Abstract
In this study, we investigated molecular mechanisms underlying low susceptibility to apoptosis induced by the nucleoside analog azidothymidine (AZT) and the role of nuclear factor-κB (NF-κB) activation in these phenomena. A preliminary screening in different cell lines indicated U937 monocytic cell line as suitable to this purpose. Treatment of U937 cells even with suprapharmacological concentrations of AZT induced only moderate levels of apoptosis. Surprisingly, SuperArray analysis showed that AZT induced the transcriptional activity of both pro- and anti-apoptotic genes. Interestingly, moreover, several genes upregulated by AZT were NF-κB related. In fact, AZT, after an initial inhibition of NF-κB activation with respect to control, induced a transient, but consistent, increase in NF-κB-binding activity. Inhibition of NF-κB activation in U937 cells, stably transfected with a dominant-negative IκBα or by pharmacological treatment, sensitized them to apoptosis induced by AZT and impaired the upregulation of anti-apoptotic genes in response to AZT treatment, with respect to control cells. These results indicate that NF-κB activation by AZT has a role in protecting target cells from apoptotic cell death, improving our understanding of the toxicology and the therapeutic usage of this drug.
Keywords: apoptosis, AZT, DNA damage, NF-κB, chemotherapy
Azidothymidine (3′-azido-3′-deoxythymidine, AZT) was the first anti-HIV compound introduced in clinics in 1987.1 This drug is considered the prototype of a class of anti-retrovirals (ARV), sharing the common nucleoside/nucleotide structure and the mechanism of action.2 After its failure as an anti-HIV monotherapeutic agent, AZT became one of the pillars of the combination therapy strategy with three or more ARV in HIV-infected adults and for preventing HIV infection in infants by antepartum, intrapartum and postpartum regimens. Particularly, lower cost connected with patent expiration in 2004 and availability of fixed-dose formulations in which AZT is combined with other ARV, greatly favors AZT usage in resource-limited countries, where access to ARV therapy exponentially grew in the recent years.3
Although benefits associated with AZT-based anti-HIV treatment are unequivocal, a series of severe adverse reactions, including bone marrow suppression, were observed in patients after prolonged assumption of the drug since the beginning of the era of ARV therapy.4 In particular, more recent studies identified cytotoxic, genotoxic and teratogenetic potentials of AZT as a main possible cause of undesirable pathological consequences in adults and infants.5, 6, 7, 8 Regarding cytotoxicity, AZT was shown to cause cell cycle arrest in S phase by directly targeting cell polymerases or proteins involved in cell cycle regulation.9, 10, 11 Checkpoint-arrested cells are known to switch on repair mechanisms,12 which potentially generate mutations. This could link the cytotoxic with the mutagenic activity of AZT.13 Alternatively, DNA damage could trigger apoptosis.14 In fact, AZT has been reported to induce apoptosis15 and this form of programmed cell death has been hypothesized to have a role in AZT-induced pathologies.16 However, no conclusive evidence exists supporting a noticeable pro-apoptotic activity of AZT and molecular mechanisms involved. In particular, our recent results highlighted that acute treatment of peripheral blood mononuclear cells with AZT in vitro induced a remarkable expression/activation of caspases, although in the absence of a reliable induction of apoptosis.17 Our hypothesis was that the apparent resistance to cell death was owed to a sort of ‘precommitment' to apoptosis by AZT, lacking sufficient downstream apoptotic signals. In fact, the lack of final commitment to apoptosis could explain the genotoxic/teratogenetic/ pro-carcinogenic effects of AZT.5, 6, 7, 18
One of the main systems involved in the resistance to chemotherapy-induced apoptosis is the nuclear factor-κB (NF-κB) signaling pathway.19, 20 The NF-κB system has a key role as regulator of inflammations, cell proliferation and of early pathogen response.21, 22 Transcriptional regulation of NF-κB generally promotes survival and protects cells from apoptosis by inducing expression of genes encoding anti-apoptotic proteins.23, 24 However, NF-κB has been shown to be endowed with a contradictory role in cell death and tumorigenesis,25, 26 controlling the expression of both pro-apoptotic and anti-apoptotic genes.27, 28
In this context, the primary aim of this study was to investigate molecular mechanisms underlying susceptibility to AZT-induced apoptosis to ameliorate our understanding of the toxicology and the therapeutic usage of this drug. Surprisingly, we found, using monocytic U937 cells as an experimental model, that AZT activated the transcription of both pro-apoptotic and anti-apoptotic genes belonging to the NF-κB-dependent pathway. Moreover, results demonstrated that inhibition of NF-κB activation significantly enhanced the apoptotic response to AZT in U937 cells.
Results
AZT induces apoptotic cell death in U937 cells only at suprapharmacological concentrations
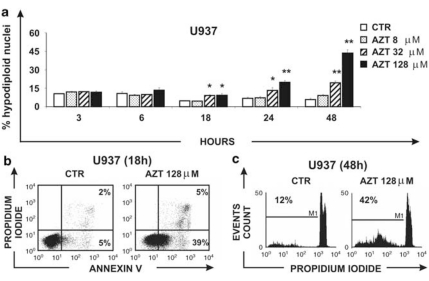
A preliminary screening in different cell lines of lymphocytic/monocytic origin showed that most of the cells were quite resistant to AZT-induced apoptosis, even when challenged at suprapharmacological concentrations of the drug. Among the others, however, U937 monocytic cells showed the best dose- and time-dependent response to apoptosis induction when exposed to AZT in vitro. Figure 1a shows the time course of the dose-effect study. Susceptibility to apoptosis was measured in U937 cells treated with vehicle, 8, 32 and 128 μM AZT at different times by flow cytometry analysis of hypodiploid events referring to propidium iodide (PI)-stained nuclei. The in vitro concentration of 8 μM AZT approximately corresponds to the pharmacological level utilized in ARV therapy in vivo, although higher concentrations of the drug were used as suprapharmacological concentrations. The results are the mean values±S.E. obtained from three independent experiments. No significant increase in apoptosis was observed after 3 and 6 h in culture, whereas after 18 h of treatment with AZT, U937 cells showed significantly increased levels of apoptosis in comparison with control samples only at 32 μM AZT and 128 μM (P=0.042 and P=0.034, respectively). A more significant increase in hypodiploid events in comparison with control samples occurred at 24 h (CTR versus 32 μM AZT, P=0.033; CTR versus 128 μM AZT, P<0.001) and 48 h (32 μM AZT and 128 μM AZT versus CTR, P<0.001) after AZT addition. However, even in the best pro-apoptotic conditions found (128 μM AZT, 48 h), only a minority of the cells (<45%) underwent apoptosis. To confirm the results with another method of apoptosis detection and to further characterize the features of cell death induced by AZT, the percentage of annexin-V+ and PI+ U937 cells, treated with the highest suprapharmacological concentration of AZT, was evaluated by two-fluorescence flow cytometry analysis (Figure 1b). After 18 h of treatment, results showed 5% annexin-V+PI+ (late apoptotic/necrotic) cells and 39% annexin-V+/PI− (early apoptotic) cells, whereas the control sample showed 2% annexin-V+PI+ cells and 5% annexin-V+/PI− cells, respectively. After 48 h of treatment with AZT, evaluation of apoptosis in the same experiment by means of hypodiploid nuclei counting showed 42% of positive events versus 12% of positive events in the control sample (Figure 1c). Thus, percentage of early apoptotic cells at 18 h, using the annexin-V/PI method (Figure 1b), was very similar to percentage hypodiploid apoptotic events observed later on at 48 h in the same sample (Figure 1c). These data indicate that cell death induced in U937 cells by AZT occurred mainly, if not exclusively, by apoptosis. On the basis of these results, U937 cells were selected as a suitable experimental model to further investigate mechanisms involved in AZT-induced sensitivity/resistance to apoptotic cell death.
Figure 1.
Effect of AZT on apoptosis in U937 cell. (a) U937 cells were treated with 0, 8, 32, 128 μM AZT for 3, 6, 18, 24 and 48 h and apoptosis was evaluated by hypodiploid nuclei analysis after DNA staining with PI. Apoptosis was evaluated by flow cytometry analysis of hypodiploid nuclei and expressed as mean values ± S.E. obtained from three independent experiments; asterisk indicates a significant difference versus CTR (*P<0.05; **P<0.010). (b) FACS analysis of annexin-V and PI in U937 cells treated with vehicle or 128 μM AZT, after 18 h in culture. (c) Percentage of hypodiploid nuclei in U937 cells treated with vehicle or 128 μM AZT, after 48 h in culture. In Figure 1b and c one representative out of the three independent experiments performed is shown
AZT modulates NF-κB activation in U937 cells
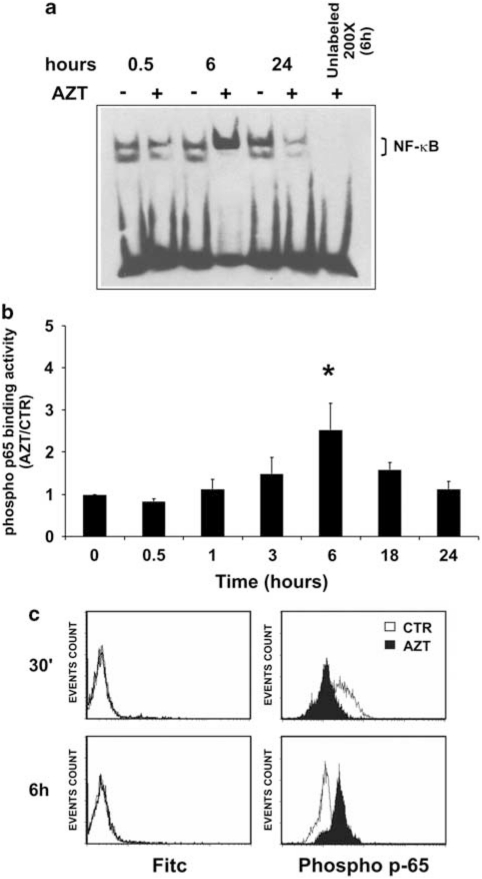
To investigate mechanisms underlying scarce sensitivity to AZT-induced apoptosis, we investigated transcriptional levels of hundred genes in response to AZT treatment in U937 cells through SuperArray analysis following exposure to 128 μM AZT for 18 h. In addition to apoptosis-related genes, also the transcriptional levels of genes involved in the cell cycle and DNA-repair pathways were evaluated. The results, shown as Supplementary materials (Supplementary Table S1), indicated a number of genes for which transcriptional levels were considered as significantly modified. As expected, mRNA levels for some caspase genes and other genes that can be classified as pro-apoptotic were found to be upregulated. Surprisingly, however, SuperArray analysis showed that AZT induced also the transcriptional activity of some anti-apoptotic genes. Interestingly, several genes whose expression was shown to be modulated by AZT were found to be involved in the NF-κB pathway. However, AZT was reported to presumably inhibit NF-κB activation.29, 30 We, then, verified the capability of AZT to directly affect the activation of the NF-κB complex in our experimental conditions. For this purpose, first U937 cells were either exposed to vehicle or treated with 128 μM AZT and, at different times after treatment, NF-κB activation was assayed in nuclear lysates from experimental samples by revealing the DNA-binding activity using non-radioactive electromobility shift assay (EMSA). As shown in Figure 2a, comparison of the bands visualized from control and treated samples at corresponding times of incubation indicated, in agreement with results from other authors, the inhibition of NF-κB-binding activity early after exposure to the drug and in the late time, that is, at 0.5 and 24 h after treatment. However, unexpectedly, a noticeable rise of the NF-κB complex-binding activity consistently occurred at 6 h of treatment. Addition of the unlabeled κB DNA probe to the 6 h sample completely abolished the band visualization, indicating its specificity for NF-κB (Figure 2a). Experiments with a shorter time interval did not add information on the kinetics of this activation, confirming a single peak at 6 h after treatment (data not shown). Moreover, supershift experiments using specific antibodies to p50 and to p65 NF-κB subunits indicated the involvement of both p65 and p50 subunits in the activated complex (Supplementary Figure S1). These data suggested that AZT induced a triphasic response in NF-κB activation.
Figure 2.
Effect of AZT on NF-κB activation. (a) U937 cells were either treated with vehicle or treated with 128 μM AZT and after 0.5, 6 and 24 h, NF-κB complex activation was assayed by non-radioactive EMSA. Specificity of signal was assessed in one sample (AZT 6 h) by addition of a 200x times excess of unlabeled κB-specific DNA (unlabeled 200x) during the binding reaction. A representative experiment out of the three performed with similar results is shown. (b) U937 cells were either treated with vehicle or treated with 128 μM AZT and after 0.5, 1, 3, 6, 18, 24 h, NF-κB activation was quantitatively measured by assaying the DNA-binding activity of the phosphorylated p65 protein by ELISA. The results are the means from three independent experiments performed on U937 cells and have been expressed as ratio of p65-binding activity in AZT-treated cell versus control samples at each time of the kinetics (AZT/CTR); asterisk indicates a significant difference versus time 0 (*P<0.05). (c) Expression of phosphorylated p65 was examined by flow cytometry in fixed U937 cells stained with a FITC-conjugated anti-rabbit IgG and rabbit anti-phospho-p65 plus goat FITC-conjugated anti-rabbit IgG 30 min (upper panels) and 6 h (lower panels) after treatment with 128 μM AZT (filled peak). The results are one representative of three experiments performed
However, non-radioactive EMSA could not be considered a quantitative method for detecting NF-κB activation. Thus, to confirm and quantitatively characterize the observed phenomenon, NF-κB activation in U937 cells, either treated with vehicle or with 128 μM AZT, was measured in cell nuclear lysates by assaying the DNA-binding activity of phosphorylated p65 at 0.5, 1, 3, 6, 18 and 24 h. The results, shown in Figure 2b, are expressed as the ratio of the DNA-binding activity of phosphorylated p65 in AZT-treated cells versus that evaluated in the corresponding control samples (AZT/CTR). After 30 minutes of treatment with AZT, an early slight inhibition of the AZT/CTR ratio was observed. Then, a progressive increase in the AZT/CTR DNA-binding activity occurred with a raised peak after 6 h of treatment (P=0.033 in comparison with time 0). The AZT/CTR DNA-binding activity of p65 decreased remarkably in the successive 18 and 24 h time points. These results clearly confirmed and improved those observed by EMSA. To further investigate the ability of AZT to directly modulate p65, the expression of phosphorylated p65 was assayed by MAb staining and flow cytometry analysis in fixed U937 cells at 0.5 and 6 h after treatment with 128 μM AZT. After 0.5 h, cells treated with 128 μM AZT showed a lower phospho-p65 expression with respect to the control sample (Figure 2d, upper right panel, filled peak versus open peak, respectively). In contrast, after 6 h of treatment with AZT, a well-defined shift of the peak toward the right side confirmed the increased p65 expression in drug-treated cells in comparison with control cells (Figure 2d, lower right panel, filled peak versus open peak, respectively). Fluorescein isothiocyanate (FITC)-only stained U937 cells did not exhibit any shift in the fluorescence peak (Figure 2d, left panels). Taken together, these data show that AZT actually induced a remarkable peak of NF-κB activation at 6 h after treatment, as demonstrated using three different techniques.
AZT-induced apoptosis is enhanced in U937 cells in which NF-κB activation is impaired by stable transfection with a dominant negative IκBα and is dependent on protein neo-synthesis
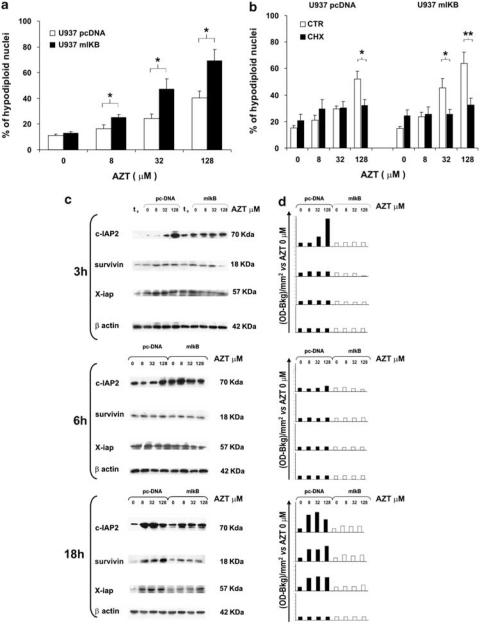
Having demonstrated that AZT induced a consistent, even if transient, increase in NF-κB DNA-binding activity, we wanted to further investigate the role of this phenomenon in AZT-induced apoptosis. For this purpose, experiments were carried out in U937 cells in which NF-κB activation was impaired by stable transfection with a dominant negative murine IκBα (U937-mIκB), previously generated for other purpose in our laboratory.31 Susceptibility to apoptosis was measured in both U937-mIκB and U937 cells transfected with the empty vector (U937-pcDNA) after 48 h of treatment with 0, 8, 32 and 128 μM AZT. The results in Figure 3a show that the percentage of hypodiploid nuclei was consistently higher in U937-mIκB cells (25, 46 and 69%) than in U937-pcDNA cells (16, 24 and 40%) at 8, 32 and 128 μM AZT, respectively. The differences between U937-mIKB values and those of control U937-pcDNA were statistically significant at all the drug concentrations assayed (8 μM AZT, P=0.042; 32 μM AZT, P=0.036; 128 μM AZT, P=0.020). Moreover, the percentage of hypodiploid nuclei in U937-mIκB cells treated with 32 and 128 μM AZT was significantly higher with respect to the control (P≤0.001), whereas in U937-pcDNA cells, only the treatment with 128 μM AZT induced a significant increase in percentage of hypodiploid nuclei in comparison with vehicle-treated cells (P=0.004). Thus, inhibition of NF-κB activation rendered target cells remarkably more susceptible to AZT-induced cell death. To assess how important was the neo-synthesis of pro-apoptotic or anti-apoptotic proteins in the observed phenomenon, U937-pcDNA and U937-mIκB cells were incubated in the presence or in the absence of 0.1 μg/ml cycloheximide (CHX) before the addition of 8, 32 and 128 μM AZT. The susceptibility to apoptosis was measured after 48 h (Figure 3b). U937-pcDNA cells treated with CHX underwent apoptosis at significantly lower level than the control counterpart at the concentration of 128 μM AZT (P=0.027). Also, U937-mIκB cells treated with CHX were susceptible to apoptosis at significant lower level in the presence of 32 and 128 μM AZT (32 μM, P=0.036; 128 μM AZT, P=0.009). These results indicated that neo-synthesized proteins fundamentally contributed in determining apoptosis levels in AZT-treated U937 cells independently on NF-κB activation.
Figure 3.
Enhancement of AZT-induced apoptosis in U937 cells stably transfected with a dominant negative IκBα and its dependency on protein synthesis and association with failure of regulation of anti-apoptotic proteins. (a) U937-mIKB cells knock out for NF-κB (filled column) and their control counterpart U937-pcDNA (empty column) were treated with 0, 8, 32 and 128 μM AZT for 48 h. Apoptosis was evaluated by flow cytometry analysis of hypodiploid nuclei and expressed as mean values ± S.E. obtained from three independent experiments; asterisks indicate significant difference between the two cell lines (*P<0.05 or **P<0.010). (b) U937-pcDNA and U937-mIκB cells were incubated in the presence (filled column) or in the absence (empty column) of an inhibitor of protein synthesis, the CHX, at 0.1 μg/ml, before addition of 8, 32 and 128 μM AZT and susceptibility to apoptosis was measured after 48 h. Data are the means of hypodiploid nuclei ± S.E. from three experiments carried on in the same culture conditions. (c) U937-mIκB and U937-pcDNA cells were treated with 0, 8, 32 and 128 μM AZT. Expression of c-IAP, survivin and X-IAP was assessed by western blot analysis before (t0) and after 3, 6 and 18 h of treatment with AZT. One experiment representative of the three independent experiments performed is shown. β-actin expression, run on the same gel of X-IAP, indicates that an equal amount of protein was loaded for each sample. (d) Western blot analysis was quantified by densitometry analysis and values are expressed as OD-B kg /mm2 with respect to vehicle
AZT upregulates the expression of anti-apoptotic proteins in U937-pcDNA but not in U937-mIκB cells
Results of previous experiments indicated that the downregulation of NF-κB signaling rendered AZT-treated cells more prone to undergo apoptosis, with respect to the NF-κB competent control cells. We then hypothesized that the reason for this might lie in the fact that impairment of NF-κB activation in AZT-treated U937-mIκB inhibited the upregulation of some NF-κB-dependent anti-apoptotic proteins by AZT, as predicted by SuperArray analysis. To test this hypothesis, we investigated the dose- and time-dependent expression of some NF-κB-dependent anti-apoptotic proteins in U937-pcDNA and in U937-mIκB transfectants. Proteins of the IAP family, such as c-IAP2, X-IAP and survivin, were assayed by western blot analysis before (t0) and after 3, 6 and 18 h of treatment with 8, 32 and 128 μM AZT. Figure 3c and d report immunoblot visualization and densitometry analysis, respectively, from one representative experiment out of the three performed with similar results. Results show that c-IAP2 expression increased in AZT-treated U937-pcDNA cells with respect to the control at 3 h after treatment with the higher concentration, as well as with all the concentrations assayed at 18 h. Conversely, practically identical levels of c-IAP2 expression were detected, independently of the AZT concentration and the time of observation, in U937-mIκB cells. In U937-pcDNA cells, the survivin expression increased at 18 h after addition of 8, 32 and 128 μM AZT with respect to the control, whereas in U937-mIκB cells, survivin expression was not modulated at all by AZT. Similarly, also the expression of X-IAP in AZT-treated U937-pcDNA cells showed a peak after 18 h in culture. Essentially, no evident upregulation of X-IAP was observed in U937-mIκB cells after AZT treatment at all times assayed. These data confirmed at protein level the increased transcriptional expression of some anti-apoptotic genes by AZT and demonstrated that NF-κB activation was, at least in part, responsible for this upregulation.
Treatment with a pharmacological inhibitor of NF-κB enhances the susceptibility to AZT-induced cell death in U937 cells
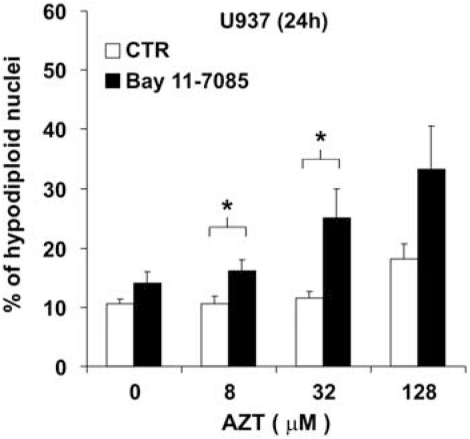
The above reported data indicated that AZT directly affect NF-κB activation and that this activation could, in turn, control the apoptotic response to AZT. We then investigated whether the combination of a pharmacological inhibitor of NF-κB with AZT could affect drug-induced cell death. To this purpose, U937 cells were treated with AZT in the absence or in the presence of the chemical inhibitor of NF-κB, Bay 11–7085, at a concentration of 1 μM. This concentration was considered to be suitable for a combination treatment with AZT on the basis of preliminary dose-dependent experiments which showed that Bay 11–7085 at 1 μM did not induce significant apoptosis by itself on U937 cells (data not shown). Figure 4 shows that the co-treatment with Bay 11–7085 significantly increased the susceptibility of U937 cells to apoptosis already at 24 h after addition of 8, 32 and 128 μM AZT, in comparison with cells which were not incubated with the NF-κB inhibitor, in a dose-dependent manner. Moreover, combination treatment with Bay 11–7085 and 8 or 32 μM AZT significantly enhanced the susceptibility of U937 cells to apoptosis in comparison with the AZT-treated control cells (8 μM AZT, P=0.032; 32 μM AZT, P=0.039). Combination treatment with Bay 11–7085 and 128 μM AZT induced an increased level of apoptosis with respect to AZT alone, but the difference was not statistically significant. Thus, the combination of low, non-toxic concentrations of both AZT and a pharmacological NF-κB inhibitor sensitized U937 cells to apoptotic cell death.
Figure 4.
Increase in susceptibility to AZT-induced cell death by pharmacological inhibition of NF-κB. U937 cells were treated for 24 h with vehicle or 8, 32, 128 μM AZT in the absence (open column) or in the presence (filled column) of a chemical inhibitor of NF-κB, Bay 11–7085, at a concentration of 1 μM. The means±S.E. of hypodiploid nuclei percentages from three independent experiments are shown; asterisks indicate significant differences versus control samples at each concentration of AZT (*P<0.05)
Discussion
In this study, we addressed the issue of mechanisms underlying potential pathological effects of AZT-based therapy and the possible roles of apoptosis modulation and NF-κB activation in these adverse effects. Having observed, using U937 cells as an experimental model, that AZT induced apoptotic cell death only at relatively high concentration of 128 μM, after 48 h in culture and in a minority of the cell population, the unexpected, novel finding of this first part of our study was that in the same experimental conditions, we found the upregulation of both pro-apoptotic and anti-apoptotic gene transcripts. Thus, variability in the balance of upregulated pro-apoptotic and anti-apoptotic gene products could account for the variable susceptibility or resistance to AZT-induced apoptosis observed at different drug concentrations as well as, at the same drug concentration, at individual cell level. In this context, it is worthy to also note that neo-synthesis of proteins was necessary for induction of significant levels of apoptosis by AZT and that the kinetics of the effect was characterized by a delayed induction of apoptosis, with maximal values at 48 h. Moreover, we must take into consideration that SuperArray analysis revealed that transcripts for the DNA-repair genes, GADD45A, MDM2 and ATM, were upregulated by AZT (Supplementary Table S1). Upregulation of GADD45A and MDM2 transcripts by AZT was also confirmed at protein level (Supplementary Figure S2). Thus, the general picture which emerges from these results suggests that the appearance of apoptotic cell death in part of the cells following exposure to high concentration of AZT could be the final consequence of the cell response to DNA damage. In addition, lack of functional p53 protein in U937 cells excludes the involvement of this functional protein in the observed phenomena.
In this context, we were particularly attracted by the fact that treatment of U937 cells with AZT was associated with the transcriptional activation of several genes related to the NF-κB survival pathway. Therefore, we investigated whether this observation was associated with a direct capacity of AZT to modulate the NF-κB activation process. In fact, we demonstrated that AZT induced an early and transitory NF-κB inhibition, as demonstrated by different techniques. Surprisingly, however, a longer observation revealed that after 6 h of treatment, a remarkable activation of NF-κB could be consistently detected in all experiments. These data are apparently in contrast with studies sustaining that the AZT-induced apoptosis in B-cell malignancy occurred through inhibition of NF-κB activation.29, 30 However, we noted that in one of these studies, actually, after an initial inhibition, the NF-κB-binding activity of AZT-treated samples was increased in comparison with that of control samples.30
Interestingly, our study ascertained that the NF-κB system is actually able to affect the susceptibility to AZT-induced apoptosis. Experiments carried out in dominant negative murine IκBα U937 cells revealed that the impairment of the machinery of NF-κB activation remarkably increased the susceptibility of U937 cells to AZT-induced apoptosis. Coherently, U937-mIκB cells were unable to upregulate anti-apoptotic genes upon treatment with AZT. Therefore, our results fundamentally agree with previous reports from our and other laboratories showing that AZT has a negligible pro-apoptotic effect, but also indicate for the first time a possible explanation for this observation. In fact, the demonstration that AZT by itself induces a consistent NF-κB activation, following an initial inhibition, could be the reason why an anti-apoptotic response, rather then a pro-apoptotic response, tends to prevail in some conditions following AZT treatment. However, at the same time, results of our study suppose that a biological or pharmacological condition associated with inhibition of NF-κB activation could considerably increase the cytotoxic potential of the drug. This should be taken into particular consideration when treating pregnant women for additional risk of toxic effects to fetus or newborn. The other side of the medal is that our findings suggest that a combination treatment with NF-κB inhibitors should be considered in case the therapeutic goal would be represented by a decreased risk of genotoxic/mutagenic response to AZT treatment. The task of such a combination therapy with AZT and NF-κB inhibitors could be to route DNA-damaging events toward induction of apoptosis, tipping the balance toward death rather than toward accumulation of gene mutations to decrease the mutagenic/transforming potential of AZT. This should be taken into consideration when treating HIV+ patients at high risk to develop cancer or in whom cancer has been already diagnosed, with AZT. In particular, such a combination therapy could be of particular effectiveness in pathological situations in which cancer is associated with retroviral infections, such as HIV-associated neoplasias or adult T-cell leukemia.
Besides, our study could contribute to define a possible, anti-tumoral potential of AZT, or other nucleoside compounds, in combination with NF-κB inhibitors, also for the control of neoplastic diseases other than those associated with retroviral infections. Moreover, our study sustains the general idea that effects of chemotherapeutic drugs do not exclusively lie in their ability to directly induce DNA damage and alteration of the cell cycle control, but that repair mechanisms of DNA or cell cycle damage in response to chemotherapy could, finally, indirectly lead to apoptosis.
Taken together, our results disclose new mechanisms underlying the effect of AZT on survival/death of target cells. Given the basic knowledge regarding the biology of both viral infections and tumors, modulation of NF-κB activation could be a critical step in determining the adverse or desired effects of AZT in patients.
Materials and Methods
Cell cultures
Human monocytic U937 cells, originally obtained from Zooprofilactic Institute (Brescia, Italy), were grown in suspension culture at a density of 4 × 105 cells/ml in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 2 mM glutamine (Hyclone, Cramlington, England, UK), 50 U/ml penicillin and 50 U/ml streptomycin (Hyclone) at 37°C in a 5% CO2-humified atmosphere, in the presence or not of the dideoxynucleoside analog 3′-azido-3′-deoxythymidine (AZT, Wellcome Research labs, Beckenham, Kent, UK), for different times and at different concentrations (range: 8–128 μM). In some experiments, U937 cells were pre-treated with a chemical IκBα inhibitor, Bay 11–7085 (SuperArray Bioscience Corporation, Frederick, MD, USA) 1 h before adding AZT, and apoptosis was evaluated at 24 h. U937 stable transfectants carrying a dominant negative murine IκBα (U937-mIκB) or a control vector pcDNA3.1 (U937-pcDNA)31 were cultured at a density of 4 × 105 cells/ml in complete RPMI 1640 supplemented with 10% FBS and 400 μg/ml of G418 (Invitrogen) and treated with or without AZT for various times. In some experiments, both U937-mIκB and U937-pcDNA were pre-treated with CHX 0.1 μg/ml (Sigma, St. Louis, MO, USA) for 1 h, and apoptosis was evaluated 48 h in presence or in absence of AZT. Following incubation in the various culture conditions above described, cells were harvested and washed three times with cold phosphate-buffered saline (PBS) for 5 min, at 1000 × g, +4°C, and then used for RNA and protein extractions or for intracellular staining to evaluate protein expression and apoptosis.
Apoptosis assays
Apoptosis was evaluated by flow cytometry analysis of isolated nuclei following detergent treatment and PI staining, using a method that distinguishes nuclei from apoptotic, necrotic or viable cells, as previously described.32 Briefly, harvested cells were treated with a solution of PI at 25 μg/ml (PI, Sigma) plus 0.05% sodium citrate (Sigma) and with detergent at a high concentration (20% Triton X-100, Sigma) for 30 min. Isolated nuclei were then analyzed using a FACScan flow cytometry (BD Biosciences, Mountain View, CA, USA). Detectors and amplifier gains for forward and orthogonal scatter were adequately selected to simultaneously detect nuclei from viable, apoptotic and necrotic cells. Events were gated on forward versus orthogonal scatter in such a way that degraded DNA from cell debris or from doublets was excluded and nuclei from viable, apoptotic and necrotic cells were assayed. Data acquisition and analysis were performed using CellQuest software on a minimum of 5000 events for each sample (BD Biosciences, San Jose, CA, USA). In some experiments, the Annexin V-FITC Apoptosis Detection Kit (BD-Bioscience Pharmingen, San Diego, CA, USA) was used, according to the manufacturer's instructions, to detect apoptosis. Briefly, 5 × 105 cells were incubated for 15 min with annexin-V-FITC and then washed in annexin buffer. Cells were analyzed immediately after staining by flow cytometry analysis using a FACScan flow cytometer and Cell Quest software.
Immune staining and flow cytometry analysis of phospho NF-κB p65
For analysis of intranuclear NF-κB p65 proteins, 2 × 106 cells were fixed in 0.5 ml of 1% paraformaldehyde in PBS for 10 min at 37°C, and successively after being washed twice in PBS, in 90% methanol for 10 min at 4°C. Samples were first incubated with a rabbit monoclonal antibody against phospho NF-κB p65 Ser (536) (Cell Signaling Technology, Danvers, MA, USA) for 30 min at 4°C and then stained with goat FITC-conjugated anti-rabbit IgG (Harlan Sera-Lab Ltd., Belton Loughborough, UK). Cells were analyzed immediately after staining by flow cytometry using a FACScan flow cytometry and CellQuest software.
NF-κB-binding assays
For detecting NF-κB activation by non-radioactive EMSA, nuclear extracts from cells subjected to different experimental conditions were prepared as follows. Aliquots of 107 cells were suspended in 400 μl of buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.2 mM phenylmethylsulphonyl fluoride, all from Sigma), incubated on ice for 15 min and homogenized by 15 passages through a 25-gauge needle. The nuclei were pelleted by centrifugation at 10 000 × g for 40 s at 4°C, washed in 150 ml of buffer A and re-suspended in buffer B (20 mM HEPES, pH 7.9, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulphonyl fluoride) supplemented with 1 × protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). After 30 min of incubation on ice, the nuclear extracts were collected by centrifugation at 10 000 × g for 2 min. The extracts were rapidly frozen on dry ice and stored at −80°C until processed for EMSA. Before freezing, the protein concentration was estimated using an assay kit (Bio-Rad Laboratoires, Richmond, CA, USA). After thawing, groups of nuclear extracts were collectively subjected to EMSA using reagents provided in the ‘LightShift chemiluminescent EMSA Kit' (Pierce, Rockford, IL, USA). Aliquots of ∼10 μg of the extracts were mixed with 1–10 pmoles of biotin-labeled κB DNA probe (AGTTGAGGGGACTTTCCCAGGC) and poly(dI-dC) in binding buffer (10 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 5% glycerol, pH 7.5) in a final volume of 15 μl. Binding reactions were incubated for 20 min at room temperature. The dye solution was then added and samples were directly loaded onto a 5% polyacrylamide gel in 0.5 × TBE buffer for running. The gels were then transferred to ‘Zeta-probe GT Genomic' blotting membranes (Bio-Rad) and visualized using the ‘chemiluminescent nucleic acid detection module' provided in the EMSA kit. Specificity of the protein–DNA complex was verified by incubating the nuclear extracts for 30 min on ice with 2 μg of polyclonal antibodies to p65 (Rel A) or to p50, before the binding reaction and, in competition experiments, with a 200x excess of unlabeled κB-specific DNA.
For quantization of NF-κB p65 binding by an enzyme-linked immunosorbent assay (ELISA), nuclear extracts from cells cultured for different times with or without AZT were obtained as described above. Protein concentration of each sample was determined by BCA protein Assay Kit (Pierce). The DNA-binding activity of NF-κB p65 was measured using a commercial ELISA according to the manufacturer's protocol (Trans-AM NF-κB p65 Transcription Factor Assay Kit; Active Motif North America, Carlsbad, CA, USA). The absorbance was determined at 450 nm with wavelength correction 650 nm using a Labsystem Multiskan Bichromatic spectrophotometer (Helsinki, Finland).
Western blot analysis
A total number of 3 × 106 cells were solubilized at 4°C in lysis buffer (50 mM Tris-HCL pH 7.4, 1 mM EDTA, 1 mM EGTA pH 7.4, 1% Triton-X, 150 mM NaCl, 0.25% sodium deoxycholate, 1% NP-40 and, freshly added, 1 mM PMSF, 5 μM DTT, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 2 μg/ml aprotinin, 1 mM Na3VO4, 20 mM Na3F, all from Sigma) and centrifuged at 10 000 × g for 20 min. An certain amount of protein obtained from 5 × 105 cells was loaded onto a 10% SDS-polyacrylamide gel, subjected to electrophoresis and transferred to nitrocellulose membrane (Bio-Rad laboratories, Hercules, CA, USA), which was subsequently stained with 0.2% Ponceau red to ensure equal protein loading and transfer. After blocking the membrane in 10% non-fat dried milk and 3% BSA in TTBS (20 mM Tris-HCl pH 8.0, 0.9% NaCl, 0.03% Tween 20, all from Sigma), the blots were incubated overnight at 4°C with diluted primary antibody and, subsequently, washed and then incubated with anti-mouse (Bio-Rad laboratories) or anti-rabbit or anti-goat IgG chain-specific conjugated to peroxidase (Calbiochem, Merck Biosciences, Darmstadt, Germany). Binding of antibodies was detected by chemiluminescence staining using the ECL detection kit (Amersham Biosciences, Little Chalfont, UK). The following antibodies where used: rabbit polyclonal antibodies against human survivin (1 : 1 000, R&D System, Minneapolis, MN, USA), human X-IAP (1 : 1 000, R&D Systems), human c-IAP2 (H-85; 1 : 1 000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), human MDM2 (1 : 500, Chemicon International, Temecula, CA, USA) and mouse monoclonal antibodies against GADD-45A (1 : 200, Abnova Corporation, Taipei, Taiwan) and human β-actin (1 : 8000, Novus Biologicals, Inc., Littleton, CO, USA). Comparative analysis of the bands was performed by quantitative densitometry using the Tina software (version 2.10, Raytest, Straubenhardt, Germany).
SuperArray
RNA isolation was performed using RNAzol (Invitrogen, Grand Island, NY, USA), according to the manufacturer's instructions. An amount of 3.5 μg of total RNA was used in a reverse transcription (RT) reaction with biotin-16-dUTP (Roche Diagnostics GmbH). RT reaction was performed using Ampolabelling LPR kit (SABioscience Corporation, Frederick, MD, USA). The labeled cDNA was incubated with GEArray-Q Series human apoptosis and cell cycle membranes (SuperArray, SABioscience) at 60°C overnight. The membrane used in the present study contained 96 genes that were closely related to apoptosis and cell cycle pathways, in addition to positive control genes (glyceraldehydes-3-phosphate dehydrogenase, GAPDH, cyclophillin A and β actin). After being washed, the membrane was incubated with streptavidin-alkaline phosphatase and was finally exposed to CDP-Star chemiluminescent substrate (SuperArray). Signal detection was performed using a high Performance chemiluminescence film (Amersham Biosciences). Analysis of results was performed by GEArray Expression Analysis Suite software (http://geasuite.superarray.com). According to this analysis, transcriptional levels of genes showing fold change values of >1.50 or <0.66 were considered significantly modified. SuperArray analysis results are provided as Supplementary material (Supplementary Table S1).
Statistical analysis
Data analysis was performed using the SPSS statistical software system (Chicago, IL, USA). Statistical probabilities were expressed as P<0.05 (*) or P<0.010 (**). Comparison of means of apoptosis or p65 activation levels in response to AZT treatment was carried out using Bonferroni's post hoc multiple comparison ANOVA test. Comparison of means of values between different cell lines or after treatment with CHX or NF-κB inhibitor was carried out using the independent samples Student's t-test.
Acknowledgments
We wish to thank Martino Tony Miele for his linguistic assistance. This work was supported by grants from: the Italian Ministry of University and Research, Research Projects of National Interest; Istituto Superiore di Sanità, AIDS Project; the University of Rome ‘Tor Vergata' and the University of Messina.
Glossary
- AZT
azidothymidine
- ARV
anti-retrovirals
- CHX
cycloheximide
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehydes -3- phosphate dehydrogenase
- mIκB
dominant negative murine IκBα
- NF-κB
nuclear factor-κB
- PI
propidium iodide
- PBS
phosphate-buffered saline
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Supplementary Material
References
- De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery. Int J Antimicrob Agents. 2009;33:307–320. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Warnke D, Barreto J, Temesgen Z. Antiretroviral Drugs. J Clin Pharmacol. 2007;47:1570–1579. doi: 10.1177/0091270007308034. [DOI] [PubMed] [Google Scholar]
- WHO, UNAIDS and UNICEF Towards Universal Access: Scaling up Priority HIV/AIDS Interventions in the Health SectorProgress reportWorld Health Organization: Geneva; 2009(http://www.who.int/hiv/pub/2009progressreport/en/index.html , accessed on 14 May 2010). [Google Scholar]
- Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol Appl Pharmacol. 2004;199:151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Olivero OA. Mechanisms of genotoxicity of nucleoside reverse transcriptase inhibitors. Environ Mol Mutagen. 2007;48:215–223. doi: 10.1002/em.20195. [DOI] [PubMed] [Google Scholar]
- Watts DH. Teratogenicity risk of antiretroviral therapy in pregnancy. Curr HIV/AIDS Rep. 2007;4:135–140. doi: 10.1007/s11904-007-0020-y. [DOI] [PubMed] [Google Scholar]
- Martin F, Taylor GP. The safety of highly active antiretroviral therapy for the HIV-positive pregnant mother and her baby: is ‘the more the merrier'. J Antomicrob Chemother. 2009;64:895–900. doi: 10.1093/jac/dkp303. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Kute TE, Duch DS. Synchronization of cells in the S phase of the cell cycle by 3′-azido-3′-deoxythymidine: implications for cell cytotoxicity. Cancer Chemother Pharmacol. 1995;35:489–495. doi: 10.1007/BF00686833. [DOI] [PubMed] [Google Scholar]
- Olivero OA, Tejera AM, Fernandez JJ, Taylor BJ, Das S, Divi RL, et al. Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005;20:139–146. doi: 10.1093/mutage/gei019. [DOI] [PubMed] [Google Scholar]
- Escobar PA, Olivero OA, Wade NA, Abrams EJ, Nesel CJ, Ness RB, et al. Genotoxicity assessed by the comet and GPA assays following in vitro exposure of human lymphoblastoid cells (H9) or perinatal exposure of mother-child pairs to AZT or AZT-3TC. Environ Mol Mutagen. 2007;48:330–343. doi: 10.1002/em.20285. [DOI] [PubMed] [Google Scholar]
- Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt RA, Von Tungeln LS, Shaddock JG, Dobrovolsky VN, Beland FA, Heflich RH. Analysis of mutations in the Tk gene of Tk+/− mice treated as neonates with 3′-azido-3′-deoxythymidine (AZT) Mutat Res. 2004;547:63–69. doi: 10.1016/j.mrfmmm.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23:2797–2808. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- Collier AC, Helliwell RJ, Keelan JA, Paxton JW, Mitchell MD, Tingle MD. 3′Azido-3′ deoxythymidine (AZT) induces apoptosis and alters metabolic enzyme activity in human placenta. Toxicol Appl Pharmacol. 2003;192:164–173. doi: 10.1016/s0041-008x(03)00274-6. [DOI] [PubMed] [Google Scholar]
- Scruggs ER, Dirks Naylor AJ. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008;82:83–88. doi: 10.1159/000134943. [DOI] [PubMed] [Google Scholar]
- Matteucci C, Minutolo A, Balestrieri E, Ascolani A, Grelli S, Macchi B, et al. Effector caspase activation, in the absence of a conspicuous apoptosis induction, in mononuclear cells treated with azidothymidine. Pharmacol Res. 2009;59:125–133. doi: 10.1016/j.phrs.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Walker DM, Malarkey DE, Seilkop SK, Ruecker FA, Funk KA, Wolfe MJ, et al. Trasplacental carcinogenicity of 3′-azido-3′deoxythymidine in B6C3F1 mice and F344 rats. Environ Mol Mutagen. 2007;48:283–298. doi: 10.1002/em.20297. [DOI] [PubMed] [Google Scholar]
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappa B. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-κB response. Cell Death Diff. 2006;13:773–784. doi: 10.1038/sj.cdd.4401843. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-{kappa}B signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- Chen F, Beezhold K, Castranova V. Tumor promoting or tumor suppressing of NF-kappa B a matter of cell context dependency. Int Rev Immunol. 2008;27:183–204. doi: 10.1080/08830180802130327. [DOI] [PubMed] [Google Scholar]
- Shetty S, Graham BA, Brown JG, Hu X, Vegh-Yarema N, Harding G, et al. Transcription factor NF-kappaB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol Cell Biol. 2005;25:5404–5416. doi: 10.1128/MCB.25.13.5404-5416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SB. Epidermal growth factor and trail interactions in epithelial-derived cells. Vitam Horm. 2004;67:207–227. doi: 10.1016/S0083-6729(04)67012-9. [DOI] [PubMed] [Google Scholar]
- Ghosh SK, Wood C, Boise LH, Mian AM, Deyev VV, Feuer G, et al. Potentiation of TRAIL-induced apoptosis in primary effusion lymphoma through azidothymidine-mediated inhibition of NF-kappa B. Blood. 2003;101:2321–2327. doi: 10.1182/blood-2002-08-2525. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Ghosh SK, Ramos JC, Mian AM, Toomey NL, Cabral L, et al. Azidothymidine inhibits NF-kappaB and induces Epstein-Barr virus gene expression in Burkitt lymphoma. Blood. 2005;106:235–240. doi: 10.1182/blood-2004-09-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici MA, Sciortino MT, Perri D, Amici C, Avitabile E, Ciotti M, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J Biol Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- Matteucci C, Grelli S, De Smaele E, Fontana C, Mastino A. Identification of nuclei from apoptotic, necrotic and viable lymphoid cells by using multiparameter flow cytometry. Cytometry. 1999;35:145–153. doi: 10.1002/(sici)1097-0320(19990201)35:2<145::aid-cyto6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.