Abstract
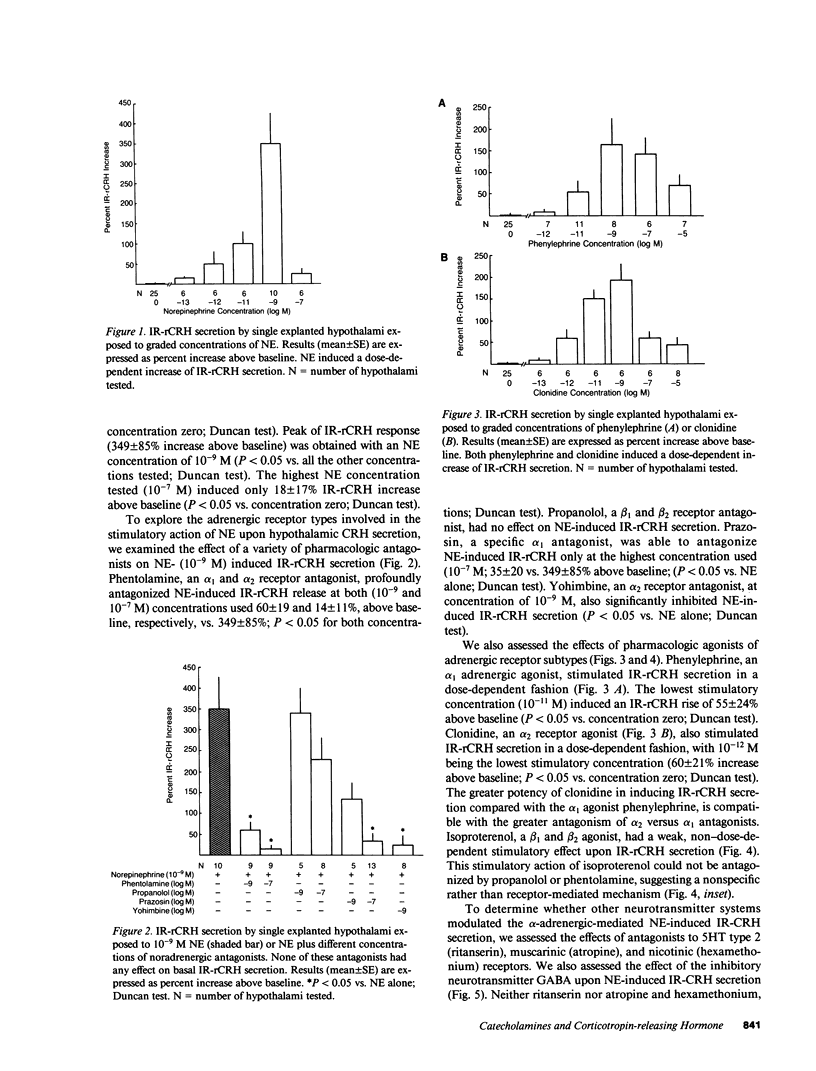
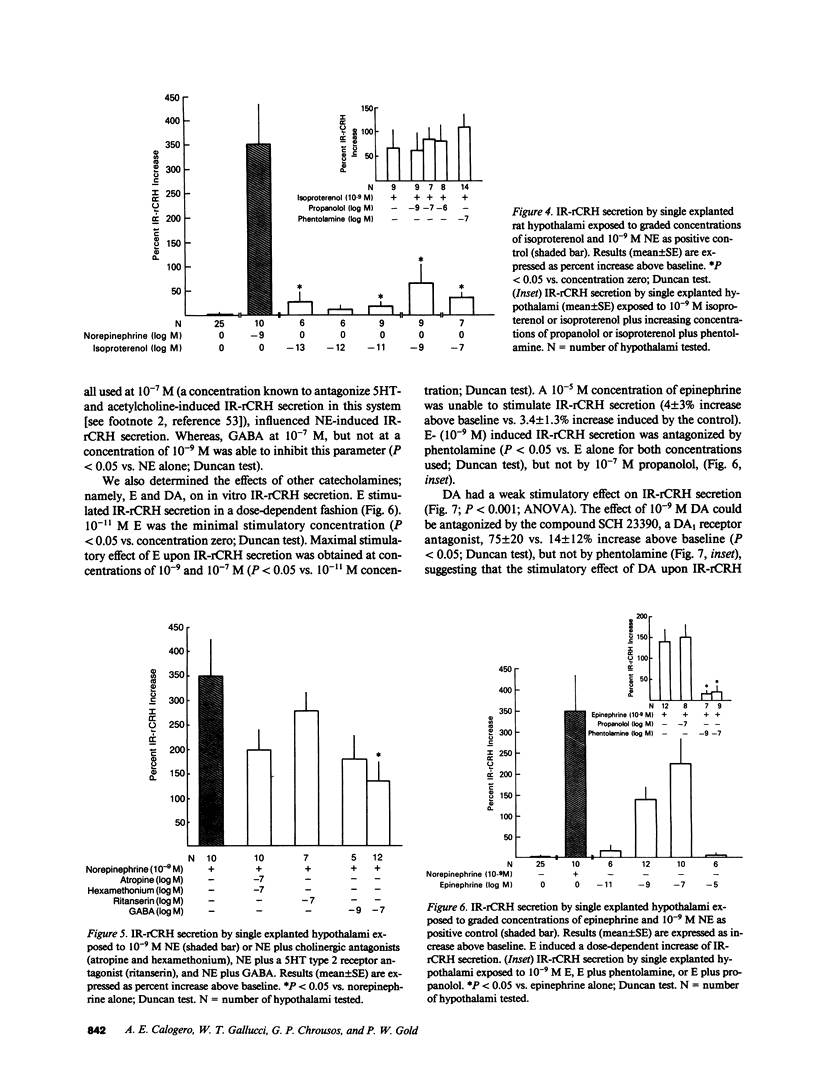
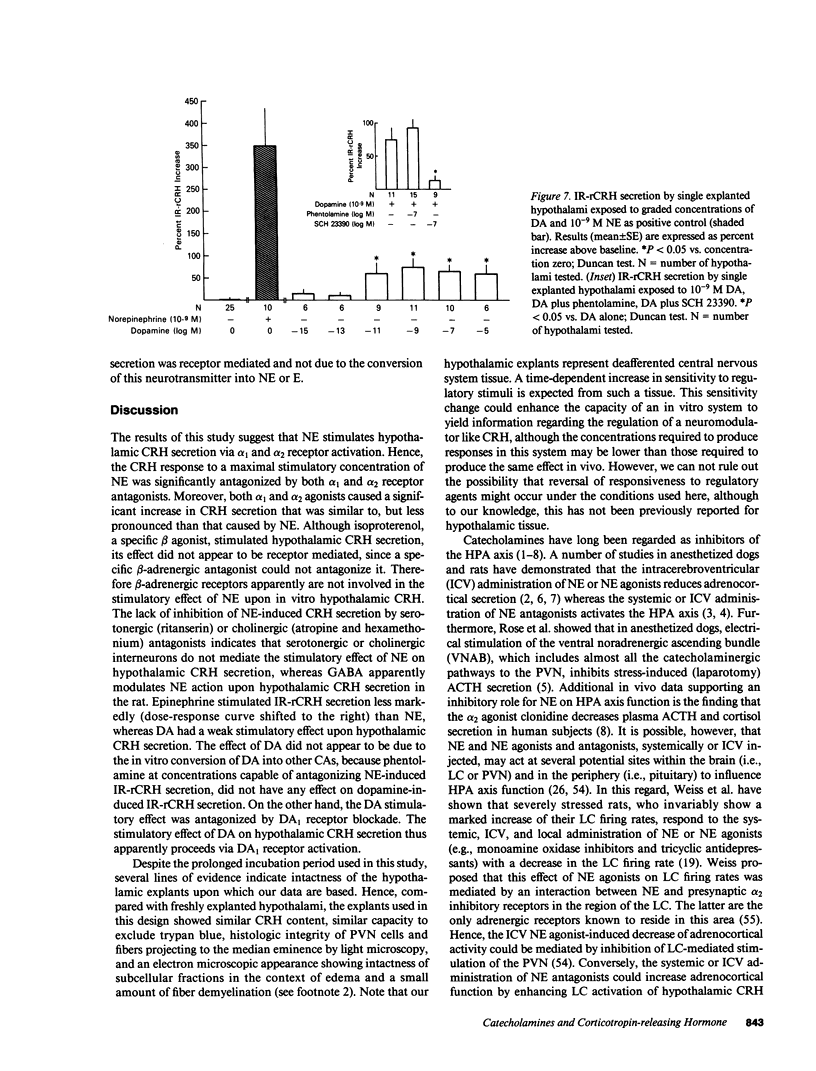
To further our understanding of the functional role of catecholaminergic systems in regulating hypothalamic corticotropin-releasing hormone (CRH) secretion, we assessed the direct effects of a multiplicity of catecholamine agonists and antagonists on hypothalamic CRH secretion. To accomplish this, we used an in vitro rat hypothalamic organ culture system in which CRH secretion from single explants was evaluated by a specific RIA (IR-rCRH). Norepinephrine (NE) stimulated IR-rCRH secretion dose dependently, with peak effects in the nanomolar range. The effect of NE was antagonized by the mixed alpha antagonist phentolamine, the alpha 1 antagonist prazosin, and the alpha 2 antagonist yohimbine, but not by the beta blocker, L-propanolol. Compatible with these data were the findings that the alpha 1 agonist phenylephrine and the alpha 2 agonist clonidine both stimulated IR-rCRH secretion in a dose-dependent fashion. On the other hand, whereas the beta agonist, isoproterenol, caused a weak, non-dose-dependent increase in IR-rCRH secretion, this effect could not be antagonized by L-propanolol. Despite pretreatment with serotonin and acetylcholine antagonists, the effect of NE upon IR-rCRH secretion was undiminished, suggesting that NE-induced CRH secretion is not mediated by either neurotransmitter. On the other hand, pretreatment with gamma-aminobutyric acid (GABA) attenuated NE-induced IR-rCRH secretion. Whereas epinephrine (E) stimulated IR-rCRH secretion, this occurred only at higher concentrations, and was antagonized by phentolamine, but not by L-propanolol. Dopamine (DA) had a weak stimulatory effect that could be antagonized by the DA1 receptor antagonist, SCH 23390, but not by phentolamine. We conclude that NE and E stimulate hypothalamic IR-rCRH secretion via alpha 1 and alpha 2 receptors. The effect of NE upon IR-rCRH secretion is not apparently mediated by serotonergic or cholinergic interneurons, but is modulated by the inhibitory neurotransmitter, GABA. These data support the idea that the central catecholaminergic systems are excitatory rather than inhibitory upon CRH secretion when acting directly at the hypothalamic level.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Cedarbaum J. M., Wang R. Y. Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons. Brain Res. 1977 Nov 18;136(3):570–577. doi: 10.1016/0006-8993(77)90083-x. [DOI] [PubMed] [Google Scholar]
- Al-Damluji S., Perry L., Tomlin S., Bouloux P., Grossman A., Rees L. H., Besser G. M. Alpha-adrenergic stimulation of corticotropin secretion by a specific central mechanism in man. Neuroendocrinology. 1987 Jan;45(1):68–76. doi: 10.1159/000124705. [DOI] [PubMed] [Google Scholar]
- Andersson K., Agnati L. F., Fuxe K., Eneroth P., Härfstrand A., Benfenati F. Corticotropin-releasing factor increases noradrenaline turnover in the median eminence and reduces noradrenaline turnover in the paraventricular region of the hypophysectomized male rat. Acta Physiol Scand. 1984 Apr;120(4):621–624. doi: 10.1111/j.1748-1716.1984.tb07430.x. [DOI] [PubMed] [Google Scholar]
- Antoni F. A. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986 Nov;7(4):351–378. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- Antoni F. A., Palkovits M., Makara G. B., Linton E. A., Lowry P. J., Kiss J. Z. Immunoreactive corticotropin-releasing hormone in the hypothalamoinfundibular tract. Neuroendocrinology. 1983 Jun;36(6):415–423. doi: 10.1159/000123492. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Mueller R. A., Henry J. P., Stephens P. M. Changes in enzymes involved in the biosynthesis and metabolism of noradrenaline and adrenaline after psychosocial stimulation. Nature. 1970 Mar 14;225(5237):1059–1060. doi: 10.1038/2251059a0. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Buckingham J. C., Hodges J. R. Hypothalamic receptors influencing the secretion of corticotrophin releasing hormone in the rat. J Physiol. 1979 May;290(2):421–431. doi: 10.1113/jphysiol.1979.sp012780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero A. E., Gallucci W. T., Bernardini R., Saoutis C., Gold P. W., Chrousos G. P. Effect of cholinergic agonists and antagonists on rat hypothalamic corticotropin-releasing hormone secretion in vitro. Neuroendocrinology. 1988 Apr;47(4):303–308. doi: 10.1159/000124929. [DOI] [PubMed] [Google Scholar]
- Calogero A. E., Gallucci W. T., Gold P. W., Chrousos G. P. Multiple feedback regulatory loops upon rat hypothalamic corticotropin-releasing hormone secretion. Potential clinical implications. J Clin Invest. 1988 Sep;82(3):767–774. doi: 10.1172/JCI113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. J., Brandsborg O. The relationship between plasma catecholamine concentration and pulse rate during exercise and standing. Eur J Clin Invest. 1973 Jul;3(4):299–306. doi: 10.1111/j.1365-2362.1973.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. M. Further evidence of a central alpha-adrenergic inhibitory influence on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology. 1975;17(2):154–166. doi: 10.1159/000122351. [DOI] [PubMed] [Google Scholar]
- Fehm H. L., Voigt K. H., Lang R. E., Pfeiffer E. F. Effects of neurotransmitters on the release of corticotropin releasing hormone (CRH) by rat hypothalamic tissue in vitro. Exp Brain Res. 1980;39(2):229–234. doi: 10.1007/BF00237553. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong W. F., Kramer N., Salmon J., Reid I. A., Lovinger R., Scapagnini U., Boryczka A. T., Shackelford R. Pharmacological evidence for inhibition of ACTH secretion by a central adrenergic system in the dog. Neuroscience. 1976 Jun;1(3):167–174. doi: 10.1016/0306-4522(76)90073-7. [DOI] [PubMed] [Google Scholar]
- Ganong W. F. Neurotransmitters and pituitary function: regulation of ACTH secretion. Fed Proc. 1980 Sep;39(11):2923–2930. [PubMed] [Google Scholar]
- Gold P. W., Loriaux D. L., Roy A., Kling M. A., Calabrese J. R., Kellner C. H., Nieman L. K., Post R. M., Pickar D., Gallucci W. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986 May 22;314(21):1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- Guillaume V., Conte-Devolx B., Szafarczyk A., Malaval F., Pares-Herbute N., Grino M., Alonso G., Assenmacher I., Oliver C. The corticotropin-releasing factor release in rat hypophysial portal blood is mediated by brain catecholamines. Neuroendocrinology. 1987 Aug;46(2):143–146. doi: 10.1159/000124811. [DOI] [PubMed] [Google Scholar]
- Henry J. P., Stephens P. M., Axelrod J., Mueller R. A. Effect of psychosocial stimulation on the enzymes involved in the biosynthesis and metabolism of noradrenaline and adrenaline. Psychosom Med. 1971 May-Jun;33(3):227–237. doi: 10.1097/00006842-197105000-00004. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987 Oct;23(1):1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hillhouse E. W., Burden J., Jones M. T. The effect of various putative neurotransmitters on the release of corticotrophin releasing hormone from the hypothalamus of the rat in vitro. I. The effect of acetylcholine and noradrenaline. Neuroendocrinology. 1975;17(1):1–11. doi: 10.1159/000122335. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol. 1986;27(2):183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Jones M. T., Hillhouse E. W. Neurotransmitter regulation of corticotropin-releasing factor in vitro. Ann N Y Acad Sci. 1977 Oct 28;297:536–560. doi: 10.1111/j.1749-6632.1977.tb41881.x. [DOI] [PubMed] [Google Scholar]
- Koslow S. H., Maas J. W., Bowden C. L., Davis J. M., Hanin I., Javaid J. CSF and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis. Arch Gen Psychiatry. 1983 Sep;40(9):999–1010. doi: 10.1001/archpsyc.1983.01790080081011. [DOI] [PubMed] [Google Scholar]
- Krieger H. P., Krieger D. T. Chemical stimulation of the brain: effect on adrenal corticoid release. Am J Physiol. 1970 Jun;218(6):1632–1641. doi: 10.1152/ajplegacy.1970.218.6.1632. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R., Mikulaj L. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology. 1970 Oct;87(4):738–743. doi: 10.1210/endo-87-4-738. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R., Sun C. L., Lake C. R., Thoa N., Torda T., Kopin I. J. Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology. 1978 Nov;103(5):1868–1874. doi: 10.1210/endo-103-5-1868. [DOI] [PubMed] [Google Scholar]
- Lake C. R., Pickar D., Ziegler M. G., Lipper S., Slater S., Murphy D. L. High plasma norepinephrine levels in patients with major affective disorder. Am J Psychiatry. 1982 Oct;139(10):1315–1318. doi: 10.1176/ajp.139.10.1315. [DOI] [PubMed] [Google Scholar]
- Lanes R., Herrera A., Palacios A., Moncada G. Decreased secretion of cortisol and ACTH after oral clonidine administration in normal adults. Metabolism. 1983 Jun;32(6):568–570. doi: 10.1016/0026-0495(83)90026-4. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- Liposits Z., Phelix C., Paull W. K. Electron microscopic analysis of tyrosine hydroxylase, dopamine-beta-hydroxylase and phenylethanolamine-N-methyltransferase immunoreactive innervation of the hypothalamic paraventricular nucleus in the rat. Histochemistry. 1986;84(2):105–120. doi: 10.1007/BF00499821. [DOI] [PubMed] [Google Scholar]
- Luger A., Deuster P. A., Kyle S. B., Gallucci W. T., Montgomery L. C., Gold P. W., Loriaux D. L., Chrousos G. P. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med. 1987 May 21;316(21):1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- Makara G. B., Stark E., Kárteszi M., Palkovits M., Rappay G. Effects of paraventricular lesions on stimulated ACTH release and CRF in stalk-median eminence of the rat. Am J Physiol. 1981 Apr;240(4):E441–E446. doi: 10.1152/ajpendo.1981.240.4.E441. [DOI] [PubMed] [Google Scholar]
- Murakami K., Hashimoto K., Ota Z. Effect of angiotensin II, catecholamines and glucocorticoid on corticotropin releasing factor (CRF)-induced ACTH release in pituitary cell cultures. Acta Med Okayama. 1984 Aug;38(4):349–355. doi: 10.18926/AMO/30312. [DOI] [PubMed] [Google Scholar]
- Plotsky P. M. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987 Sep;121(3):924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- Plotsky P. M., Vale W. Hemorrhage-induced secretion of corticotropin-releasing factor-like immunoreactivity into the rat hypophysial portal circulation and its inhibition by glucocorticoids. Endocrinology. 1984 Jan;114(1):164–169. doi: 10.1210/endo-114-1-164. [DOI] [PubMed] [Google Scholar]
- Rose J. C., Goldsmith P. C., Holland F. J., Kaplan S. L., Ganong W. F. Effect of electrical stimulation of the canine brain stem on the secretion of ACTH and growth hormone (GH). Neuroendocrinology. 1976;22(4):352–362. doi: 10.1159/000122644. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P. P., Uhde T. W., Post R. M., Gallucci W., Chrousos G. P., Gold P. W. The corticotropin-releasing hormone stimulation test in patients with panic disorder. Am J Psychiatry. 1986 Jul;143(7):896–899. doi: 10.1176/ajp.143.7.896. [DOI] [PubMed] [Google Scholar]
- Roy A., Pickar D., Linnoila M., Chrousos G. P., Gold P. W. Cerebrospinal fluid corticotropin-releasing hormone in depression: relationship to noradrenergic function. Psychiatry Res. 1987 Mar;20(3):229–237. doi: 10.1016/0165-1781(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981 Nov 6;214(4521):685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc. 1985 Jan;44(1 Pt 2):221–227. [PubMed] [Google Scholar]
- Scapagnini U., Van Loon G. R., Moberg G. P., Preziosi P., Ganong W. F. Evidence for central norepinephrine-mediated inhibition of ACTH secretion in the rat. Neuroendocrinology. 1972;10(3):155–160. doi: 10.1159/000122113. [DOI] [PubMed] [Google Scholar]
- Smythe G. A., Bradshaw J. E., Vining R. F. Hypothalamic monoamine control of stress-induced adrenocorticotropin release in the rat. Endocrinology. 1983 Sep;113(3):1062–1071. doi: 10.1210/endo-113-3-1062. [DOI] [PubMed] [Google Scholar]
- Suda T., Yajima F., Tomori N., Sumitomo T., Nakagami Y., Ushiyama T., Demura H., Shizume K. Inhibitory effect of norepinephrine on immunoreactive corticotropin-releasing factor release from the rat hypothalamus in vitro. Life Sci. 1987 Apr 27;40(17):1645–1649. doi: 10.1016/0024-3205(87)90012-9. [DOI] [PubMed] [Google Scholar]
- Svensson T. H., Bunney B. S., Aghajanian G. K. Inhibition of both noradrenergic and serotonergic neurons in brain by the alpha-adrenergic agonist clonidine. Brain Res. 1975 Jul 11;92(2):291–306. doi: 10.1016/0006-8993(75)90276-0. [DOI] [PubMed] [Google Scholar]
- Svensson T. H., Usdin T. Feedback inhibition of brain noradrenaline neurons by tricyclic antidepressants: alpha-receptor mediation. Science. 1978 Dec 8;202(4372):1089–1091. doi: 10.1126/science.213833. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A., Alonso G., Ixart G., Malaval F., Assenmacher I. Diurnal-stimulated and stress-induced ACTH release in rats is mediated by ventral noradrenergic bundle. Am J Physiol. 1985 Aug;249(2 Pt 1):E219–E226. doi: 10.1152/ajpendo.1985.249.2.E219. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A., Malaval F., Laurent A., Gibaud R., Assenmacher I. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinology. 1987 Sep;121(3):883–892. doi: 10.1210/endo-121-3-883. [DOI] [PubMed] [Google Scholar]
- Udelsman R., Norton J. A., Jelenich S. E., Goldstein D. S., Linehan W. M., Loriaux D. L., Chrousos G. P. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987 May;64(5):986–994. doi: 10.1210/jcem-64-5-986. [DOI] [PubMed] [Google Scholar]
- Uhde T. W., Boulenger J. P., Post R. M., Siever L. J., Vittone B. J., Jimerson D. C., Roy-Byrne P. P. Fear and anxiety: relationship to noradrenergic function. Psychopathology. 1984;17 (Suppl 3):8–23. doi: 10.1159/000284127. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Vale W., Vaughan J., Smith M., Yamamoto G., Rivier J., Rivier C. Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, catecholamines, neurohypophysial peptides, and other substances on cultured corticotropic cells. Endocrinology. 1983 Sep;113(3):1121–1131. doi: 10.1210/endo-113-3-1121. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Foote S. L., Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983 Jul 4;270(2):363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Foote S. L. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987 Jan;45(1):28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Van Loon G. R., Scapagnini U., Cohen R., Ganong W. F. Effect of intraventricular administration of adrenergic drugs on the adrenal venous 17-hydroxycorticosteroid response to surgical stress in the dog. Neuroendocrinology. 1971;8(5):257–272. doi: 10.1159/000122013. [DOI] [PubMed] [Google Scholar]
- Van Loon G. R., Scapagnini U., Moberg G. P., Ganong W. F. Evidence for central adrenergic neural inhibition of ACTH secretion in the rat. Endocrinology. 1971 Dec;89(6):1464–1469. doi: 10.1210/endo-89-6-1464. [DOI] [PubMed] [Google Scholar]
- Weiss J. M., Simson P. G. Depression in an animal model: focus on the locus ceruleus. Ciba Found Symp. 1986;123:191–215. doi: 10.1002/9780470513361.ch11. [DOI] [PubMed] [Google Scholar]
- Wiegand S. J., Price J. L. Cells of origin of the afferent fibers to the median eminence in the rat. J Comp Neurol. 1980 Jul 1;192(1):1–19. doi: 10.1002/cne.901920102. [DOI] [PubMed] [Google Scholar]



