Abstract
Lithium therapy mainly used in curing some psychiatric diseases responsible for numerous undesirable side effects on different organs in humans. The present study explores the beneficial effect of sobatum, a purified compound of Solanum trilobatum, on lithium carbonate (Li2CO3)-induced multiple organ toxicity in rats. Li2CO3 (150 mg/kg body weight) was administered orally in drinking water for a period of 30 days to induce toxicity in rats. Li2CO3 could induce lipid peroxidation to a significant extent that was accompanied by marked reduction in reduced glutathione, SOD, CAT, GST, GPX activities, and parallel decline in ATP in tissues. Toxicity resulted in abnormal elevation of lipids such as cholesterol, triglycerides, phospholipids, and fatty acids in liver tissues. Treatment with sobatum affords substantial protection in liver and heart by altering all the parameters to near normal levels that were further confirmed by histological examination. Sobatum prevents Li2CO3-induced oxidative damage of DNA by reducing DNA fragmentation indicating its block on cell death. However, these results demonstrated that sobatum has the ability to suppress the drug-induced toxicity.
Keywords: Antioxidants, lithium toxicity, oxidative damage, sobatum, Solanum trilobatum
INTRODUCTION
Lithium is widely used in the treatment of bipolar disorders, refractory depressive disorders, and in the long-term prophylaxis of cluster headache. It has a narrow therapeutic index. Chronic lithium intoxication is more common. There is gradual accumulation of lithium, usually due to decreased excretion. Lithium poisoning causes impaired renal function, drug interactions, volume depletion, and concurrent illnesses such as congestive heart failure or cirrhosis.[1,2] Lithium exerts its effects on a wide range of cellular functions by inhibiting inositol production, affects the protein kinase C signaling pathway, and inhibits glycogen synthase kinase 3 (GSK3).[3] Early recognition and management of lithium-induced organ toxicity can save lives and reduce significant morbidity.
In the Ayurvedic system of medicine, Solanum trilobatum L. (Solanaceae) (common name: Tuduvalai) have been used for respiratory, cardiac, liver, renal, and cancer diseases.[4] Sobatum, alpha-solamarine, solanine, solasodine, glycoalkaloid, diosogenin, and tomatidine are the constituents isolated from this plant.[5] Previous studies in our lab proved that treatment with S. trilobatum extract enhance the recovery from carbon tetrachloride-induced hepatic damage in rats.[6] It was also reported that S. trilobatum possesses anti-inflammatory, anti-ulcerogenic, and antimicrobial activities.[7,8] In our laboratory, sobatum has been isolated and purified to homogeneity from herb S. trilobatum and believed to be an active principle responsible for its beneficial action on vital organs. Earlier studies proved that sobatum has the antitumor property and produce no toxicities.[9] Sobatum also possesses anti-inflammatory and free radical scavenging activities by inhibiting superoxide radical production.[10,11] These points made us interested to do research on sobatum against lithium-induced toxicity. However, no studies have been performed to study whether sobatum have beneficial effects on lithium carbonate (Li2CO3)-induced toxicity in experimental models of organ injury, which may have implications in managing humans with accidental exposures to such compounds.
Considering above information, the present study aims to investigate the effect of sobatum on toxicity caused by Li2CO3 in liver, heart, and kidney tissues of experimental rats through evaluating lipid peroxidative content, hepatic lipid levels, antioxidant enzymes, adenosine triphosphate (ATP) levels, apoptotic cell death, and histological changes.
MATERIALS AND METHODS
Chemicals
All chemicals and reagents were of analytical grade or of highest purity, commercially available. Lithium carbonate, glutathione, disodium phenyl phosphate, and bovine serum albumin were purchased from Sigma Chemical Co., St Louis, MO, USA. EDTA, DNAase, and proteinase K were purchased from British Drug House Pvt. Ltd Glaxo Division, Mumbai, India.
Plant materials and extraction of sobatum
The whole plant of Solanum trilobatum was collected in and around Chennai, India, and authenticated in the Department of Pharmacognosy, Captain Srinivasa Murthi Drug Research Institute for Ayurveda, Chennai, India. A voucher specimen has been deposited in the same institute (No: 1785). The plant samples were shade-dried and powdered using an electric blending machine. For the preparation of the extract, 100 g of powdered plant material was subjected to extraction with 90% petroleum ether in a Soxhlet apparatus up to four cycles. After filtration through Whatman filter paper no. 40, the filtrate was dried using a lyophilizer.
The petroleum ether extract of S. trilobatum was subjected to chromatography using Spherisorb-ODS2 25 cm × 4.6 cm, 5 cm, C18 silica column using water and petroleum ether/ethyl acetate (75:25) as a solvent at a flow rate of 1 ml/min. These fractions were concentrated and crystallized from solvent giving only one pure crystalline compound that was identified and compared with authentic sample. For oral administration, the sobatum compound was dissolved in 0.5 ml of 10% dimethyl sulphoxide (DMSO) immediately before use.
Animals
Adult male albino rats of Wistar strain weighing about 150-200 g were purchased from Fredrick Institute, Padapai, India. The animals were housed at 27 ± 2°C temperature, 55% humidity, and a 12 h-light/dark cycle. They were fed with standard laboratory chow (Hindustan Lever Foods, Bangalore, India) and provided with water ad libitum. Experimental protocols were approved by our Institute ethical committee, which follows the guidelines of Institutional Animals Ethics Committee (IAEC) (360/01/a/CPSEA).
Experimental design
Animals were divided into four groups each containing six animals. Group I animals served as control who received the vehicle (0.5 ml of 10% DMSO) alone orally. Group II animals constituted the toxicity group, who received Li2CO3 (150 mg/kg body weight) orally in drinking water for a period of 30 days.[12] Group III animals received Li2CO3 (150 mg/kg body weight) orally in drinking water for a period of 30 days and treated simultaneously with sobatum orally at doses (30 mg/kg body weight/day)[13] dissolved in 0.5 ml of 10% DMSO. Group IV rats received sobatum orally at doses (30 mg/kg body weight/day) dissolved in 0.5 ml of 10% DMSO.
After 24 h of sobatum last dose treatment, animals were sacrificed by cervical decapitation under pentobarbitone sodium (60 mg/kg) anesthesia and blood was collected without an anticoagulant to separate serum for biochemical investigation. The liver and heart organ were dissected out immediately and washed in ice-cold saline. The tissues were then homogenized in ice-cold Tris-HCl buffer (0.1 M, pH 7.4) and 10% homogenate was obtained and used for the following biochemical investigations.
Determination of lipid peroxidation and reduced glutathione levels
LPO was measured in hepatic homogenates according to the method of Okhawa et al. based on the formation of thiobarbituric acid reactive substances (TBARS) and expressed as the extent of malondialdehyde (MDA) production.[14]
Glutathione (GSH) levels were quantified by the method of Moron et al.(1979).[15] The tissue homogenate was treated with 1 ml of 5% TCA in 1 mM EDTA and centrifuged at 2000 g for 10 min. After that 1 ml of the filtrate was mixed with 5 ml of 0.1 M phosphate buffer (pH 8.0), and 0.4 ml of 5,5’-dithiobis (2-nitrobenzoic acid). The absorbance of the solutions was estimated at 412 nm against blank. The level of ATP in the heart tissue was determined by the method of Ryder.[16]
Estimation of antioxidant enzymes
The activity of superoxide dismutase (SOD) was measured, using an assay based on the ability of SOD to inhibit the autoxidation of pyrogallol by 50%. One unit of enzyme is defined as the amount of enzyme that causes half maximal inhibition of pyrogallol autooxidation/mg protein/min.[17] The assay of catalase was performed by following the method of Aebi. One unit of enzyme activity is defined as the amount of enzyme required to decomposed 1 mmol of H2O2/mg protein/min.[18] The activity of glutathione peroxidase (GPx) was measured using a coupled enzyme assay as described by Lawrence and Burk.[19]
Estimation of lipid profile
Lipid was extracted from liver tissues by the method of Folch et al. using the chloroform-methanol mixture (2:1 v/v).[20] The total cholesterol, free cholesterol, free fatty acids (FFA), triglyceride (TG), phospholipids (PL) after perchloric acid digestion have been determined in liver tissues of sobatum-treated rats.[21–25]
Histopathology
The heart tissue samples were preserved in 10% neutral buffered formalin for 24 h, processed for routine paraffin block preparation, sections of 5μm thick were cut and stained with hematoxylin and eosin. These were examined under the light microscope for histological changes in tissues.
Studies on DNA damage
The kidney tissue was used for the isolation of DNA by homogenizing in PBS-E (50 mM sodium phosphate buffer containing 0.9% saline and 20 mM EDTA, pH 8) and suspended in 2 ml of PBS-E containing 0.5 mg/ ml of collagenase. The suspension was incubated at 37°C for 1 h with stirring, followed by the addition of pronase E (1 mg ml), and further incubated for 15 min at 37°C. It was then centrifuged at 1000 rpm for 5 min. The pellet was dispersed and incubated with 2 ml of a lysis buffer containing 50 mM Tris-HCl−, pH 8, 20 mM EDTA, 10 mM NaCl and 1% w/v SDS for 15 min. It was centrifuged again at 14,000 × g for 15 min, and DNA was isolated from the lysate by a phenol-chloroform extraction procedure.[26] DNA was dissolved in 10 mM Tris-HCl−, pH 8, containing 1 mM EDTA by gentle shaking at 65°C. Residual contaminating RNA was removed by incubating the DNA solution with 1 μg/ml DNase-free RNase at 37°C for 1 h followed by 0.1 mg ml proteinase K for 3 h. Phenol-chloroform extraction was repeated to obtain purified DNA that was dissolved in 10 mM Tris-HCl2 buffer, pH 8, containing 1 mM EDTA. To study DNA fragmentation, DNA was loaded on to a 1.5% agarose gel. In addition to compare the test samples with the standard antioxidant 1 μg of catalase was used and electrophoresis was carried out at 100 V for 1.5 h in TBE (90 mM Tris borate, 2 mM EDTA, pH 8) buffer and DNA was visualized by UV exposure after staining with ethidium bromide.
Statistical analysis
Data are expressed as mean ± SD. The results were computed statistically (SPSS software package, version 11.0) using one-way analysis of variance. Post hoc testing was performed for inter-group comparison using the Dunnett’s ‘t’ test. P < 0.001, P < 0.01, P < 0.05 were considered to be statistically significant.
RESULTS
Compared with controls, lithium-toxicated rats were accompanied by increased TBARS production and decreased antioxidant defense enzyme activities, suggesting oxidative stress in liver tissue [Table 1]. Administration of sobatum led to a marked reduction in the level of TBARS and significant enhancement in antioxidant defense enzyme activities as compared to lithium-treated rats. On the other hand, in sobatum-alone-treated rats, no significant change in enzyme activities could be observed, as compared to that of normal control.
Table 1.
Effect of sobatum on the changes of liverantioxidant defense enzyme activities in Li2CO3 administered rats
| Groups | SOD | CAT | GPx | TBARS | GSH |
|---|---|---|---|---|---|
| I | 8.61±0.45 | 4.81±0.19 | 0.02±0.001 | 0.56±0.04 | 5.98±0.03 |
| II | 6.51±0.32* | 2.98±0.15* | 0.05±0.003* | 1.02±0.09* | 3.99±0.02* |
| III | 8.17±0.35a | 4.01±0.23a | 0.04±0.002a | 0.70±0.05a | 4.82±0.03a |
| IV | 8.64±0.41 | 4.83±0.31 | 0.02±0.001 | 0.54±0.04 | 6.01±0.05 |
Results are expressed as mean±SD for groups of six animals each; SOD: Superoxide dismutase; CAT: Catalase; GPx- Glutathione peroxidase: U/mg protein. TBARS-nmoles/mg protein; GSH-reduced glutathione - μg of GSH/mg protein
Values are expressed as significant difference at *P <0.001 as compared with control group
significant difference at aP <0.001 as compared with Li2CO3 alone administered rats
Li2CO3 caused a significant increase in free cholesterol, total cholesterol, triglycerides, phospholipds, and fatty acid content as compared to the corresponding control group. Sobatum treatment showed a marked protection against this enhanced lipid levels in the liver tissue of Group II rats [Figure 1]. No significant differences were observed between normal and sobatum-treated rats. Lipid levels and other biochemical parameters were not altered upon sobatum treatment.
Figure 1.
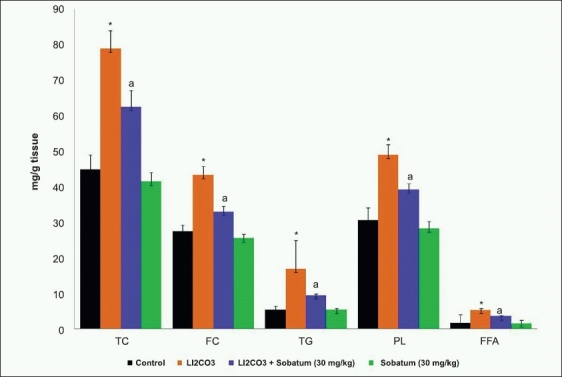
Effect of sobatum on the levels of total cholesterol (TC), free cholesterol (FC), triglyceride (TG), phospholipid (PL) and free fatty acid (FFA) in the liver tissue of Li2CO3 administered rats. Results are expressed as mean ±SD for groups of six animals each. *P<0.001 as compared to control; aP<0.01 as compared to Li2CO3 alone administered rats
Figure 2 shows the level of myocardial ATP content in normal and experimental groups of rats. Significant reduction in the level of ATP content in the heart tissue of lithium-induced rats as compared to that of Group I rats. The administration of sobatum maintained the level of ATP at near normalcy in Group III rats as compared to that of Group II rats, reflecting its ability to maintain the function of the heart mitochondria at near normal status.
Figure 2.
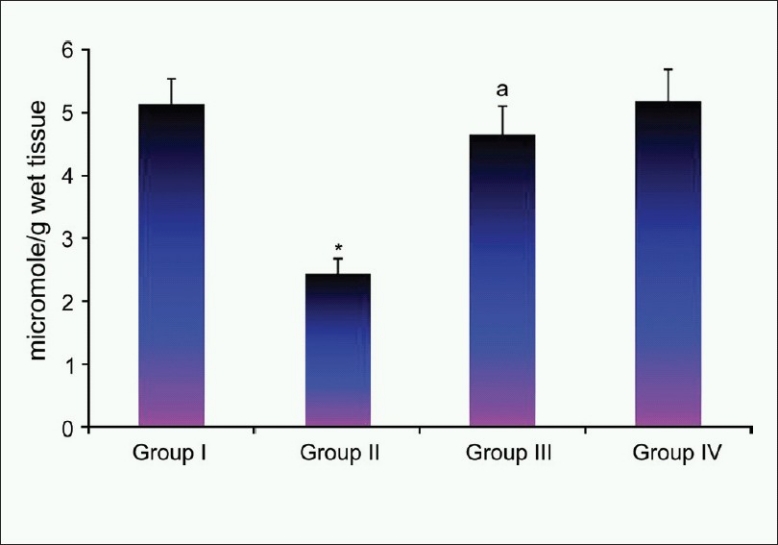
Effect of sobatum level of adenosine triphosphate in heart tissue of control and experimental rats
Microscopic examination of heart tissue of the Group I control rats showed normal myocardial fibers and muscle bundles with normal architecture. Heart tissue of Group II rats showed separation of myocardial fibers with inflammatory mononuclear collections, edema, and myocardial necrosis. Sobatum-alone-treated Group IV rats showed normal myocardial fibers with no pathological changes. Myocardial section of sobatum-treated Group III rats showed slightly separated myocardial fibers with small focus of inflammatory mononuclear collections with the absence of necrotic damage [Figure 3].
Figure 3.
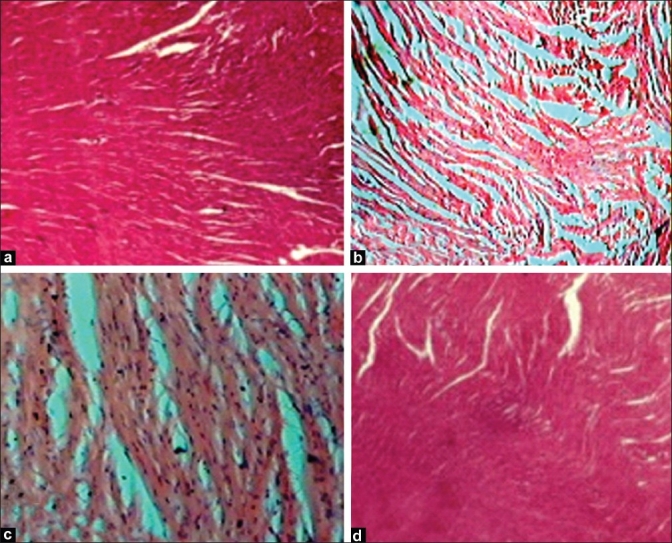
Sections of heart tissue obtained from rats of control groups and treated with sobatum in lithium carbonate-induced rats. (H and E, ×100)
Agarose gel electrophoresis of kidney tissue DNA, treated with or without sobatum in Li2CO3-treated rats is shown in Figure 4. Control rat and sobatum-treated rat showed the absence of DNA fragmentation in kidney tissue, which was observed in Lane 3 and Lane 4. In Lane 1, the toxic effects of Li2CO3 were reflected in a significantly higher rate of apoptosis in kidney tissue of Group II rats so no DNA band was observed. However, in Lane 2, Li2CO3-induced DNA fragmentation was markedly reduced by treatment with sobatum suggesting its role to prevent cell death. Sobatum provides significant protection against DNA damage in comparison with the already known antioxidant, catalase (Lane 5) suggesting its antiapoptotic role during oxidative damage.
Figure 4.
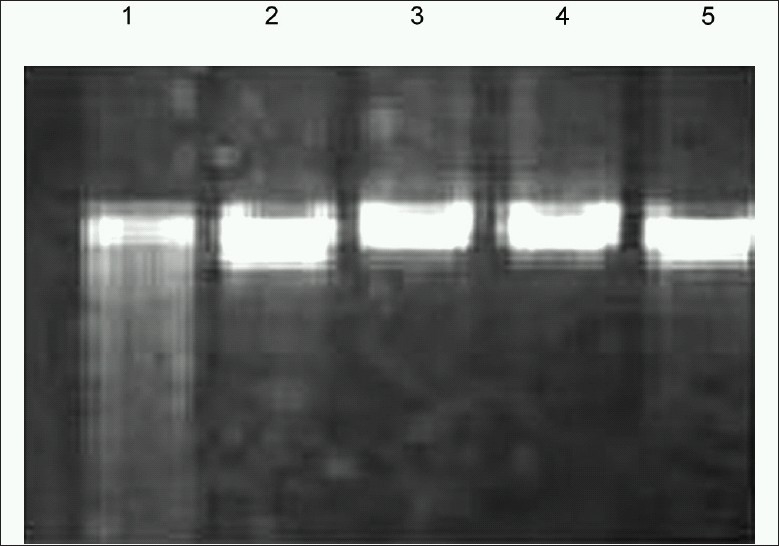
Effect of sobatum on lithium carbonate-induced DNA fragmentation in rats
DISCUSSION
Lithium toxicity represents a state of increased oxidative stress, which is mainly based on the evidence of increased lipid peroxidation (LPO), or by indirect evidence of reduced antioxidant reserve, such as SOD and catalase enzymes, in animal models.[27] Sobatum have been reported to play a beneficial role in the reduction of free radical production.[11] In our present study, the administration of sobatum exerted beneficial effects against lithium toxicity followed by the fall in lipid peroxidation products. During physiological states, SOD metabolizes superoxide anion (O2−), producing hydrogen peroxide (H2O2), which can react with iron to generate highly reactant hydroxyl radicals via the Fenton reaction. CAT is the most important peroxidase in detoxifying excess hydrogen peroxide to prevent hydroxyl production. Thus an increase in SOD or CAT levels per se does not necessarily indicate increased oxidative stress, whereas an imbalance between SOD and CAT activities could lead to an excessive generation of free radicals.[28] The antioxidative defense system like SOD, CAT, and GPx showed lower activities in liver during toxicity condition. Treatment of the lithium-induced animals with sobatum restored the altered activities of SOD, CAT, and GPx. This study suggests that possible mechanism of this protective activity against lithium toxicity may be due to free radical-scavenging and antioxidant activities.[5]
Lithium-induced elevation in lipid levels could be due to increase in biosynthesis and decrease in its utilization. Li2CO3 induces free radicals, which may cause cellular cholesterol accumulation, by increasing cholesterol biosynthesis and its esterification. The significant increase in the rate of TG, PL, and FFA synthesis by the liver is a well-known risk of toxicity caused by Li2CO3.[29] Abnormal activities of lipid-metabolizing enzymes contributed to these hyperlipid changes in liver caused by Li2CO3. S. trilobatum is a hypolipidemic agent, which reduces the rise in total cholesterol, free cholesterol, TG, FFA, and PL.[30] In addition, antioxidants such as sobatum could be beneficial in preventing the increase in lipidemic status, suggesting that it would prevent the toxicity of Li2CO3 on lipid metabolism.
In the heart, ATP is synthesized mainly in the mitochondria through oxidative phosphorylation and transported to the contractile apparatus, where it is consumed by myosin ATPase to generate force. The viability of the cell depends for the most part on the integrity of several vital subcellular systems, and the major source of energy for contraction comes from the oxidative metabolism of mitochondria in the cell. In the present study, administration of sobatum maintained the level of ATP at near normalcy in Group III rats as compared to that of lithium-induced rats, reflecting its ability to maintain the function of the heart mitochondria at near normal status. Many recent evidences indicated that mainly heart damage may result from Li2CO3 intoxication[31] which was evident in the present study by histopathologic findings. Protection against heart damage in sobatum-treated rats showed normal functional cytoarchitecture of heart tissues.
Involvement of ROS, oxidative damage of DNA, and DNA fragmentation has also been evident in apoptotic cell death in renal injury.[32] Cytotoxic effects of Li 2CO3 that affect the liver tissue are manifested by the disturbances of NO, a key mediator of signaling events linked to apoptotic cell death.[33] Sobatum prevents Li2CO3-induced DNA fragmentation, suggesting its antiapoptotic role to block cell death during damage. Sobatum could have a unique capacity to block this oxidative damage similar to that shown by H2O2 scavenger, catalase, indicating its potent antioxidant role to protect DNA from the attack of ROS.
CONCLUSION
The results of the present study indicate that administration of sobatum attenuates lithium-induced organ toxicity in rats through counteraction of free radicals by its antioxidant property, antiapoptotic effect by blocking DNA fragmentation and attenuation of toxic changes in vital organs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Yip KK, Yeung WT. Lithium overdose causing non-convulsive status epilepticus-the importance of lithium levels and the electroencephalography in diagnosis. Hong Kong Med J. 2007;13:471–4. [PubMed] [Google Scholar]
- 2.Warick LH. Lithium poisoning report of a case with neurologic, cardiac and hepatic sequelae. West J Med. 1979;130:259–63. [PMC free article] [PubMed] [Google Scholar]
- 3.Pilcher HR. Drug research: The ups and downs of lithium. Nature. 2003;425:118–20. doi: 10.1038/425118a. [DOI] [PubMed] [Google Scholar]
- 4.Nadkarni KM. 3rd (Eds.) vol.1. Bombay: Popular Prakasan Pvt. Ltd; 1976. Indian Material Medical; pp. 1153–4. [Google Scholar]
- 5.Subramani J, Josekutty PC, Mehta AR, Bhatt PN. Solasodine levels in Solanum sisymbriifolium Lam. Indian J Exp Biol. 1989;27:189. [PubMed] [Google Scholar]
- 6.Shahjahan M, Sabitha KE, Mallika J, Shyamala Devi CS. Effect of Solanum trilobatum in carbon tetrachloride induced hepatotoxicity in rats. Indian J Med Res. 2004;192:94–8. [PubMed] [Google Scholar]
- 7.Swapnalatha P, Kannabiran K. Antimicrobial activity and phytochemicals of Solanum trilobatum Linn. African J Biotech. 2006;5:2402–4. [Google Scholar]
- 8.Emmanuel S, Ignacimuthu S, Perumalsamy R, Amalraj T. Antiinflammatory activity of Solanum trilobatum. Fitoterapia. 2006;77:611–2. doi: 10.1016/j.fitote.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Mohanan PV, Devi KS. Toxicological evaluation of sobatum. Cancer Lett. 1998;127:135–40. doi: 10.1016/s0304-3835(98)00022-6. [DOI] [PubMed] [Google Scholar]
- 10.Mohanan PV, Devi KS. Effect of sobatum on tumour development and chemically induced carcinogenesis. Cancer Lett. 1997;112:219–23. doi: 10.1016/s0304-3835(96)04574-0. [DOI] [PubMed] [Google Scholar]
- 11.Mohanan PV, Rathinam K, Devi KS. Effect of sobatum on ultraviolet-induced damage and superoxide production. Indian J Pharmacol. 1997;29:129–31. [Google Scholar]
- 12.Oktem F, Ozguner F, Sulak O, Olgar S, Akturk O, Yilmaz HR, et al. Lithium-induced renal toxicity in rats: Protection by a novel antioxidant caffeic acid phenethyl ester. Mol Cell Biochem. 2005;277:109–15. doi: 10.1007/s11010-005-5426-5. [DOI] [PubMed] [Google Scholar]
- 13.Vijai M, Mallika J, Subramaniyam S. Proceedings of International Conference on Genetic and Molecular Diagnosis in Modern Medicine: Hyderabad; 2008. Attenuation of brain damage with sobatum in rat brain neuronal cells; pp. 7–9. [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–7. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase reductase and glutathione-S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–72. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 16.Ryder JM. Determination of adenosine triphosphate and its breakdown products in fish muscle by high performance liquid chromatography. J Agri Food Chem. 1985;33:678–80. [Google Scholar]
- 17.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 18.Aebi H, Wyss SR, Scherz B, Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974;48:137–45. doi: 10.1111/j.1432-1033.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–8. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–501. [PubMed] [Google Scholar]
- 21.Parekh AC, Jung DH. Cholesterol determination with ferric acetate-uranyl acetate and sulphuric acid-ferrous sulfate reagents. Anal Chem. 1970;42:1423–7. [Google Scholar]
- 22.Leffler HH, McDougald CH. Estimation of cholesterol in serum by means of improved techniques. Am J Clin Path. 1963;39:311–3. [PubMed] [Google Scholar]
- 23.Rice RW. Triglycerides (neutral fat) in serum. In: Roederick P, Mac-Donald RP, editors. Standard methods of clinical chemistry. New York: Academic Press; 1970. pp. 215–22. [Google Scholar]
- 24.Fiske CV, Subbarrow Y. Colorimetric determination of phosphorous. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 25.Hron WT, Menahan LA. A sensitive method for the determination of free fatty acids in plasma. J Lipid Res. 1981;22:377–9. [PubMed] [Google Scholar]
- 26.Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, et al. Selective inhibition of the induciable nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1998;233:119–22. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 27.Tandon A, Dhawan DK, Nagpaul JP. Effect of lithium on hepatic lipid peroxidation and antioxidative enzymes under different dietary protein regimens. J Appl Toxicol. 1998;18:87–90. doi: 10.1002/(sici)1099-1263(199805/06)18:3<187::aid-jat495>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Andrades M, Ritter C, Moreira JC, Dal-Pizzol F. Oxidative parameters differences during non-lethal and lethal sepsis development. J Surg Res. 2005;125:68–72. doi: 10.1016/j.jss.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Sharma SD, Iqbal M. Lithium induced toxicity in rats: A hematological, biochemical and histopathological study. Biol Pharm Bull. 2005;28:834–7. doi: 10.1248/bpb.28.834. [DOI] [PubMed] [Google Scholar]
- 30.Pillai CK. Materia Medica., India: Ratna Naicker and Sons; 1973. Siddha Vaidya Padhartha Guna Vilakam (Moolavarkam) pp. 428–9. [Google Scholar]
- 31.Lithium carbonate, Eskalith, Lithane, Lithobid Pharmacology. http:/ www. HealthyPlace_com.html [homepage on the Internet]
- 32.Kim SH, Kim YS, Kang SS, Bae K, Hung TM, Lee SM. Anti-apoptotic and hepatoprotective effects of gomisin A on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice. J Pharmacol Sci. 2008;106:225–33. doi: 10.1254/jphs.fp0071738. [DOI] [PubMed] [Google Scholar]
- 33.Tajes M, Gutierrez-Cuesta J, Folch J, Ferrer I, Caballero B, Smith MA, et al. Lithium treatment decreases activities of kinases in a murine model of senescence. J Neuropathol Exp Neurol. 2008;67:12–3. doi: 10.1097/NEN.0b013e3181776293. [DOI] [PubMed] [Google Scholar]


