Abstract
Glucose uptake into the mammalian nervous system is mediated by the family of facilitative glucose transporter proteins (GLUT). In this work we investigate how the expression of the main neuronal glucose transporters (GLUT3, GLUT4 and GLUT8) is modified during cerebellar cortex maturation. Our results reveal that the levels of the three transporters increase during the postnatal development of the cerebellum. GLUT3 localizes in the growing molecular layer and in the internal granule cell layer. However, the external granule cell layer, Purkinje cell cytoplasm and cytoplasm of the other cerebellar cells lack GLUT3 expression. GLUT4 and GLUT8 have partially overlapping patterns, which are detected in the cytoplasm and dendrites of Purkinje cells, and also in the internal granule cell layer where GLUT8 displays a more diffuse pattern. The differential localization of the transporters suggests that they play different roles in the cerebellum, although GLUT4 and GLUT8 could also perform some compensatory or redundant functions. In addition, the increase in the levels and the area expressing the three transporters suggests that these roles become more important as development advances. Interestingly, the external granule cells, which have been shown to express the monocarboxylate transporter MCT2, express none of the three main neuronal GLUTs. However, when these cells migrate inwardly to differentiate in the internal granule cells, they begin to produce GLUT3, GLUT4 and GLUT8, suggesting that the maturation of the cerebellar granule cells involves a switch in their metabolism in such a way that they start using glucose as they mature.
Keywords: cerebellum, development, external granule cells, glucose transport, glucose transporters (GLUT)
Introduction
Glucose uptake into the mammalian nervous system is mediated by the family of facilitative glucose transporter proteins (GLUT). Of the 13 GLUT family members, several are present in the brain (Wood & Trayhurn, 2003; Uldry & Thorens, 2004), and their alteration can result in important alterations of functions of the central nervous system (Wang et al. 2005; Zhao et al. 2009).
The distribution of GLUT in the nervous system suggests that the transport of glucose across the blood–brain barrier and within nervous cells is tightly regulated and compartmentalized. It is assumed that, whereas GLUT1 is located in the ependymal and glial cells and mediates glucose transport across the blood–brain barrier, GLUT3 is considered the main neuronal transporter. Nonetheless, GLUT4 and GLUT8 have also been described as being present in neurons (McEwen & Reagan, 2004). Other transporters are produced by astrocytes (GLUT2) and microglia (GLUT5) (McEwen & Reagan, 2004). In addition, increasing evidence shows that neurons can also express GLUT2, GLUT6 and even GLUT1 under specific conditions (Simpson et al. 2008).
Several works have described the regional and cellular distribution of neuronal GLUT transporters in the adult brain. GLUT3 is widely distributed in the central nervous system (Nagamatsu et al. 1993; Maher et al. 1994; Choeiri et al. 2002), localized in the neuropil, but absent in cell bodies (Gerhart et al. 1995; McCall et al. 1994). The insulin-responsive transporter GLUT4 is also widely expressed in the brain in a somatodendritic localization in both the plasma membrane and cytoplasm (El Messari et al. 1998, 2002; Choeiri et al. 2002). GLUT8, which is considered to be probably located in intracellular compartments in both neurons (Piroli et al. 2002) and other cell types (Gomez et al. 2006, 2009; Romero et al. 2009), also exhibits a widespread distribution in the brain by localizing in both neuronal cell bodies and apical dendrites (Reagan et al. 2001, 2002; Ibberson et al. 2002; Sankar et al. 2002).
GLUT3, GLUT4 and GLUT8 are present in the cerebellum (Brant et al. 1993; Rayner et al. 1994; Kobayashi et al. 1996; Leloup et al. 1996; Vannucci et al. 1997, 1998b; El Messari et al. 1998, 2002; Apelt et al. 1999; Choeiri et al. 2002; Ibberson et al. 2002). However, their variation and distribution during the complex process of cerebellar maturation is not well known. Consequently, the levels of glucose transporters can be modified in accordance with energy demand (Vannucci et al. 1998b; Vannucci & Simpson, 2003). This is especially important for the cerebellar cortex as its development is postnatal in mice. In this work, we investigate how the expression of the main neuronal glucose transporters is modified during cerebellar cortex maturation.
Materials and methods
The C57/BL6 mice from Harlan (Barcelona, Spain) were housed, bred and killed according to European Council legislation (86/609/EEC) on experimental animal protection. Breeding pairs of mice were monitored daily for litters, and the date of birth was taken as day 0. All of the animals were maintained on a 12-h day/night cycle at constant room temperature (22 °C) with free access to water and standard mouse fodder. Adult mice (8–10 weeks old) and mice pups of 7, 15 and 21 days of age were killed by decapitation or an overdose of pentobarbital (100 mg/Kg intraperitoneally). All of the experimental protocols were approved by the Ethics Committee of the Cardenal Herrera-CEU University.
Materials
All of the generic reagents were obtained from Sigma-Aldrich (St Louis, MO, USA) or Roche Diagnostics (Barcelona, Spain). A rabbit polyclonal antibody against a synthetic peptide corresponding to the 11 C-terminal residues (466–477) of mouse GLUT8 was prepared by Q-Biogene (Illkrich, France), as previously described (Gomez et al. 2006). Rabbit anti-GLUT3 antibodies were obtained from Calbiochem (San Diego, CA, USA) and Abcam (Cambridge, UK) and demonstrated similar results. Rabbit anti-GLUT4 and rabbit anti-GLUT1 antibodies were obtained from Calbiochem. Rabbit polyclonal antibodies against mouse anti-actin bound to peroxidase were obtained from Sigma-Aldrich. The Hybond membranes enhanced chemiluminescence (ECL) detection system and anti-rabbit IgG secondary antibody were obtained from GE Healthcare (Chalfont St Giles, UK). The Bradford and western blotting reagents were obtained from Bio-Rad (Hercules, CA, USA).
Western blot
Proteins were obtained as described elsewhere (Romero et al. 2009). Tissues were homogenized in Tris-EDTA-sucrose (20 mm Tris, 1 mm EDTA, 255 mm sucrose, pH 7.1) plus a protease inhibitory cocktail and 10 mm phenylmethylsulfonyl fluoride (both from Roche Diagnostics), with an Ultra Turrax T25 basic (IKA Labortechnik, Staufen, Germany). The homogenate was then sonicated, and kept on ice. Samples were then centrifuged at 16 000 g for 20 min at 4 °C. The pellet was resuspended in 2 mm Tris/50 mm mannitol pH 7.1 buffer. The supernatant was used to analyze GLUT4 levels and the pellet was used to analyze GLUT3 and GLUT8. Protein concentration was determined by the Bradford method. Western blots were performed with 40 μg of total protein as described elsewhere. Protein samples were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 11% acrylamide gels, and the proteins transferred to the Hybond-ECL membranes, which were blocked for 2 h in 5% non-fat dried milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T). Blots were probed overnight with the anti-GLUT8 antibody (1 : 500) at 4 °C, the GLUT3 and GLUT4 antibodies (dilution 1 : 500) for 1 h at room temperature, and then overnight at 4 °C. Primary antibodies were detected with an anti-rabbit IgG antibody coupled to horseradish peroxidase (dilution 1 : 5000) using the ECL detection system. Blots were reprobed after stripping in 200 mm glycine, pH 2.5, 0.4% sodium dodecyl sulfate. Nitrocellulose sheets were rinsed in TBS-T and then reblotted for 60 min in 5% non-fat dried milk in TBS-T before being probed with an anti-actin antibody used at a 1 : 20 000 dilution. Blots were quantified by densitometry, and the GLUT : β-actin protein ratio was calculated from the films with the Quantity One Image Analysis Software (Bio-Rad).
Histological and immunohistochemical methods
The immunohistochemical methods were performed as previously described (Gomez et al. 2006, 2009). Cerebella (three animals per group) were placed overnight in 4% paraformaldehyde. After fixation, tissues were dehydrated at increasing ethanol concentrations, embedded in paraffin, serially sectioned (3 μm) in an HM 310 Microm microtome and collected on polylysine-coated slides. Sections were deparaffinized and rehydrated. Antigen retrieval was performed by heating the sections at 100 °C in a water-bath for 15 min in citrate buffer (10 mm, pH 8). The sections were then washed three times in 0.1 m phosphate buffer with 0.2% Triton X-100, incubated with 3% H2O2 in methanol for 20 min to quench endogenous peroxidase activity, and processed for immunohistochemical analysis with the corresponding primary antibody. Immunohistochemistry for GLUT8 (Gomez et al. 2006, 2009) and GLUT1 was performed using the immunoperoxidase procedure corresponding to the Vectastain Elite ABC kit from Vector Laboratories (Burlingame, CA, USA). Sections were incubated overnight with the rabbit anti-GLUT8 or the rabbit anti-GLUT1 polyclonal antisera (dilution 1 : 500) at 4 °C. The non-specific signal was blocked with 10% normal goat serum. The GLUT3 and GLUT4 immunohistochemistry procedures (at 1 : 300 dilution for both) were similar to that used for GLUT8, except for 2 h, which was performed with the primary antibody at room temperature followed by overnight incubation at 4 °C.
Statistical analysis
The results are expressed as mean ± SD. The statistical significance of the differences was determined by one-way anova.
Results
We first analyzed the levels of the GLUT3, GLUT4 and GLUT8 proteins in the cerebellar cortex on postnatal day (P)7, P15, P21 and in adults by western blot. Our results show that the levels of the three transporters were low on P7 and that they subsequently increased progressively to adulthood (Fig. 1). The GLUT4 levels gradually increased from P7 to adulthood reaching 10-fold higher intensities in adult cerebella than in the first age analyzed. GLUT8 displayed a similar pattern but its final levels were sixfold compared with P7. GLUT3 levels also rapidly increased to reach similar levels (or even slightly higher) at P21 as in adults, where they were approximately sevenfold higher than those found at P7.
Fig. 1.
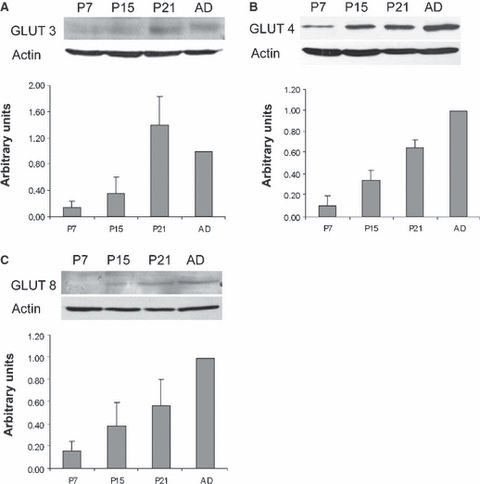
Detection of the GLUT3 (A), GLUT4 (B) and GLUT8 (C) proteins by western blot and quantification of the GLUT transporters in the cerebellum on postnatal day (P)7, P15, P21 and in adult mice (AD). The results (mean + SD) are expressed as densitometric arbitrary units. Histograms represent the GLUT levels of three (for GLUT4 and GLUT8) or four (GLUT3) independent experiments. The levels of the three transporters significantly increase (P < 0.01) throughout cerebellar development.
Regarding the specific localization of the transporters, GLUT3 immunoreactivity (IR) (Fig. 2) was appreciable in the molecular layer (ML) and in the internal granule cell layer (IGL) on P7. GLUT3 IR was also evident in the periphery of the Purkinje cells, whereas their cytoplasm lacked GLUT3 IR. As a result, the growing ML was labeled. Conversely, the external granule cell layer (EGL) did not express the transporter, except for some isolated cells located at the outermost level close to the piamater (Fig. 2A, arrowheads). Likewise, the cytoplasm of the cells in the IGL was devoid of GLUT3 IR, whereas intense immunolabeling was apparent in the dots corresponding to the cerebellar glomeruli. One week later, GLUT3 IR (Fig. 2B) expanded, mainly because of the larger extension of the ML which, at this age, had almost completed growth at the expense of the EGL that had practically disappeared. GLUT3 IR was detected in the neuropil, and the lack of labeling in the cytoplasm of the cells located in the ML was evident. In addition, both the IGL and Purkinje cells expressed the transporter similarly to P7. Few changes were observed in the localization of the transporter on P21 (Fig. 2C). The non-expressing EGL had recently disappeared and GLUT3 labeling was intense in both the ML and IGL. In adults, the expression pattern of GLUT3 was similar to that observed on P21 when it was detected in the neuropil of the whole cerebellar cortex (Fig. 2D).
Fig. 2.
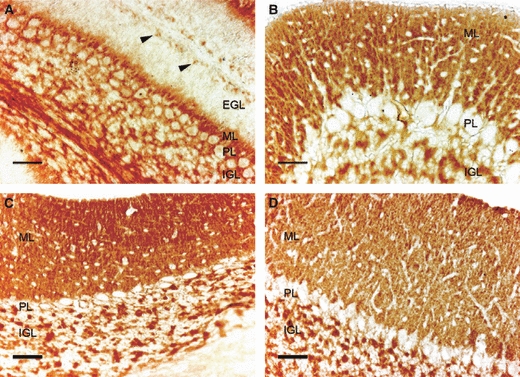
Immunodetection of GLUT3 in the mouse cerebellar cortex on postnatal day (P)7 (A), P15 (B), P21 (C) and in adults (D). EGL, external granule cell layer; IGL, internal granule cell layer; ML, molecular layer; PL, Purkinje cell layer. Observe GLUT3 immunoreactivity (IR) in both the ML and IGL, whereas the EGL, present on P7, lacks GLUT3 expression except for some isolated cells on the outermost part of the layer (arrowheads). The GLUT3 IR is absent in the cell bodies. Scale bar: 25 μm.
Conversely to GLUT3, GLUT4 IR on P7 was only evident in the cytoplasm of the Purkinje cells and hardly surpassed the background levels in the ML, EGL and IGL (Fig. 3A). On P15 it was detectable, in addition to the Purkinje cell cytoplasm, in the dendrites of the Purkinje cells in the ML and at low levels in the IGL cells (Fig. 3B). Few changes were detected subsequently on P21 and, although the western blot indicated that the levels had increased, the IR found was no stronger than that noted on P15 (Fig. 3C). Finally, in the adult mice, apart from the strong labeling previously observed in the Purkinje cell soma, GLUT4 IR was also seen in both the ML and IGL (Fig. 3D).
Fig. 3.
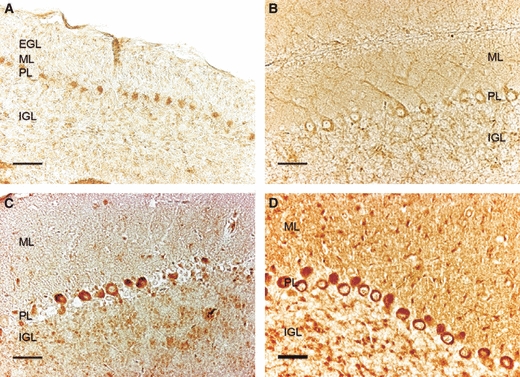
Immunodetection of GLUT4 in the mouse cerebellar cortex on postnatal day (P)7 (A), P15 (B), P21 (C) and in adults (D). EGL, external granule cell layer; IGL, internal granule cell layer; ML, molecular layer; PL, Purkinje cell layer. GLUT4 immunoreactivity is detected in both PL bodies and dendrites. Staining in adults is more intense, labeling the ML, PL and IGL. Scale bar: 25 μm.
The GLUT8 distribution on P7 was, to some extent, similar to the GLUT4 distribution. In addition to the cytoplasm of the Purkinje cells, however, GLUT8 was also noticeable in the dendritic tree of these cells in the ML. Both the IGL and EGL apparently did not express GLUT8 (Fig. 4A). One week later, the expression of GLUT8 increased and it was especially evident in both the cytoplasm and dendrites of the Purkinje cells, and relatively less strong in the rest of the ML and in the IGL (Fig. 4B). At P21 (Fig. 4C), GLUT8 IR displayed no major changes when compared with the previous stage. In adults, however, other than the labeling in the ML and Purkinje layer, the IGL expressed the transporter in a diffuse and less concentrated manner than GLUT3 and GLUT4 (Fig. 4D).
Fig. 4.
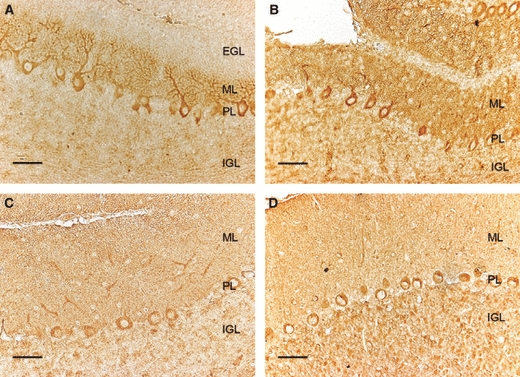
Immunodetection of GLUT8 in the mouse cerebellar cortex on postnatal day (P)7 (A), P15 (B), P21 (C) and in adults (D). EGL, external granule cell layer; IGL, internal granule cell layer; ML, molecular layer; PL, Purkinje cell layer. GLUT8 immunoreactivity (IR) is detected in PL cell bodies and dendrites, and also shows labeling in the ML, especially in the youngest ages. In the adult, GLUT8 IR is located in the ML, PL and diffusely in the IGL. Scale bar: 25 μm.
It is interesting to note that the EGL, which gradually reduces in size until approximately P15 when it disappears after providing the cells that compose the IGL, apparently expressed none of the three transporters under study. In order to investigate whether GLUT1 (which, although it is not usually expressed by neurons, has been detected both in cultured neurons and in vivo under conditions of stress) (Simpson et al. 2008) was expressed by EGL cells, GLUT1 IR was studied in cerebellar sections. Our results showed that GLUT1 was not expressed by EGL cells or any other neuronal cell type in the cerebellum (Fig. 5). GLUT1 IR was detected in microvessels, where levels were very low on P7 but gradually increased throughout postnatal development.
Fig. 5.
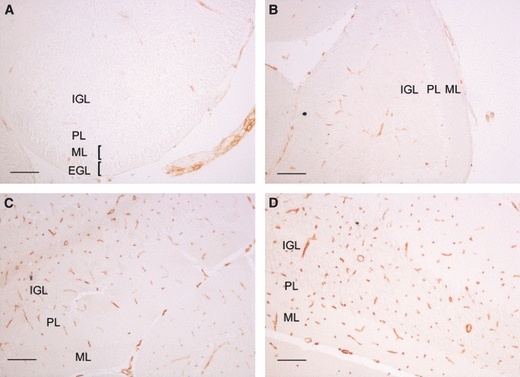
Immunodetection of GLUT1 in the mouse cerebellar cortex on postnatal day (P)7 (A), P15 (B), P21 (C) and in adults (D). EGL, external granule cell layer; IGL, internal granule cell layer; ML, molecular layer; PL, Purkinje cell layer. GLUT1 immunoreactivity is detected in microvessels with increasing intensity as development advances. Scale bar: 25 μm in A and 50 μm in B–D.
Discussion
During early postnatal development the brain requires supplies of fuel for growth and process formation (Vannucci & Vannucci, 2000). Thus, the regulation of the glucose transporter expression during this period might be critical for the glucose supply for brain maturation and, more precisely, for cerebellar development.
Here we show that the levels of the main neuronal transporters increase during cerebellar development and that they are expressed in a developmentally regulated manner. Our work shows that GLUT3 is localized in the ML and granular layers of young and adult cerebella, thus corroborating the results observed in adults (Choeiri et al. 2002). It localizes in the neuropil as the cell bodies of the cerebellar cortex lack GLUT3 IR. Previous works have shown that Glut3 mRNA is expressed in the granule cells of the cerebellum (Kobayashi et al. 1996; Vannucci et al. 1998b) and in Purkinje cells (Kobayashi et al. 1996), this most probably being the origin of the protein observed in the ML and IGL. In addition, our results confirm the postnatal increase of the GLUT3 protein levels reported by Vannucci et al. (1998a), which showed increased levels in the cerebellum in a similar way to that reported herein. This is probably due to the expansion of the ML and IGL, as the localization of GLUT3 in the ML runs in concert with ML growth. It is also interesting to note that the EGL does not express GLUT3, except for a few externally located cells.
Although GLUT3 is considered the ideal choice as a neuronal glucose transporter (Simpson et al. 2008), GLUT4 and GLUT8 are also produced by neurons. Here we show that GLUT4 expression increases considerably during postnatal development and to such an extent that suggests that its role is probably more important in adults than in the developmental stages. Indeed, GLUT4 is the transporter whose localization and levels display more differences in adults and previous stages. Some controversial results have been published about GLUT4 localization in the cerebellum. Some reports describe GLUT4 in both Purkinje cells and in the IGL to a lesser extent (Kobayashi et al. 1996), heterogeneously distributed, but mainly concentrated in Purkinje cells, and in cells and processes of the ML and IGL (El Messari et al. 1998, 2002). Apelt et al. (1999) also described GLUT4 localization in Purkinje cells, whereas Vannucci et al. (1998b) documented an absence of staining from both the Purkinje layer and ML. Our results agree with those of Choeiri et al. (2002) who described that GLUT4 can be detected in the three layers. However, we observe that it is mainly located in the Purkinje cell cytoplasm in early postnatal stages.
Previous studies have shown that GLUT8 is produced by neurons (Ibberson et al. 2002; Piroli et al. 2002; Reagan, 2002; Sankar et al. 2002; Augustin et al. 2005; Widmer et al. 2005; Zhao et al. 2009). To date, however, only a weak GLUT8 IR of the Purkinje cells has been described in relation to the cerebellar GLUT8 expression (Ibberson et al. 2002). Our results reveal that GLUT8 is mainly located in Purkinje cells, but we also observe GLUT8 IR in ML and IGL cells in such a way that it overlaps with GLUT4 to some extent, even though the distribution of GLUT8 in the IGL is more diffuse. Nevertheless, the results of a recent report showing a high expression level in the cerebellar tissue for Glut3 and Glut8 mRNA compared with Glut isoforms 1 and 4 (Weisova et al. 2009) suggest an important role for this transporter in cerebellar functions.
Nonetheless, neither the way that GLUT transporters are regulated in the cerebellum nor the specific role played by each isoform is well understood. Our results show that the levels of the three transporters in the cerebellum increase with age, but this does not seem to be a general rule for the brain as the GLUT4 and GLUT8 levels measured in whole brain homogenates increase in the first postnatal weeks, but decrease in adults (Sankar et al. 2002). Regarding the roles of the GLUT transporters, recent works suggest that, in addition to the variation observed in normal postnatal development, different pathophysiological conditions can modify the GLUT expression. GLUT3 has been suggested to be critical in mediating neuronal tolerance to excitotoxicity (Weisova et al. 2009). GLUT4, which is an insulin-dependent transporter, seems to be related with glycemia levels as chronic insulin-resistant states are associated with the increased expression of GLUT4 in the cerebellum (Alquier et al. 2006). In addition, Cheng et al. (2000) demonstrated that knocking out the neurotrophic factor insulin-like growth factor 1 in mice induces a sharp drop in glucose uptake in several brain areas of young mice, including the cerebellum. The diminished glucose uptake in the cerebellum was therefore associated with a specific decrease of Glut4 mRNA and IR in Purkinje cells, suggesting that GLUT4-mediated glucose transport may play critical roles during postnatal brain development (Cheng et al. 2000).
The role of GLUT8 in the cerebellum is less known. As in other cell types (Gomez et al. 2006, 2009; Romero et al. 2009), GLUT8 is not located in the plasma membrane of neurons, but in subcellular compartments (Piroli et al. 2002; Alquier et al. 2006), and it does not translocate to the plasma membrane on different stimulations (Shin et al. 2004; Widmer et al. 2005). Thus, it has been suggested to be an intracellular transporter involved in glycosylation processes (Piroli et al. 2002). However, a relationship has been shown between GLUT8 and the energy requirements of some cell types, such as blastocysts (Pinto et al. 2002), hepatocytes (Gorovits et al. 2003) and intestinal cells (Romero et al. 2007), suggesting that GLUT8 is associated with the physiology of cells with a high energy demand. Recently, GLUT8 knockout mice have been independently generated in two different laboratories (Membrez et al. 2006; Gawlik et al. 2008). Although knockout mice were viable with normal growth and presented no major abnormalities, they were hyperactive, which is perhaps the result of alterations in intracellular transport (Schmidt et al. 2008, 2009). It is reasonable to believe that the absence of GLUT8 in the cerebellum may be involved in the phenotype of those knockout mice.
One intriguing result of this work is that the EGL cells lack evidence of the expression of GLUT1, GLUT3, GLUT4 and GLUT8. To our knowledge, this is the first report showing such data. We may ask, then, how the EGL cells uptake fuel if they do not express any of the main neuronal transporters. One possible way could be through monocarboxylate transporters (MCTs). MCTs are responsible for the transport of lactate, pyruvate, acetoacetate and β-hydroxybutyrate, and comprise a gene family with 14 members (Halestrap & Meredith, 2004). MCT2 is the main neuronal monocarboxylate transporter (Pierre et al. 2002; Pierre & Pellerin, 2005; Bergersen, 2007; Simpson et al. 2007) and is widely distributed in the nervous system. In the cerebellum, intense MCT2 IR is found in Purkinje cell bodies and their processes, as well as on mossy fibers (Pierre et al. 2002; Rafiki et al. 2003; Pierre & Pellerin, 2005).
There has been debate about whether neuronal activity is fueled primarily by glucose (which is transported by GLUTs) or lactate (transported by MCTs) (Simpson et al. 2007). Thus, the developmental pattern of the transporters can reflect the type of metabolism at a given developmental stage, and changes in their expression and levels can be a sign of metabolic modifications. Interestingly, the EGL, which proliferates and later migrates inwardly through Purkinje cells to form internal granule cells (Wechsler-Reya, 2003), expresses MCT2 and this expression lowers concomitantly with the EGL regression (Rafiki et al. 2003). In their work the authors had already suggested a complementary pattern of the MCT2 and GLUT distribution as a gradual developmental decline in MCT2 expression seemed to be balanced by an increase in GLUT1 (at the blood–brain barrier) and GLUT3 (in neurons) (Rafiki et al. 2003). Our results sustain this hypothesis, which, at least in the cerebellum, can be generalized for other transporters like GLUT8 and GLUT4. More precisely, the granule cells of the cerebellum emerge as a model to study such a complementary pattern: the EGL cells express MCT2 and lack GLUT expression, but when they migrate inwardly and mature, they start to express glucose transporters. This also strongly suggests that the transition from the EGL to the IGL implicates a switch from a monocarboxylate-based metabolism to another metabolism based on glucose uptake and utilization. Nevertheless, it would be interesting to investigate whether other neuroblasts or immature neurons display a similar switch in MCT towards GLUT expression during maturation.
Concluding remarks
The increased levels of the three neuronal transporters, GLUT3, GLUT4 and GLUT8, observed during cerebellar development suggest that these transporters develop a more important role in the mature cerebellum and, as a general rule, in mature neurons. This statement is reinforced by the fact that the granule precursor cells located in the EGL do not produce the three transporters studied herein, but express them as a more mature status when they form the IGL. The expression of the monocarboxylate transporter MCT2 by these cells suggests that they use lactate or ketones when they are immature and that they change the metabolism after migration and differentiation. Moreover, the differential localization of the transporters suggests that they play different roles in the cerebellum, although GLUT4 and GLUT8 could also perform some compensatory or redundant functions.
Acknowledgments
This research was supported by grants from the Cardenal Herrera-CEU University (PRUCH), the Copernicus-Santander Research Program and by grant SAF 2004–00228 from the Spanish Government and the European Regional Development Fund (ERDF/FEDER). The research group is a member of the Network for Cooperative Research on Membrane Transport Proteins (REIT), co-funded by the Spanish Ministry of Education and Science and by the European Regional Development Fund (ERDF) (grant BFU2007-30688-E/BFI). Fellowship support for B.B.-L. from the Generalitat Valenciana is gratefully acknowledged.
References
- Alquier T, Leloup C, Lorsignol A, et al. Translocable glucose transporters in the brain: where are we in 2006? Diabetes. 2006;55(suppl. 2):S131–S138. [Google Scholar]
- Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res. 1999;57:693–705. [PubMed] [Google Scholar]
- Augustin R, Riley J, Moley KH. GLUT8 contains a [DE]XXXL[LI] sorting motif and localizes to a late endosomal/lysosomal compartment. Traffic. 2005;6:1196–1212. doi: 10.1111/j.1600-0854.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–19. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- Brant AM, Jess TJ, Milligan G, et al. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun. 1993;192:1297–1302. doi: 10.1006/bbrc.1993.1557. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Reinhardt RR, Lee WH, et al. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci U S A. 2000;97:10236–10241. doi: 10.1073/pnas.170008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choeiri C, Staines W, Messier C. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience. 2002;111:19–34. doi: 10.1016/s0306-4522(01)00619-4. [DOI] [PubMed] [Google Scholar]
- El Messari S, Leloup C, Quignon M, et al. Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J Comp Neurol. 1998;399:492–512. doi: 10.1002/(sici)1096-9861(19981005)399:4<492::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- El Messari S, Ait-Ikhlef A, Ambroise DH, et al. Expression of insulin-responsive glucose transporter GLUT4 mRNA in the rat brain and spinal cord: an in situ hybridization study. J Chem Neuroanat. 2002;24:225–242. doi: 10.1016/s0891-0618(02)00058-3. [DOI] [PubMed] [Google Scholar]
- Gawlik V, Schmidt S, Scheepers A, et al. Targeted disruption of Slc2a8 (GLUT8) reduces motility and mito-chondrial potential of spermatozoa. Mol Membr Biol. 2008;25:224–235. doi: 10.1080/09687680701855405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart DZ, Leino RL, Borson ND, et al. Localization of glucose transporter GLUT 3 in brain: comparison of rodent and dog using species-specific carboxyl-terminal antisera. Neuroscience. 1995;66:237–246. doi: 10.1016/0306-4522(94)00544-f. [DOI] [PubMed] [Google Scholar]
- Gomez O, Romero A, Terrado J, et al. Differential expression of glucose transporter GLUT8 during mouse spermatogenesis. Reproduction. 2006;131:63–70. doi: 10.1530/rep.1.00750. [DOI] [PubMed] [Google Scholar]
- Gomez O, Ballester B, Romero A, et al. Expression and regulation of insulin and the glucose transporter GLUT8 in the testes of diabetic rats. Horm Metab Res. 2009;41:343–349. doi: 10.1055/s-0028-1128146. [DOI] [PubMed] [Google Scholar]
- Gorovits N, Cui L, Busik JV, et al. Regulation of hepatic GLUT8 expression in normal and diabetic models. Endocrinology. 2003;144:1703–1711. doi: 10.1210/en.2002-220968. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family – from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Ibberson M, Riederer BM, Uldry M, et al. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology. 2002;143:276–284. doi: 10.1210/endo.143.1.8587. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Nikami H, Morimatsu M, et al. Expression and localization of insulin-regulatable glucose transporter (GLUT4) in rat brain. Neurosci Lett. 1996;213:103–106. doi: 10.1016/0304-3940(96)12845-7. [DOI] [PubMed] [Google Scholar]
- Leloup C, Arluison M, Kassis N, et al. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Brain Res Mol Brain Res. 1996;38:45–53. doi: 10.1016/0169-328x(95)00306-d. [DOI] [PubMed] [Google Scholar]
- Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- McCall AL, Van Bueren AM, Moholt-Siebert M, et al. Immunohistochemical localization of the neuron-specific glucose transporter (GLUT3) to neuropil in adult rat brain. Brain Res. 1994;659:292–297. doi: 10.1016/0006-8993(94)90896-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Membrez M, Hummler E, Beermann F, et al. GLUT8 is dispensable for embryonic development but influences hippocampal neurogenesis and heart function. Mol Cell Biol. 2006;26:4268–4276. doi: 10.1128/MCB.00081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu S, Sawa H, Kamada K, et al. Neuron-specific glucose transporter (NSGT): CNS distribution of GLUT3 rat glucose transporter (RGT3) in rat central neurons. FEBS Lett. 1993;334:289–295. doi: 10.1016/0014-5793(93)80697-s. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Pinto AB, Carayannopoulos MO, Hoehn A, et al. Glucose transporter 8 expression and translocation are critical for murine blastocyst survival. Biol Reprod. 2002;66:1729–1733. doi: 10.1095/biolreprod66.6.1729. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Hoskin EK, et al. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J Comp Neurol. 2002;452:103–114. doi: 10.1002/cne.10368. [DOI] [PubMed] [Google Scholar]
- Rafiki A, Boulland JL, Halestrap AP, et al. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Rayner DV, Thomas ME, Trayhurn P. Glucose transporters (GLUTs 1–4) and their mRNAs in regions of the rat brain: insulin-sensitive transporter expression in the cerebellum. Can J Physiol Pharmacol. 1994;72:476–479. doi: 10.1139/y94-069. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Glucose, stress, and hippocampal neuronal vulnerability. Int Rev Neurobiol. 2002;51:289–324. doi: 10.1016/s0074-7742(02)51009-6. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Gorovits N, Hoskin EK, et al. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci U S A. 2001;98:2820–2825. doi: 10.1073/pnas.051629798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Alves SE, et al. GLUT8 glucose transporter is localized to excitatory and inhibitory neurons in the rat hippocampus. Brain Res. 2002;932:129–134. doi: 10.1016/s0006-8993(02)02308-9. [DOI] [PubMed] [Google Scholar]
- Romero A, Terrado J, Brot-Laroche E, et al. Glucose transporter GLUT8 mRNA expression in intestinal Caco-2 cells is regulated by growth and metabolism. Horm Metab Res. 2007;39:62–64. doi: 10.1055/s-2007-957351. [DOI] [PubMed] [Google Scholar]
- Romero A, Gomez O, Terrado J, et al. Expression of GLUT8 in mouse intestine: identification of alternative spliced variants. J Cell Biochem. 2009;106:1068–1078. doi: 10.1002/jcb.22090. [DOI] [PubMed] [Google Scholar]
- Sankar R, Thamotharan S, Shin D, et al. Insulin-responsive glucose transporters – GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res. 2002;107:157–165. doi: 10.1016/s0169-328x(02)00487-4. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Gawlik V, Holter SM, et al. Deletion of glucose transporter GLUT8 in mice increases locomotor activity. Behav Genet. 2008;38:396–406. doi: 10.1007/s10519-008-9208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Joost HG, Schurmann A. GLUT8, the enigmatic intracellular hexose transporter. Am J Physiol Endocrinol Metab. 2009;296:E614–E618. doi: 10.1152/ajpendo.91019.2008. [DOI] [PubMed] [Google Scholar]
- Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75:835–844. doi: 10.1002/jnr.20054. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Dwyer D, Malide D, et al. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Glucose metabolism in the developing brain. Semin Perinatol. 2000;24:107–115. doi: 10.1053/sp.2000.6361. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Clark RR, Koehler-Stec E, et al. Glucose transporter expression in brain: relationship to cerebral glucose utilization. Dev Neurosci. 1998a;20:369–379. doi: 10.1159/000017333. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Koehler-Stec EM, Li K, et al. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res. 1998b;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Wang D, Pascual JM, Yang H, et al. Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol. 2005;57:111–118. doi: 10.1002/ana.20331. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ. Analysis of gene expression in the normal and malignant cerebellum. Recent Prog Horm Res. 2003;58:227–248. doi: 10.1210/rp.58.1.227. [DOI] [PubMed] [Google Scholar]
- Weisova P, Concannon CG, Devocelle M, et al. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer M, Uldry M, Thorens B. GLUT8 subcellular localization and absence of translocation to the plasma membrane in PC12 cells and hippocampal neurons. Endocrinology. 2005;146:4727–4736. doi: 10.1210/en.2005-0668. [DOI] [PubMed] [Google Scholar]
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fung C, Shin D, et al. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2009;15:286–299. doi: 10.1038/mp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]


