33. doi: 10.1002/ar.1092340104. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Kato S, Miura M, et al. Fine structure and distribution of lymphatic vessels in the human dental pulp: a study using an enzyme-histochemical method. Cell Tissue Res. 1997;288:79–85. doi: 10.1007/s004410050794. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Zhang B, Kato S. Lymphatic networks in the periodontal tissue and dental pulp as revealed by histochemical study. Microsc Res Tech. 2002;56:50–59. doi: 10.1002/jemt.10006. [DOI] [PubMed] [Google Scholar]
- Matthews B, Andrew D. Microvascular architecture and exchange in teeth. Microcirculation. 1995;2:305–313. doi: 10.3109/10739689509148275. [DOI] [PubMed] [Google Scholar]
- Mouta Carreira C, Nasser SM, di Tomaso E, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- Noyes FB. Lymphatics of the dental region. J Am Dent Assoc. 1928;15:1911–1913. [Google Scholar]
- Oehmke MJ, Knolle E, Oehmke HJ. Lymph drainage in the human dental pulp. Microsc Res Tech. 2003;62:187–191. doi: 10.1002/jemt.10378. [DOI] [PubMed] [Google Scholar]
- Pimenta FJ, Sa AR, Gomez RS. Lymphangiogenesis in human dental pulp. Int Endod J. 2003;36:853–856. doi: 10.1111/j.1365-2591.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- Rodd HD, Boissonade FM. Immunocytochemical investigation of neurovascular relationships in human tooth pulp. J Anat. 2003;202:195–203. doi: 10.1046/j.1469-7580.2003.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben MP, Prieto-Hernandez JR, Gott FK, et al. Visualization of lymphatic microcirculation of oral tissues. II. Vital retrograde lymphography. J Periodontol. 1971;42:774–784. doi: 10.1902/jop.1971.42.12.774. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nakagami H, Morishita R, et al. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation. 2006;114:1177–1184. doi: 10.1161/CIRCULATIONAHA.105.602953. [DOI] [PubMed] [Google Scholar]
- Sawa Y, Yoshida S, Ashikaga Y, et al. Immunohistochemical demonstration of lymphatic vessels in human dental pulp. Tissue Cell. 1998;30:510–516. doi: 10.1016/s0040-8166(98)80030-x. [DOI] [PubMed] [Google Scholar]
- Schmidt H. Hamburg, Germany: University of Hamburg; 1984. Altersbedingte Veränderungen der Pulpen-Anatomie im Gebiss des Beagle-Hundes. PhD Thesis. [Google Scholar]
- Schweitzer G. Über die Lymphgefässe des Zahnfleisches und der Zähne beim Menschen und bei Säugetieren. I. Die Lymphgefässe des Zahnfleisches beim Menschen. II. Lymphgefässe der Zähne. Arch Mikrosk Anat. 1907;69:807–908. [Google Scholar]
- Sleeman JP, Krishnan J, Kirkin V, et al. Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech. 2001;55:61–69. doi: 10.1002/jemt.1157. [DOI] [PubMed] [Google Scholar]
- Staszyk C, Duesterdieck KF, Gasse H, et al. Immunohistochemical identification of lymphatic vessels in the periodontium of equine cheek teeth. J Vet Dent. 2005;22:227–232. doi: 10.1177/089875640502200402. [DOI] [PubMed] [Google Scholar]
- Sulzmann R. Beiträge zur Histologie der Zahnpulpa. I. Mitteilung. Über das Vorkommen von epitheloidzellhaltigen Gefäßstrecken und Sperrarterien (Polsterarterien und Arterienwülsten) in der Eckzahnpulpa des Schäferhundes. Z Mikrosk Anat Forsch. 1955;61:281–303. [PubMed] [Google Scholar]
- Thibodeau B, Teixeira F, Yamauchi M, et al. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680–689. doi: 10.1016/j.joen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Tonder KJ. Vascular reactions in the dental pulp during inflammation. Acta Odontol Scand. 1983;41:247–256. doi: 10.3109/00016358309162331. [DOI] [PubMed] [Google Scholar]
- Tonder KJ, Kvinnsland I. Micropuncture measurements of interstitial fluid pressure in normal and inflamed dental pulp in cats. J Endod. 1983;9:105–109. doi: 10.1016/S0099-2399(83)80106-X. [DOI] [PubMed] [Google Scholar]
- Watts A, Paterson RC. Migration of materials and microorganisms in the dental pulp of dogs and rats. J Endod. 1982;8:53–58. doi: 10.1016/S0099-2399(82)80258-6. [DOI] [PubMed] [Google Scholar]
- Wilting J, Papoutsi M, Christ B, et al. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002;16:1271–1273. doi: 10.1096/fj.01-1010fje. [DOI] [PubMed] [Google Scholar]
- Yanpiset K, Trope M. Pulp revascularization of replanted immature dog teeth after different treatment methods. Endod Dent Traumatol. 2000;16:211–217. doi: 10.1034/j.1600-9657.2000.016005211.x. [DOI] [PubMed] [Google Scholar]
- Zander HA, Smith HW. Penetration of silver nitrate into Dentin. J Dent Res. 1945;24:121–128. [Google Scholar]


