Abstract
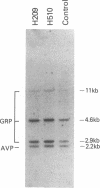
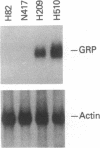
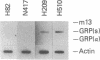
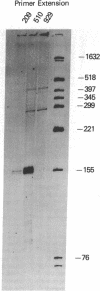
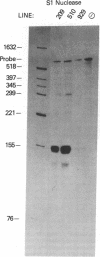
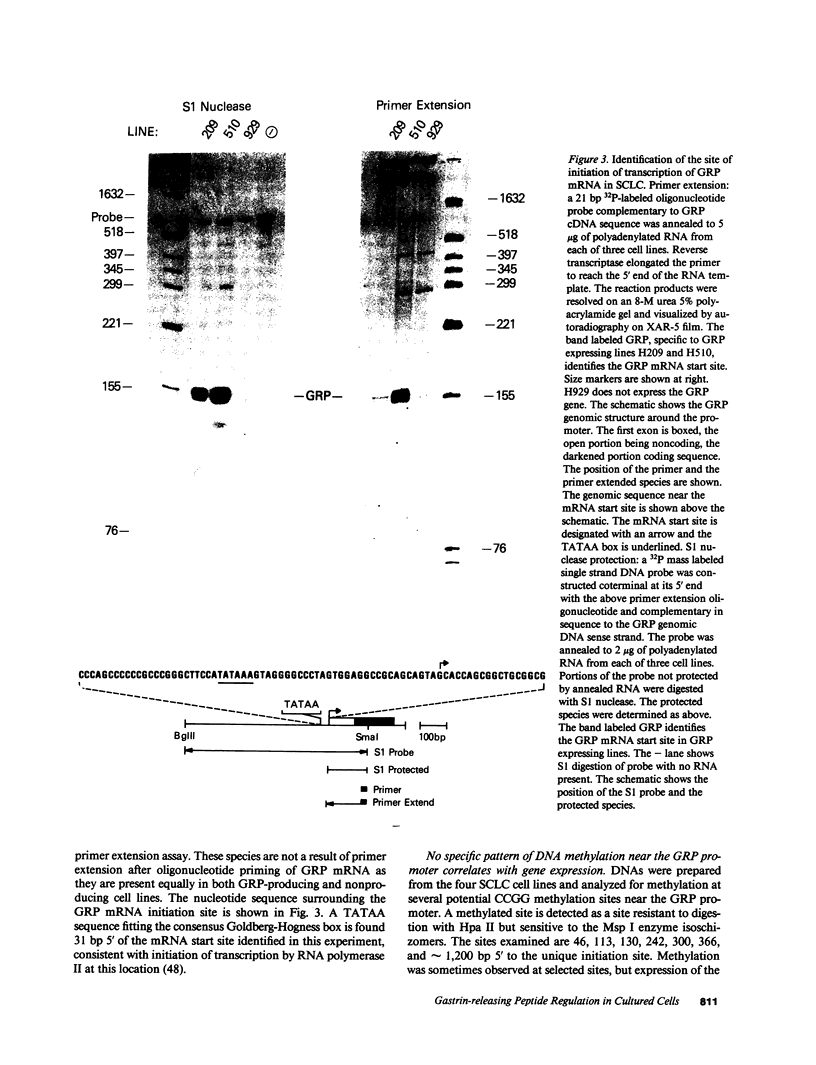
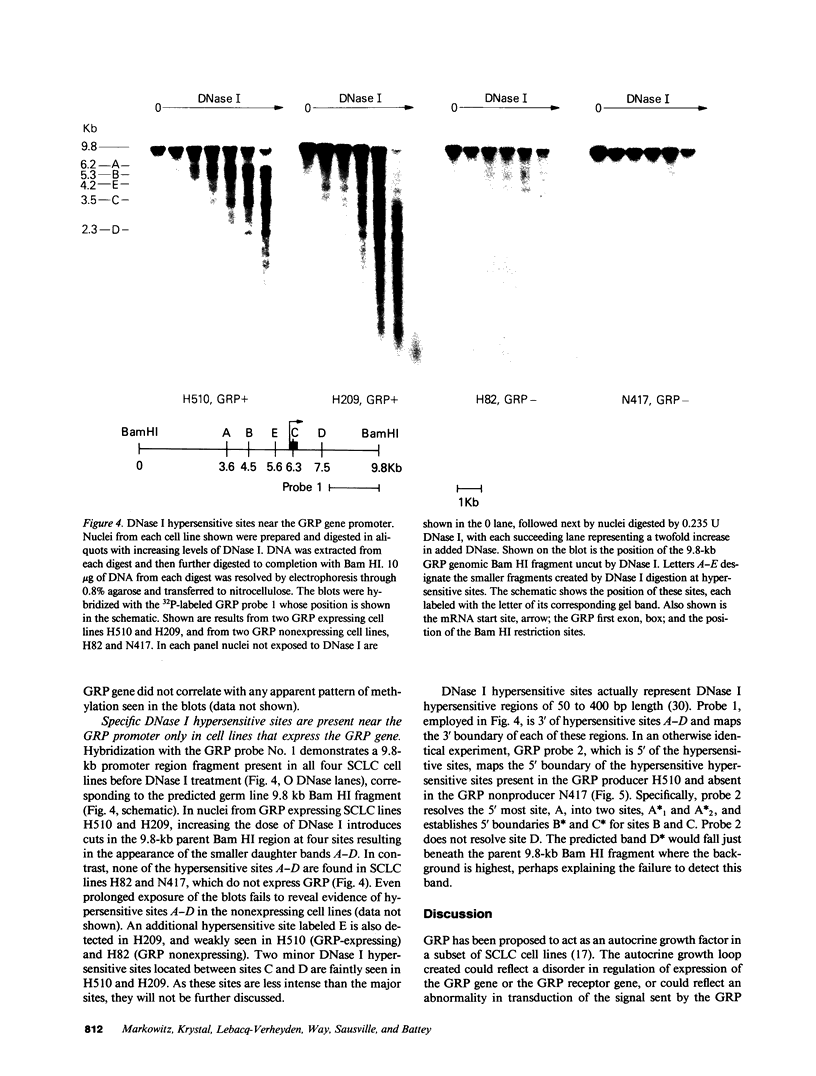
The gastrin-releasing peptide (GRP) is a neuropeptide hormone and growth factor produced normally by neural and neuroendocrine cells, as well as by human small-cell lung cancer (SCLC) tumors and derived cell lines. This study compares the structure of the human prepro-GRP gene in four SCLC cell lines that express variable levels of steady-state GRP mRNA. The regulation of GRP gene expression appears to be at the level of primary transcription based on nuclear run on studies. In the two SCLC cell lines expressing GRP we find a single transcription start site for GRP mRNA, and near this site we find four DNase I hypersensitive sites. These hypersensitive sites are absent in the two cell lines that do not express GRP. The presence of DNase hypersensitive sites in the promoter region of the GRP gene is the structural feature that best correlates with transcriptional activation. These four DNase hypersensitive sites are candidates for cis acting regulatory regions, which may be important in determining the level of transcription of the human prepro GRP gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Benvenisty N., Reshef L. Developmental acquisition of DNase I sensitivity of the phosphoenolpyruvate carboxykinase (GTP) gene in rat liver. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1132–1136. doi: 10.1073/pnas.84.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Brown M., Allen R., Villarreal J., Rivier J., Vale W. Bombesin-like activity: radioimmunologic assessment in biological tissues. Life Sci. 1978 Dec 31;23(27-28):2721–2728. doi: 10.1016/0024-3205(78)90652-5. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Cuttitta F., Moody T. W., Minna J. D. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987 Feb 1;47(3):821–825. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Carr F. E., Need L. R., Chin W. W. Isolation and characterization of the rat thyrotropin beta-subunit gene. Differential regulation of two transcriptional start sites by thyroid hormone. J Biol Chem. 1987 Jan 25;262(3):981–987. [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Eissenberg J. C., Cartwright I. L., Thomas G. H., Elgin S. C. Selected topics in chromatin structure. Annu Rev Genet. 1985;19:485–536. doi: 10.1146/annurev.ge.19.120185.002413. [DOI] [PubMed] [Google Scholar]
- Ghatei M. A., Springall D. R., Nicholl C. G., Polak J. M., Bloom S. R. Gastrin-releasing peptide-like immunoreactivity in medullary thyroid carcinoma. Am J Clin Pathol. 1985 Nov;84(5):581–586. doi: 10.1093/ajcp/84.5.581. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Jackson P. D., Felsenfeld G. A method for mapping intranuclear protein-DNA interactions and its application to a nuclease hypersensitive site. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2296–2300. doi: 10.1073/pnas.82.8.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongstra J., Reudelhuber T. L., Oudet P., Benoist C., Chae C. B., Jeltsch J. M., Mathis D. J., Chambon P. Induction of altered chromatin structures by simian virus 40 enhancer and promoter elements. Nature. 1984 Feb 23;307(5953):708–714. doi: 10.1038/307708a0. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Jörnvall H., Nilsson G., Vagne M., Ghatei M., Bloom S. R., Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B. Bombesin-like peptides in rat brain: quantitation and biochemical characterization. Biochem Biophys Res Commun. 1979 Sep 12;90(1):7–14. doi: 10.1016/0006-291x(79)91582-1. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Jr, Carney D. N., Gazdar A. F., Battey J. F., Sausville E. A., Minna J. D. Human small-cell lung cancers show amplification and expression of the N-myc gene. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1092–1096. doi: 10.1073/pnas.83.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Weber E., Barchas J. D. Distribution of gastrin releasing peptide--bombesin-like immunostaining in rat brain. Brain Res. 1982 Nov 18;251(2):277–282. doi: 10.1016/0006-8993(82)90744-2. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausville E. A., Lebacq-Verheyden A. M., Spindel E. R., Cuttitta F., Gazdar A. F., Battey J. F. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J Biol Chem. 1986 Feb 15;261(5):2451–2457. [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Spindel E. R., Chin W. W., Price J., Rees L. H., Besser G. M., Habener J. F. Cloning and characterization of cDNAs encoding human gastrin-releasing peptide. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5699–5703. doi: 10.1073/pnas.81.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel E. R., Zilberberg M. D., Chin W. W. Analysis of the gene and multiple messenger ribonucleic acids (mRNAs) encoding human gastrin-releasing peptide: alternate RNA splicing occurs in neural and endocrine tissue. Mol Endocrinol. 1987 Mar;1(3):224–232. doi: 10.1210/mend-1-3-224. [DOI] [PubMed] [Google Scholar]
- Tamai S., Kameya T., Yamaguchi K., Yanai N., Abe K., Yanaihara N., Yamazaki H., Kageyama K. Peripheral lung carcinoid tumor producing predominantly gastrin-releasing peptide (GRP). Morphologic and hormonal studies. Cancer. 1983 Jul 15;52(2):273–281. doi: 10.1002/1097-0142(19830715)52:2<273::aid-cncr2820520214>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Wong H. C., Dockray G. J. Bombesin-like peptides in mammals. Fed Proc. 1979 Aug;38(9):2315–2319. [PubMed] [Google Scholar]
- Weber S., Zuckerman J. E., Bostwick D. G., Bensch K. G., Sikic B. I., Raffin T. A. Gastrin releasing peptide is a selective mitogen for small cell lung carcinoma in vitro. J Clin Invest. 1985 Jan;75(1):306–309. doi: 10.1172/JCI111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bloom S. R., Ghatei M. A., Solcia E., Brown M. R., Pearse A. G. Bombesin-like immunoreactivity in the lung. Nature. 1978 Jun 29;273(5665):769–770. doi: 10.1038/273769a0. [DOI] [PubMed] [Google Scholar]
- Willey J. C., Lechner J. F., Harris C. C. Bombesin and the C-terminal tetradecapeptide of gastrin-releasing peptide are growth factors for normal human bronchial epithelial cells. Exp Cell Res. 1984 Jul;153(1):245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]
- Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984 Sep 6;311(5981):81–84. doi: 10.1038/311081a0. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984 May 17;309(5965):229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Abe K., Adachi I., Suzuki M., Kimura S., Kameya T., Yanaihara N. Concomitant production of immunoreactive gastrin-releasing peptide and calcitonin in medullary carcinoma of the thyroid. Metabolism. 1984 Aug;33(8):724–727. doi: 10.1016/0026-0495(84)90212-9. [DOI] [PubMed] [Google Scholar]
- Yang K., Ulich T., Taylor I., Cheng L., Lewin K. J. Pulmonary carcinoids. Immunohistochemical demonstration of brain-gut peptides. Cancer. 1983 Sep 1;52(5):819–823. doi: 10.1002/1097-0142(19830901)52:5<819::aid-cncr2820520512>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]