Abstract
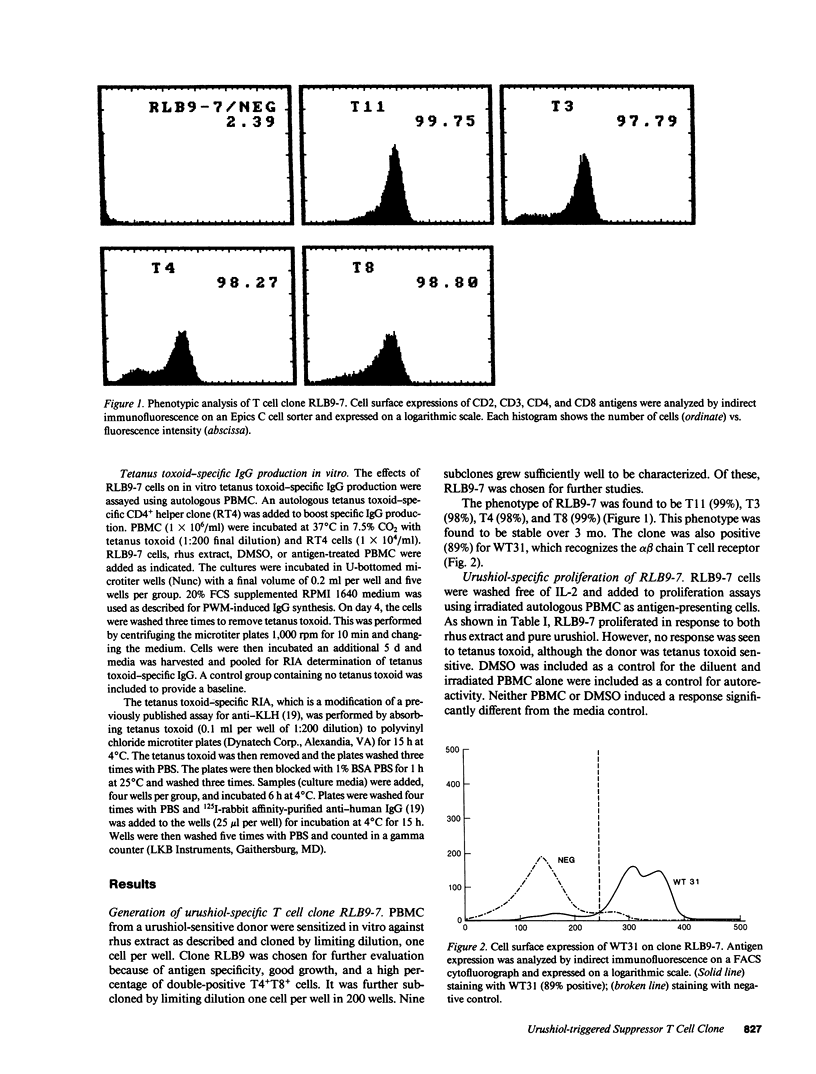
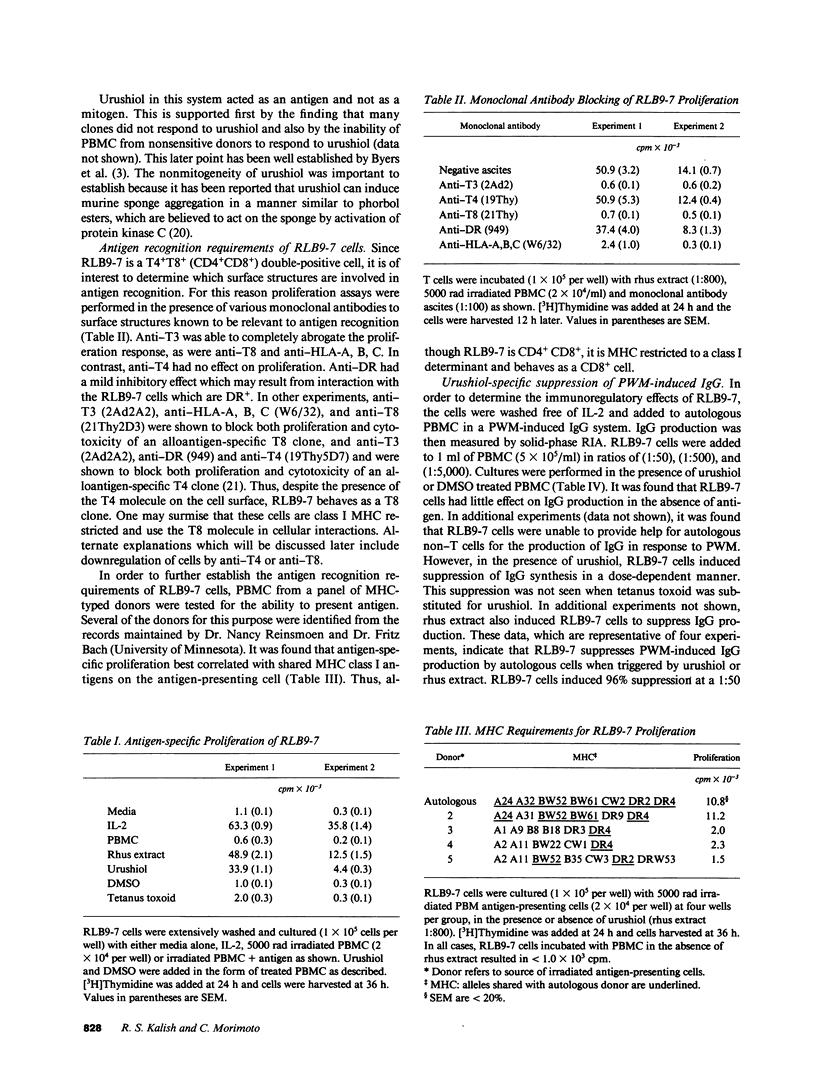
Allergic contact dermatitis to Toxicodendron radicans (poison ivy) is mediated by the hapten urushiol. An urushiol-specific, interleukin 2 (IL-2)-dependent T cell clone (RLB9-7) was generated from the peripheral blood of a patient with a history of allergic contact dermatitis to T. radicans. This clone proliferated specifically to both leaf extract and pure urushiol. Although the clone had the phenotype CD3+CD4+CD8+, proliferation to antigen was blocked by anti-CD8 and anti-HLA-A, B, C, but not by anti-CD4, suggesting that CD4 was not functionally associated with the T cell receptor. Furthermore, studies with antigen-presenting cells from MHC-typed donors indicated that the clone was MHC class 1 restricted. RLB9-7 was WT31 positive, indicating it bears the alpha beta T cell receptor. The clone lacked significant natural killer cell activity and produced only low levels of IL-2 or gamma-interferon upon antigen stimulation. Addition of RLB9-7 to autologous peripheral blood mononuclear cells in the presence of urushiol inhibited the pokeweed mitogen-driven IgG synthesis. This suppression was resistant to irradiation (2,000 rad) and was not seen when RLB9-7 was added to allogeneic cells, even in the presence of irradiated autologous antigen-presenting cells, suggesting that suppression was MHC restricted and not mediated by nonspecific soluble factors. However, RLB9-7 cells in the presence of urushiol inhibited the synthesis of tetanus toxoid-specific IgG by autologous lymphocytes, indicating that the suppression, although triggered specifically by urushiol, was nonspecific.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai S., Yamamoto H., Itoh K., Kumagai K. Suppressive effect of human natural killer cells on pokeweed mitogen-induced B cell differentiation. J Immunol. 1983 Aug;131(2):651–657. [PubMed] [Google Scholar]
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue M. L., Hafler D. A., Daley J. F., Levine H., Craig K. A., Breitmeyer J. B., Schlossman S. F. Regulation of T cell clone function via CD4 and CD8 molecules. Anti-CD4 can mediate two distinct inhibitory activities. J Immunol. 1988 Jan 15;140(2):376–383. [PubMed] [Google Scholar]
- Byers V. S., Epstein W. L., Castagnoli N., Baer H. In vitro studies of poison oak immunity. I. In vitro reaction of human lymphocytes to urushiol. J Clin Invest. 1979 Nov;64(5):1437–1448. doi: 10.1172/JCI109602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonica G. W., Caria M., Bagnasco M., Cosulich M. E., Giordano G., Moretta L. Proliferation of T8-positive cytolytic T lymphocytes in response to thyroglobulin in human autoimmune thyroiditis: analysis of cell interactions and culture requirements. Clin Immunol Immunopathol. 1985 Jul;36(1):40–48. doi: 10.1016/0090-1229(85)90037-6. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Dunn I. S., Liberato D. J., Castagnoli N., Jr, Byers V. S. Influence of chemical reactivity of urushiol-type haptens on sensitization and the induction of tolerance. Cell Immunol. 1986 Jan;97(1):189–196. doi: 10.1016/0008-8749(86)90388-6. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Hercend T., Beveridge R., Schlossman S. F. Characterization of an antigen expressed by human natural killer cells. J Immunol. 1983 Jun;130(6):2947–2951. [PubMed] [Google Scholar]
- Hafler D. A., Benjamin D. S., Burks J., Weiner H. L. Myelin basic protein and proteolipid protein reactivity of brain- and cerebrospinal fluid-derived T cell clones in multiple sclerosis and postinfectious encephalomyelitis. J Immunol. 1987 Jul 1;139(1):68–72. [PubMed] [Google Scholar]
- Hausman P. B., Sherr D. H., Dorf M. E. An in vitro system for the generation of suppressor cells and the requirement for B cells in their induction. J Immunol. 1985 Mar;134(3):1388–1396. [PubMed] [Google Scholar]
- Hoffmann R. M., Pape G. R., Rieber P., Eisenburg J., Döhrmann J., Zachoval R., Paumgartner G., Riethmüller G. Cytolytic T cell clones derived from liver tissue of patients with chronic hepatitis B. Eur J Immunol. 1986 Dec;16(12):1635–1638. doi: 10.1002/eji.1830161227. [DOI] [PubMed] [Google Scholar]
- Jones B., Khavari P. A., Conrad P. J., Janeway C. A., Jr Differential effects of antibodies to Lyt-2 and L3T4 on cytolysis by cloned, Ia-restricted T cells expressing both proteins. J Immunol. 1987 Jul 15;139(2):380–384. [PubMed] [Google Scholar]
- KLIGMAN A. M. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958 Feb;77(2):149–180. doi: 10.1001/archderm.1958.01560020001001. [DOI] [PubMed] [Google Scholar]
- Kapp J. A., Araneo B. A. Antigen-specific suppressor T cell interactions. I. Induction of an MHC-restricted suppressor factor specific for L-glutamic acid50-L-tyrosine50. J Immunol. 1982 Jun;128(6):2447–2452. [PubMed] [Google Scholar]
- Kapp J. A., Sorensen C. M., Pierce C. W. Antigen-specific suppressor T cell interactions. II. Characterization of two different types of suppressor T cell factors specific for L-glutamic acid50-L-tyrosine50 (GT) and L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). J Exp Med. 1983 Dec 1;158(6):1962–1978. doi: 10.1084/jem.158.6.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg M. L., Res P., Bos J. D., Schootemijer A., Teunissen M. B., Van Schooten W. Nickel-specific T lymphocyte clones derived from allergic nickel-contact dermatitis lesions in man: heterogeneity based on requirement of dendritic antigen-presenting cell subsets. Eur J Immunol. 1987 Jun;17(6):861–865. doi: 10.1002/eji.1830170620. [DOI] [PubMed] [Google Scholar]
- Löfström A., Wigzell H. Antigen specific human T cell lines specific for cobalt chloride. Acta Derm Venereol. 1986;66(3):200–206. [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Brenner M. B., Krangel M. S., Duby A. D., Bloom B. R. T-cell receptors of human suppressor cells. Nature. 1987 Oct 8;329(6139):541–545. doi: 10.1038/329541a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Wong L., Fujimiya Y., Chang W. C., Horwitz D. A., Bloom B. R., Rea T. H., Pattengale P. K. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986 Nov 1;137(9):2831–2834. [PubMed] [Google Scholar]
- Morimoto C., Distaso J. A., Borel Y., Schlossman S. F., Reinherz E. L. Communicative interactions between subpopulations of human T lymphocytes required for generation of suppressor effector function in a primary antibody response. J Immunol. 1982 Apr;128(4):1645–1650. [PubMed] [Google Scholar]
- Morimoto C., Distaso J. A., Cheney J. J., Reinherz E. L., Schlossman S. F. Cellular interaction between subsets of T8 population for maximal suppression of antigen-specific antibody response. Cell Immunol. 1984 Oct 1;88(1):75–84. doi: 10.1016/0008-8749(84)90053-4. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Borel Y., Schlossman S. F. Direct demonstration of the human suppressor inducer subset by anti-T cell antibodies. J Immunol. 1983 Jan;130(1):157–161. [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Schlossman S. F. Primary in vitro anti-KLH antibody formation by peripheral blood lymphocytes in man: detection with a radioimmunoassay. J Immunol. 1981 Aug;127(2):514–517. [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Todd R. F., Distaso J. A., Schlossman S. F. Generation of antigen-specific suppressor cells in vitro in man. J Immunol. 1983 Sep;131(3):1209–1213. [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., Klatser P. R., de Vries R. R. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. 1986 Jul 31-Aug 6Nature. 322(6078):462–464. doi: 10.1038/322462a0. [DOI] [PubMed] [Google Scholar]
- Preffer F. I., Colvin R. B., Leary C. P., Boyle L. A., Tuazon T. V., Lazarovits A. I., Cosimi A. B., Kurnick J. T. Two-color flow cytometry and functional analysis of lymphocytes cultured from human renal allografts: identification of a Leu-2+3+ subpopulation. J Immunol. 1986 Nov 1;137(9):2823–2830. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Scheidegger D., Garotta G., Scheper R., Pletscher M., Lanzavecchia A. Isolation and characterization of Ni-specific T cell clones from patients with Ni-contact dermatitis. J Immunol. 1985 Dec;135(6):3929–3932. [PubMed] [Google Scholar]
- Takeuchi T., Rudd C. E., Schlossman S. F., Morimoto C. Induction of suppression following autologous mixed lymphocyte reaction; role of a novel 2H4 antigen. Eur J Immunol. 1987 Jan;17(1):97–103. doi: 10.1002/eji.1830170117. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Meuer S. C., Romain P. L., Schlossman S. F. A monoclonal antibody that blocks class II histocompatibility-related immune interactions. Hum Immunol. 1984 May;10(1):23–40. doi: 10.1016/0198-8859(84)90083-1. [DOI] [PubMed] [Google Scholar]
- Van Seventer G. A., Van Lier R. A., Spits H., Ivanyi P., Melief C. J. Evidence for a regulatory role of the T8 (CD8) antigen in antigen-specific and anti-T3-(CD3)-induced lytic activity of allospecific cytotoxic T lymphocyte clones. Eur J Immunol. 1986 Nov;16(11):1363–1371. doi: 10.1002/eji.1830161109. [DOI] [PubMed] [Google Scholar]
- Wang F., Blaese R. M., Zoon K. C., Tosato G. Suppressor T cell clones from patients with acute Epstein-Barr virus-induced infectious mononucleosis. J Clin Invest. 1987 Jan;79(1):7–14. doi: 10.1172/JCI112810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Azaroff L., Davidson S., Dunham P. Synergy between phorbol esters, 1-oleyl-2-acetylglycerol, urushiol, and calcium ionophore in eliciting aggregation of marine sponge cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2914–2918. doi: 10.1073/pnas.83.9.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]



