Abstract
Background
Sex-specific trajectories in white matter development during adolescence may help explain cognitive and behavioral divergences between males and females. Knowledge of sex differences in typically developing adolescents can provide a basis for interpreting sexual dimorphisms in abilities and actions.
Method
We examined 58 healthy adolescents (12–14 years of age) with diffusion tensor imaging (DTI). Diffusion parameters fractional anisotropy (FA), and mean (MD), radial (RD), and axial diffusivities (AD) were subjected to whole-brain voxel-wise group comparisons using tract-based spatial statistics. Sex differences in white matter microstructure were examined in relation to pubertal development.
Results
Early adolescent females (n=29) evidenced higher FA in the right superior corona radiata, higher FA and AD in bilateral corticospinal tracts (≥164 µl, p<.01), and lower MD in the right inferior longitudinal fasciculus (ILF) and left forceps major (≥164 µl, p<.01) than age-matched males (n=29). Males did not show any areas of higher FA or lower MD than females, but had higher AD in the right superior longitudinal fasciculus, ILF, and forceps minor (≥ 164 µl, p<.01). Pubertal stage did not account for sex disparities.
Conclusion
In early adolescence, females’ motor tracts may reflect widespread changes, while males may undergo relatively more microstructural change in projection and association fibers.
Keywords: Diffusion tensor imaging, Adolescence, White matter, Sex differences, Development, Maturation
1. Introduction
Adolescence marks a time of major biological and psychosocial change. Increasing divergences in male and female physiology during this period of transition underscore the importance of understanding sexual dimorphisms in brain structure (Christakou et al., 2009; Plante et al., 2006; Rubia et al., 2010). Morphometric studies suggest that a number of structural differences between the male and female brain are evident even from a young age. Male children and adolescents consistently show larger overall brain volumes (Caviness et al., 1996; Giedd et al., 1999; Giedd et al., 1997; Reiss et al., 1996; Rubia et al., 2010), and proportionally larger amygdala and globus pallidus sizes, while females demonstrate larger caudate nuclei and cingulate gyrus volumes (Blanton et al., 2004; Caviness et al., 1996; Giedd et al., 1997). Such differences may contribute to specific psychopathological vulnerabilities in males and females and have significant influence during early adolescence, before the onset of most mental health disorders (Paus et al., 2008). Knowledge of underlying structural variation in typically developing male and female youth, particularly in maturing white matter pathways, will aid in understanding sex discrepancies in the physiology and progression of neurocognitive abilities and risk for mental health disorders.
Axonal growth and the establishment of new cortical connections in adolescence contribute to increasing efficiency and complexity in behavioral functions (Giedd et al., 2008; Paus et al., 1999). Increases in white matter during adolescence are most prominent in the frontal lobe for both genders (Giedd et al., 1999), though male children and adolescents have significantly larger volumes of white matter surrounding the lateral ventricles and caudate nuclei than females (Hua et al., 2009). Adolescent males also demonstrate a significantly higher rate of change in white matter volume compared to females (De Bellis et al., 2001; Giedd, 2004; Lenroot et al., 2007; Perrin et al., 2008), particularly in the occipital lobe (Perrin et al., 2009). However, growth in white matter density has only been apparent in females, observed in the corticospinal tract (CST) and fornix (Perrin et al., 2009). Despite steeper white matter volume changes in males, recent evidence suggests that maturation of white matter microstructure may occur earlier in female than male adolescents (Asato et al., 2010). Different mechanisms of white matter growth are implicated, specifically, an increase in axonal caliber in males and a growth in myelin content in females (Perrin et al., 2009). Increasing testosterone levels may influence axonal caliber in males, suggesting a role for sex hormones in white matter maturation (Perrin et al., 2008).
Descriptions of white matter pathways in the adolescent brain have gained momentum with the use of diffusion tensor imaging (DTI). DTI relies on the diffusion of water molecules in cerebral tissue to gain insight into structural organization and integrity. Fractional anisotropy (FA), the common scalar variable used in DTI, measures the directional coherence of diffusional motion. Tissues such as white matter have higher FA values due to their strong fiber regularity. High FA suggests greater fiber organization, but values may also reflect myelination and structural characteristics of the axon. Another key measure, mean diffusivity (MD), quantifies the magnitude of diffusional motion. Low MD values reflect greater white matter density (Roberts and Schwartz, 2007). Linear increases in FA and decreases in MD are typically evident in white matter during adolescence (Barnea-Goraly et al., 2005; Bonekamp et al., 2007; Giorgio et al., 2008; Mukherjee et al., 2001; Schmithorst et al., 2002). Increases in FA over this developmental period are associated with diminutions in diffusion perpendicular to fiber pathways, possibly attributable to myelination or growth in fiber density. Nonetheless, decreases in perpendicular or radial diffusivity (RD) have not been consistently found (Ashtari et al., 2007; Giorgio et al., 2010). Changes in axial diffusivity (AD), diffusion along the axis of the white matter fibers, also show discrepant trends in studies on adolescents, with some findings indicating increases and others indicating decreases in AD (Ashtari et al., 2007; Bava et al., 2010; Eluvathingal et al., 2007; Lebel et al., 2008).
Further examination of diffusion dynamics and their implications for white matter architecture in adolescents is needed, and is best achieved using sex-based comparisons. Toward this end, Schmithorst and colleagues (2008) conducted a DTI study of 106 children and adolescents ages 5 through 18 years. Across this broad age range, males had higher FA in bilateral frontal white matter areas, right arcuate fasciculus, and left parietal and occipito-parietal regions, while females showed higher FA in the splenium of the corpus callosum (Schmithorst et al., 2008). Females displayed age-related increases in FA in all regions except the left frontal lobe, whereas males did not. Males showed higher MD in the CST bilaterally and in the right frontal lobe, while females had higher MD in the arcuate fasciculus, occipito-parietal white matter, and the right superior aspect of the CST. For females, MD decreased with age in each of these regions except the CST, whereas males only showed decreases with age in the right frontal lobe. Other findings show higher FA in the genu in adolescent males compared to females (Silveri et al., 2006), and fiber tract analysis suggests no FA differences between males and females in this age range (Eluvathingal et al., 2007), but these results could be due to methodological differences.
The previous studies are limited in their examination of an expansive age range, evaluation of few brain structures, low sampling of diffusion directions, or analysis of only a few diffusion indices. Evaluation of sex differences during a circumscribed early adolescent period is needed to identify structures that may be disrupted prior to the onset of psychopathology or behaviors that could influence white matter development, such as substance use. In the present study, we examined sex differences in white matter microstructure among 12–14 year-old adolescents using whole-brain voxelwise high angular resolution diffusion imaging (HARDI) (Frank, 2001) analysis. We hypothesized that sex differences would be greatest in fronto-parietal tracts, shown to undergo significant developmental growth during adolescence (Ashtari et al., 2007; Bava et al., 2010; Giorgio et al., 2010). We predicted that males would show higher FA and lower MD than females in fronto-parietal fiber bundles as well as in the occipital lobe, and that females would demonstrate higher FA and lower MD in the corpus callosum and CST, considering previous findings (Perrin et al., 2009; Schmithorst et al., 2008). Given recent evidence of lower RD in adolescent females in association and projection fibers (Asato et al., 2010; Eluvathingal et al., 2007), we also examined sex differences in RD and AD (Le Bihan et al., 2001). The relationship between white matter growth and pubertal stage has only been explored in volumetric and density studies in adolescents. Therefore, a secondary aim was to determine whether sex differences in white matter structure would be linked to pubertal development stage.
2. Results
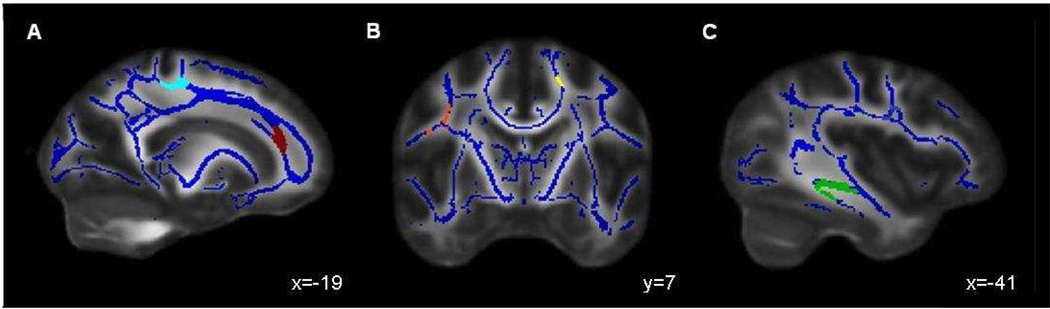
Anatomic regions showing significant differences in diffusion indices between males and females are presented in Table 1. Independent samples t-tests, corrected with intensity and cluster-based thresholding (≥ 164 contiguous voxels with each showing the effect at p<.01) revealed 3 clusters in which females showed significantly higher FA than males: 2 clusters in bilateral CST (p<.001) and 1 cluster in right superior corona radiata (SCR) (p=.001, see Figure 1); no clusters were found showing higher FA in males than females. For MD, two clusters were identified showing lower values in females than males (p<.001): right inferior longitudinal fasciculus (ILF) and left forceps major; no areas of higher MD in females were identified. Similarly, examination of AD revealed 2 clusters within bilateral CST (p<.001) where females demonstrated higher AD than males. In contrast, higher AD in males than females was observed in 3 clusters, all located in the right hemisphere (p<.001): superior longitudinal fasciculus (SLF), ILF, and forceps minor (see Figure 1). No sex differences in RD were detected. Given that sex differences in the right ILF and bilateral CST were found on two diffusion indices, bivariate correlations were performed to assay potential relationships among these diffusion measures. Significant and similar correlations between diffusion indices were found for males and females: MD and AD in the right ILF (males: r = .68; p <.001; females: r = .70; p <.001), and FA and AD in the left CST (males: r = .79, p <.001; females: r = .79; p <.001) and right CST (males: r = .77; p <.001; females: r = .78; p <.001).
Table 1.
Clusters showing significant fractional anisotropy (FA), mean diffusivity (MD), and axial diffusivity (AD) differences between males and females.
| Anatomic Region | Cluster Size (Voxels) |
MNI Coordinates* | Mean Value in Males |
Mean Value in Females |
Effect Size (Cohen’s d)† |
Group Difference |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| FA | ||||||||
| Corticospinal tract L | 503 | 19 | 18 | 46 | 0.58 | 0.62 | 1.43 | F>M |
| Corticospinal tract R | 249 | −19 | 28 | 50 | 0.57 | 0.60 | 1.28 | F>M |
| Superior corona radiata R | 185 | −26 | 18 | 34 | 0.47 | 0.49 | 0.91 | F>M |
| MD | ||||||||
| Inferior longitudinal fasciculus R | 167 | −42 | 34 | −10 | 1.13 | 1.08 | 1.14 | M>F |
| Forceps major L | 167 | 15 | 91 | 9 | 1.14 | 1.09 | 1.38 | M>F |
| AD | ||||||||
| Corticospinal tract L | 352 | 19 | 19 | 47 | 1.74 | 1.84 | 1.20 | F>M |
| Corticospinal tract R | 208 | −20 | 29 | 50 | 1.70 | 1.79 | 1.26 | F>M |
| Superior longitudinal fasciculus R | 389 | −43 | 7 | 30 | 1.60 | 1.52 | 1.67 | M>F |
| Inferior longitudinal fasciculus R | 358 | −41 | 34 | −9 | 1.91 | 1.82 | 1.45 | M>F |
| Forceps minor R | 186 | −18 | −33 | 16 | 1.91 | 1.83 | 1.01 | M>F |
Coordinates of the center of mass in clusters with significant gender differences
Effect size from the average t-value for each cluster
L=Left; R=Right; F=Female; M=Male
Figure 1.
Clusters of significant difference (≥ 164 µl, p<.01) between adolescent males and females (N=58) in: (A) right corticospinal tract FA and AD (cyan), right forceps minor AD (red); (B) right superior longitudinal fasciculus AD (orange), left corticospinal tract AD (yellow); and (C) right inferior longitudinal fasciculus MD and AD (green). Results are superimposed on the fiber skeleton (blue) and overlaid on a standardized FA template, in radiological convention.
As an expected consequence of age matching in young adolescents, females were found to have higher (i.e., further progressed) Pubertal Development Scale (PDS) scores than males (p<.001). To evaluate the influence of PDS, group differences were examined with MANCOVA (N = 58). Wilks’ criterion indicated that the group differences in FA (F(3,53) = 9.12, p<.001), MD (F(2,54) = 5.73, p=.006), and AD (F(5,51) = 13.83, p<.001) reported above persisted after controlling for PDS. To ascertain whether early adolescent age and pubertal development might account for variability in white matter development, follow-up hierarchical regressions for each sex entered age and PDS on step 1 and their interaction on step 2; no significant interaction or main effects were observed for age or pubertal stage.
3. Discussion
The current study examined sex differences in white matter anisotropy and diffusivity among young adolescents. Age- and socioeconomic status (SES)-matched males and females showed several areas of divergence in white matter microstructure. Females had higher FA than males in the right SCR and in bilateral CST. In the latter regions, females also demonstrated higher AD. Although males did not show any areas of increased FA compared to females, they demonstrated higher AD in the right ILF, SLF and forceps minor, and higher MD in the right ILF and left forceps major. Females showed no areas of increased MD compared to males. No sex differences were evident with RD, suggesting that myelination alone is not sufficient to characterize white matter disparities. Rather, variations in fiber organization, coherence and density, and axonal caliber may better capture diverging trajectories of white matter development between males and females (Perrin et al., 2009).
Differences in white matter architecture in the CST may primarily reflect greater organization and directional coherence of fiber pathways in females relative to males. Such neuroanatomical characteristics are implicated when considering the significant correlation between FA and AD values in this region. Increased white matter density in the CST of adolescent females compared to males (Perrin et al., 2009) suggests myelination as an additional physiological contributor. While Schmithorst et al., 2008 found higher MD in females than males in the right CST, it is unclear whether concomitant differences in AD were evident, and as such, we are limited in evaluating whether their findings are necessarily discrepant from current results. White matter development in the CST has been linked with high verbal abilities in children and adolescents, and may implicate earlier development of such skills in our sample of female youth (Tamnes et al., 2010).
Contrary to expectations, males did not have higher FA than females in any region; however, the largest effect in our study was that of increased AD in the SLF for males. This is consistent with previous literature indicating greater frontal and parietal FA in boys (Schmithorst et al., 2008). Higher AD in the right ILF of males showed a similarly large effect size and correlated with MD in this region. The larger extent of AD than MD difference suggests that increased parallel diffusivity may underlie the increase in MD. Greater AD in frontal, fronto-parietal and fronto-temporal pathways may lend support to a growth in axonal caliber in adolescent males that restricts diffusion along the maturing association fibers of the SLF, ILF and forceps minor. This process may be particularly active in our sample of mostly early- and mid-pubertal males, where axonal density is purportedly influenced by increasing testosterone levels (Perrin et al., 2008). Greater anisotropy in the SLF and ILF is associated with lower impulsivity (Olson et al., 2009) and may have behavioral implications with regard to risk-taking tendencies. Moreover, maturation of medial frontal cortices, such as those regions innervated by the forceps minor, is implicated in the maturation of rational decision-making and avoidance of risky choices (Crone et al., 2008). Given that risky and sensation seeking behavior peaks relative to puberty (Steinberg, 2004; Steinberg, 2008), it is not surprising that young adolescent males may be less vulnerable than age-matched females to risk- and reward- related maladaptive behaviors.
Considering the influence of sex hormones, we evaluated the contribution of pubertal stage to sex differences in diffusion indices. We did not observe pubertal effects on white matter anisotropy or diffusivity, though the restricted range of pubertal development represented in our female sample may have precluded detection of such relationships. Our reliance on a self-report measure of pubertal maturation may have also hindered accurate characterization of pubertal stage. Another consideration is the significant variation within and across individuals in the physiological processes underlying pubertal maturation (Lenroot and Giedd, 2010). Pubertal stage may therefore only serve as a proxy for more specific hormonal indicators. Indeed, higher levels of luteinizing hormone have been linked to greater white matter density (Peper et al., 2008), but elucidation of other hormonal influences on sexual dimorphisms in white matter development is needed. Age-related changes in diffusion indices were not evident in males or females within this circumscribed age range.
In summary, our findings showed higher FA and AD in females in bilateral CST and right corona radiata, and higher AD and MD in males in frontal, fronto-parietal and fronto-temporal white matter pathways. Sex differences in association fibers may be associated with differential vulnerabilities to maladaptive behaviors, considering their link to risk-taking tendencies. The neurobiological processes contributing to the differences observed here are in need of further description with in vitro methods and multi-level analysis including white matter macrostructure. Further, as our cross-sectional assessment of sex-related white matter divergences and their behavioral correlates is a mere sampling of a unit of time, longitudinal studies are needed to provide description of sex-differences that occur over the continuous developmental trajectory of adolescence.
4. Experimental Procedure
1.1 Participants
Fifty-eight typically developing adolescents (29 males and 29 females) ranging in age from 12 to 14 years (mean age 13.4 ± 0.7 years) were recruited from local middle schools as part of an adolescent brain imaging project (Anderson et al., 2005; Fryer et al., 2008; Nagel et al., 2006; Squeglia et al., 2009). Participants and their parents or legal guardians were screened with separate, private interviews to ascertain eligibility. Exclusionary criteria were: parental history of bipolar I, psychotic, or antisocial personality disorder; complicated or premature birth (<36 weeks gestation); evidence of maternal drinking or illicit drug use during pregnancy; left handedness; history of neurological disorder or head trauma with loss of consciousness >2 minutes, learning disability or mental retardation, serious medical illness, or DSM-IV Axis I disorder including any substance use disorder; lifetime use of more than 6 alcoholic beverages, 10 cigarettes, 1 marijuana use episode, or any other illicit drug uses, or use of psychoactive medications; MRI contraindications; and clinically abnormal brain anatomy as determined by neuroradiologist review.
Participants were from a range of sociocultural backgrounds, and verbal intellectual functioning (Wechsler Abbreviated Scale of Intelligence Vocabulary subtest) (Wechsler, 1999) and reading level (Wide Range Achievement Test – 3 Reading subtest) (Wilkinson, 1993) fell in the average to very superior ranges. Emotional functioning and psychopathological syndromes were within normal limits (see Table 2). Males and females were matched on age (±6 months) and SES (±12 points) (Hollingshead, 1965). Informed assent and consent were obtained from participants and their legal guardians, respectively, in accordance with UCSD Human Research Protections Program guidelines.
Table 2.
Demographic characteristics of participants.
| Females (n=29) M (SD) or % | Males (n=29) M (SD) or % | |
|---|---|---|
| Years of age (range 12–14) | 13.43 (0.72) | 13.44 (0.64) |
| Hollingshead socioeconomic level | 19.83 (12.54) | 20.38 (13.29) |
| % Caucasian* | 62% | 86% |
| Familial history of psychopathologya | 69% | 66% |
| Parental history of alcohol or drug abuse or dependence | 24% | 45% |
| Pubertal Development Scale Category Score** (median) | 4 | 3 |
| Body mass index | 19.97 (2.73) | 19.13 (2.68) |
| Spielberger State Anxiety total score | 24.31 (5.13) | 26.79 (6.83) |
| Beck Depression Inventory total score | 1.38 (1.95) | 0.86 (1.51) |
| Child Behavior Checklist Internalizing T-score | 41.72 (6.90) | 43.38 (8.64) |
| Child Behavior Checklist Externalizing T-score | 40.00 (6.72) | 41.38 (8.26) |
| Wechsler Abbreviated Scale of Intelligence Vocabulary T-score | 58.62 (8.56) | 59.90 (7.58) |
| Wechsler Wide Range Achievement Test-3 Reading standard score | 114.62 (8.69) | 117.86 (7.33) |
| Lifetime cigarette use episodes | 0 | 0.21 (0.94) |
| Lifetime marijuana use episodes | 0 | 0 |
| Lifetime alcohol drinks | 0 | 0.28 (1.16) |
| Lifetime other drug use instances | 0 | 0 |
p<.05
p<.001
Includes depression, mania, anxiety, or mental health hospitalization
1.2 Measures
1.2.1 Mental health
The Beck Depression Inventory (BDI) (Beck, 1978) and Spielberger State Trait Anxiety Inventory (STAI) (Spielberger et al., 1970) assessed mood prior to scanning. The Child Behavior Checklist (CBCL) (Achenbach and Rescorla, 2001) was completed by the parent to assess internalizing and externalizing psychopathological syndromes. The Customary Drinking and Drug use Record (Brown et al., 1998) collected from the adolescent detailed lifetime information on quantity and frequency of lifetime alcohol, cigarette, and other drug use. History of psychiatric disorder in biological parents and relatives was assessed by interview of both biological parents using the Family History Assessment Module (FHAM) (Rice et al., 1995), and history of substance use disorder (using DSM-IV criteria) in biological parents was assessed using the Computerized Diagnostic Interview Schedule (C-DIS-IV) (Robins et al., 1996).
1.2.2 Development
The Pubertal Development Scale (PDS) (Petersen, 1988) collected self-reported indicators of participants’ pubertal status. The measure is comprised of 5 questions based on the Tanner stages that pertain to changes in height, body hair, and skin, and additionally menstruation for females and voice changes for males. Responses were summed and transformed into PDS Category Scores (1 = Prepubertal, 2 = Early pubertal, 3 = Midpubertal, 4 = Late pubertal, and 5 = Postpubertal) (Carskadon and Acebo, 1993). Body mass index of each adolescent was calculated as weight (lb) / ([height (in)]2 × 703).
1.3 MR Acquisition
Participants were imaged in a 3T General Electric Excite MR system with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA). A scout scan ensured good head placement and whole-brain coverage. High angular resolution diffusion images (HARDI) (Frank, 2001) were collected along 61 noncollinear directions determined by the electrostatic repulsion model which minimizes bias in measurements by sampling with approximately uniform distribution on a sphere (Jones et al., 1999), in addition to a reference image with no diffusion weighting (b=0). The diffusion encoding scheme consisted of a single-shot dual spin echo excitation optimized for minimum TE and reduction of eddy current artifacts (Reese et al., 2003). The following sequence parameters were applied; TE/TR=93/10,900 ms, FOV=240 mm, matrix =128×128, 34 contiguous slices, 3 mm slice thickness, b-value=1500 s/mm2, one average. Two field maps were collected for unwarping to correct for signal loss and geometric distortion due to B0 field inhomogeneities (Andersson and Skare, 2002; Jezzard and Balaban, 1995). Total scan time including field maps was 16.02 minutes.
1.4 Data Processing
Datasets were visually inspected slice-by-slice for each subject, and 23 participants were removed from the analysis due to motion artifact (n=19) or technical problems (n=4). The final sample included 58 participants (29 males and 29 females). The 23 excluded participants showed a larger male to female ratio, but did not differ in age (p = .40), verbal intellectual functioning (Vocabulary T-score: p = .39; Reading standard score: p = .11), or psychopathological symptoms (CBCL Total Problems T-score: p = .48) from the 58 adolescents with valid data.
Valid datasets were corrected for head motion, eddy current distortion, and signal loss using FSL tools (FMRIB Software Library, Oxford, United Kingdom; (Smith et al., 2004). Specifically, image acquisitions for each direction were merged into a single 4D file and aligned to the first volume using affine registration with six degrees of freedom and Fourier interpolation to correct for motion (FLIRT-FMRIB's Linear Image Registration Tool; (Jenkinson et al., 2002). Each of the 61 direction files were then registered to the B0 image using a six-parameter registration in 2D to minimize eddy current distortions (FDT-FMRIB’s Diffusion Toolbox 2.0; (Behrens et al., 2003). Next, phase unwrapping (PRELUDE-Phase Region Expanding Labeler for Unwrapping Discrete Estimates; (Jenkinson, 2003) and regularization (FUGUE-FMRIB's Utility for Geometrically Unwarping EPIs; (Jenkinson and Smith, 2001) of field maps were conducted for quantifying field distortions. Resulting measurements were translated into voxel shifts, effectively assigning image intensities to correct voxel locations.
Pre-processed images were subjected to tensor decomposition to derive scalar diffusion indices FA, MD, AD, and RD (Le Bihan et al., 2001). This computation was performed in native coordinate space using Analysis of Functional NeuroImages’ (Cox, 1996) diffusion plug-in routine, 3dDWItoDT (Cox and Glen, 2006). Diffusion indices were examined with Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006), and involved the following steps. To achieve initial alignment, FA maps were registered to an averaged FA template (FMRIB-58) in MNI-152 standard space using an affine-only registration. This was followed by a non-linear transformation into 1-mm cubic voxel dimensions (FNIRT-FMRIB’s Non-linear Registration Tool). Data were examined for laterality, orientation, and cross-subject anatomical alignment. Next, transformed images were averaged across subjects to create a mean FA image, from which a white matter skeleton was derived, representing tracts common to all subjects. Individual transformed FA images were then projected onto the skeleton. To minimize partial-volume effects and areas of high inter-subject variability, values were thresholded at FA>0.2. MD, AD, and RD data were processed using the same nonlinear transformation, skeleton, and skeleton-projection vectors derived from the FA analysis (Smith et al., 2007). Data from each point on the skeleton formed the basis of voxelwise statistical comparisons.
1.5 Statistical Analysis
Voxelwise statistics on skeleton space FA, MD, RD, and AD data were carried out in AFNI using independent sample t-tests. Correction for multiple comparisons was achieved through a combination of individual voxel probability and cluster size thresholding using Monte Carlo simulation (Ward, 2000) for Type I error control. Considering that skeletonized datasets typically have an intrinsic smoothness on the order of 4 mm full width half maximum (Smith et al., 2006), this parameter was included in the multiple comparison correction. Under this criteria, only clusters ≥ 164 µl (164 contiguous 1 × 1 × 1 mm voxels) with an individual voxel probability threshold of p<.01, yielding a brain-wise p<.05 of finding such a cluster under the null hypothesis, were interpreted. Cohen’s d effect sizes (Cohen, 1988) were computed from the average t-value within each significant cluster. Anatomical identification of tract structures was confirmed using white matter atlases (Mori et al., 2008; Wakana et al., 2004). The relationship between diffusion indices in anatomically overlapping regions were examined with Pearson’s r correlation coefficients (α=.05).
Examination of PDS indicated that females fell primarily within the late- (66%) or post-pubertal (21%) stage, whereas males’ pubertal development spanned early- (24%), mid- (52%) and late-pubertal (21%) stages. Distributions of PDS Category Scores across genders precluded a voxelwise analysis of a sex × pubertal stage interaction. Instead, pubertal development was examined in a multivariate analysis of covariance (MANCOVA) to determine whether clusters with a significant sex difference persisted after accounting for PDS Category Scores (α=.05). Following this, separate regression analyses for males and females were performed to assess the unique contributions of age and PDS Category Score on FA, MD, and AD within each cluster; p-values <.05, corrected for multiple comparisons using Bonferroni adjustments, were considered significant.
Research Highlights
Adolescent females showed widespread white matter changes in motor tracts.
Adolescent males showed white matter changes in projection and association fibers.
Pubertal stage did not account for sex differences in white matter development.
Acknowledgements
This research was supported through grants from the National Institutes of Health (grant R01 AA13419 to S.F. Tapert and F32 DA024476 to S. Bava). We extend our appreciation to participants and their families and schools, as well as to Sonja Eberson, Alejandra Infante, Jesse Feng, Sonia Lentz, Andria Norman, and Drs. Lawrence Frank and MJ Meloy whose support was vital to the completion of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Anderson KG, Schweinsburg AD, Paulus MP, Brown SA, Tapert SF. Examining personality and alcohol expectancies using fMRI with adolescents. Journal of Studies on Alcohol. 2005;66:323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S. A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage. 2002;16:177–199. doi: 10.1006/nimg.2001.1039. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory (BDI) San Antonio, TX, USA: Psychological Corporation; 1978. [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Blanton RE, Levitt JG, Peterson JR, Fadale D, Sporty ML, Lee M, To D, Mormino EC, Thompson PM, McCracken JT, Toga AW. Gender differences in the left inferior frontal gyrus in normal children. Neuroimage. 2004;22:626–636. doi: 10.1016/j.neuroimage.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48:223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cox R. Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R, Glen D. Efficient, Robust, Nonlinear, and Guaranteed Positive Definite Diffusion Tensor Estimation., International Society of Magnetic Resonance in Medicine. Proc. ISMRM 14th Scientific Meeting; Seattle, WA. 2006. [Google Scholar]
- Crone EA, Bullens L, van der Plas EA, Kijkuit EJ, Zelazo PD. Developmental changes and individual differences in risk and perspective taking in adolescence. Dev Psychopathol. 2008;20:1213–1229. doi: 10.1017/S0954579408000588. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LR. Anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med. 2001;45:935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 2008;67:225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, Tossell J, Addington A, Gogtay N. Trajectories of anatomic brain development as a phenotype. Novartis Found SympTrajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–112. doi: 10.1002/9780470751251.ch9. discussion 112-8, 193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H, James AC. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Hua X, Leow AD, Levitt JG, Caplan R, Thompson PM, Toga AW. Detecting brain growth patterns in normal children using tensor-based morphometry. Hum Brain Mapp. 2009;30:209–219. doi: 10.1002/hbm.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, LLeemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Janke AL, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE. Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology. 2008;33:909–915. doi: 10.1016/j.psyneuen.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, q, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45:1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Petersen AC. Adolescent development. Annu Rev Psychol. 1988;39:583–607. doi: 10.1146/annurev.ps.39.020188.003055. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44:1210–1221. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Schwartz ES. Principles and implementation of diffusion-weighted and diffusion tensor imaging. Pediatr Radiol. 2007;37:739–748. doi: 10.1007/s00247-007-0516-z. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version 4.0 (DIS 4.0) St. Louis, MO: Washington University; 1996. [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage. 2010;51:817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magn Reson Imaging. 2006;24:833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Palo Alto, CA, USA: Consulting Psychologists Press; 1970. [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Ward DB. Simultaneous Inference for fMRI Data. Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- Wechsler D. Manual for the Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. The wide range achievement test-3 administration manual. Wilmington, DE, USA: Jastak Associates; 1993. [Google Scholar]