Abstract
Single channel currents of lysenin were measured using artificial lipid bilayers formed on a glass micropipette tip. The single channel conductance for KCl, NaCl, CaCl2, and Trimethylammonium-Cl were 474 ± 87, 537 ± 66, 210 ± 14, and 274 ± 10 pS, respectively, while the permeability ratio PNa/PCl was 5.8. By adding poly(deoxy adenine) or poly(L-lysine) to one side of the bilayer, channel currents were influenced when membrane voltages were applied to pass the charged molecules through the channel pores. Current inhibition process was concentration-dependent with applied DNA. As the current fluctuations of α-hemolysin channels is often cited as the detector in a molecular sensor, these results suggest that by monitoring channel current changes, the lysenin channel has possibilities to detect interactions between it and certain biomolecules by its current fluctuations.
Keywords: hemolytic toxin, patch clamp, single channel current, current inhibition
Introduction
Lysenin is a novel protein obtained from the body fluid of the earthworm Eisenia foetida.1,2) with a molecular weight of 33 kDa as a monomer. It specifically binds to sphingomyelin (SM) in the cell membrane3) and induces cytolysis by forming membrane-spanning channel pores.4) Because of its specific binding properties to SM, it has been used as a marker for membrane rafts since these structures are rich in sphingolipids and cholesterol.5–7) To date there are few studies that have investigated the electrophysiological properties of the lysenin protein channel.8–11) Thus the detailed features of the single lysenin channel are essentially unknown.
In this study, we measured single lysenin channel properties by using artificial lipid bilayers formed on a glass pipette tip at various ionic conditions. We found that the lysenin channel has little selectivity between monovalent cations. It does, however, show charge selectivity against divalent cations and a change in permeability depending on the radius of the ion species.
We also examined the possibility of applying the lysenin channel as a biomolecule sensor. Upon interacting with biomolecules such as poly(dA) or PLL, single lysenin channel currents responded in a predictable manner. The time course of inhibition by adding DNA molecules depended on the concentration of applied DNA. These results indicate that the lysenin channel has the potential to detect certain inhibitor or ligands by monitoring its current change in response to interactions between it and specific biomolecules.
Materials and methods
Materials.
Lysenin, asolectin, cholesterol (Cho), and phosphatidylserine (PS) were purchased from Sigma-Aldrich (St. Louis, MO); Diphytanoylphosphatidylcholine (DPhPC) and sphingomyelin (SM) from Avanti Polar Lipids (Alabaster, AL); n-decane, poly(ethylene glycol) (PEG), poly(L-lysine) (PLL) from Wako Pure Chemical Industries (Osaka, Japan); poly(deoxy adenine)100 (poly(dA)) from Eurogentec (Hampshire, UK). All other chemicals were commercial products of an analytical grade.
Membrane formation.
Artificial lipid bilayers were formed on the tip of a glass micropipette according to the Tip-Dip method12–14) with some modifications. A glass capillary (GC150T-10, Harvard, Holliston, MA) was pulled by a Pt heating puller (P-97, Sutter, Novato, CA). The tip diameter was set to approximately 20 µm as controlled by a micro forge (MF-900, Narishige, Tokyo, Japan). The pipette was plunged into the recording chamber through lipid-decane layers that were made by applying a droplet of lipids onto an aqueous solution. Because bilayer formation is more probable in thinner layers, excess lipid solution at the tip of the pipette was removed by applying pressure through the pipette by a syringe. Bilayer formation was confirmed by monitoring capacitive surge current as well as by observing binocular images.
Current recording.
In all experiments, lysenin was added only to the bottom chamber (Fig. 1a ). The membrane voltage was defined as the voltage of the upper side of the membrane with respect to the bottom side, which was defined as virtual ground. Current data was recorded with a patch clamp amplifier (CEZ-2400, Nihon Koden, Tokyo, Japan) with a bandwidth of 2 kHz and analyzed with pClamp9.02 (Molecular Devices, Sunnyvale, CA). Before every recording sequence, electrode polarization was properly corrected.
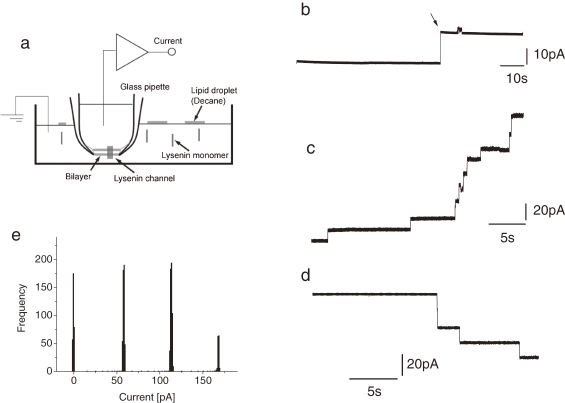
Figure 1.
Schematic drawing of the experimental setup (a), single (b) and multiple (c, d) lysenin channel currents. Channel currents were recorded in a symmetrical solution containing 100 mM KCl and 10 mM MOPS pH 7.4. The lipid composition of the bilayer was 10 mg/ml DPhPC and 4 mg/ml SM in decane. Final concentration of lysenin: 5 pM (b) and 100 pM (c, d). Holding potential (Vm): 50 mV (b), 40 mV (c) and −40 mV (d). (e) An example of a stepwise current histogram in multiple lysenin channels. Vm = 100 mV. Solution: 100 mM NaCl and 10 mM MOPS pH 7.4.
Results
Single channel properties of lysenin current.
Single and multiple channel currents of lysenin in DPhPC-SM bilayers were measured (Fig. 1b–d). A stepwise increase in current was observed several minutes after the addition of lysenin into the experimental chamber (Fig. 1a). It has been reported that lysenin channels exhibit remarkable voltage dependency when they are incorporated in a bilayer membrane formed with soybean PC asolectin,9,11) but no closing events were observed in this experimental condition. The number of observed stepwise increases in current was proportional to the final concentration of added lysenin. Intervals between peak positions were at every 60 pA (Fig. 1e).
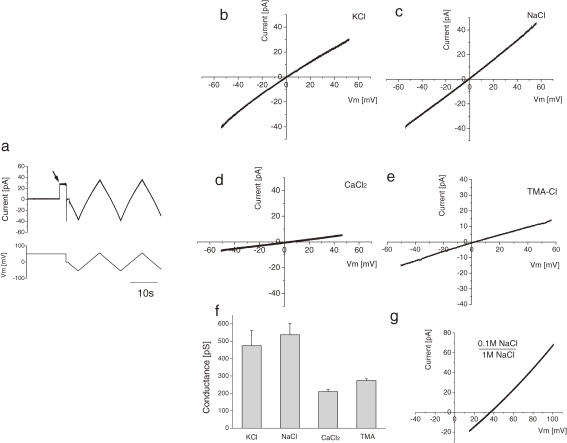
To evaluate the current–voltage relationship, menbrane potentials (Vm) ranged from −80 to +80 mV (Fig. 2a ). After a single step appeared in the current trace (Fig. 2a, arrow), a triangle wave was applied as an external command voltage. The recorded currents for the following ionic concentrations, 100 mM KCl, 100 mM NaCl, 50 mM CaCl2 and 100 mM Trimethylammonium-Cl (TMA-Cl), were plotted against Vm (Fig. 2b, c, d, e, respectively). These linear current–voltage relationships indicate the channel current property is Ohmic in this voltage range.
Figure 2.
Current–voltage relationships of single lysenin channels. (a) Experimental criteria for obtaining the I–V curve. Current trace (upper panel) was monitored. After the appearance of a single channel current step (arrow), the holding potential (lower panel) was scanned by adding a triangle wave. (b–e) I–V curves of single lysenin channels. Lipid composition of bilayer: 10 mg/ml DPhPC and 4 mg/ml SM. Final concentration of lysenin: 5 pM. Solution: (b) 100 mM KCl and 10 mM MOPS pH 7.4, (c) 100 mM NaCl and 10 mM MOPS pH 7.4, (d) 50 mM CaCl2 and 10 mM MOPS pH 7.4, (e) 100 mM Trimethylammmonium (TMA)-Cl and 10 mM MOPS pH 7.4. (f) Histogram of lysenin channel conductance calculated from I–V curves. Conductance values: 474 ± 87 pS for KCl (mean ± sd, N = 11), 537 ± 66 pS for NaCl (N = 5), 210 ± 14 pS for CaCl2 (N = 3), and 274 ± 10 pS for TMA-Cl (N = 3). (g) I–V relationship of a single lysenin channel under an asymmetric solution condition of 0.1 M NaCl and 10 mM MOPS pH 7.4 in the pipette and 1 M NaCl and 10 mM MOPS pH 7.4 in the bath. The shift of the reversal potential was approximately +35 mV.
Single channel conductance for each ion was calculated from the slope of the I–V curves. The data is summarized in Fig. 2f. The conductance for KCl, NaCl, CaCl2, and TMA-Cl were 474 ± 87, 537 ± 66, 210 ± 14, and 274 ± 10 pS, respectively.
Figure 2g shows the shift of the I–V relationship in single channel currents when a 10-fold NaCl concentration gradient exists between the pipette and the bath solution. In this condition, the shift of the reversal potential was approximately +35 mV.
Inhibition of channel current by macromolecules.
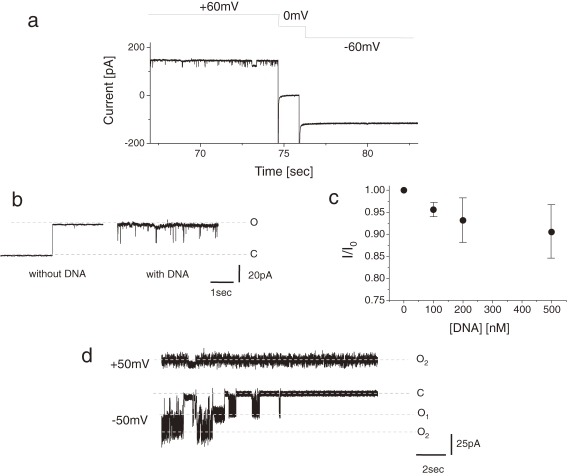
Figure 3 shows channel current inhibition induced by charged macromolecules. By adding poly(dA)100 (final conc. 2 nM) into the bottom chamber, partial inhibition of the channel was observed only when positive membrane potentials were applied (Fig. 3a). The magnitude of the current was almost half the single channel current not inhibited by DNA application. Figure 3b shows the effects of DNA on a single channel current in which short blocking events were seen. The dose response curve was obtained from multi-channel experiments in which ∼102 channel molecules were incorporated into each membrane. The mean fraction of current remaining (I/I0) in response to DNA concentration is plotted Fig. 3c.
Figure 3.
(a) Inhibition of the lysenin channel current by poly(dA)100. Upper panel: time course of applied membrane potential; lower panel: time course of channel current. (b) Effect of DNA on a single channel current. Single channel traces measured before and after addition of poly(dA)100. Final concentration of poly(dA)100: 2 nM. Holding potential: +60 mV. Solution: 100 mM KCl and 10 mM MOPS pH 7.4. Lipid contents: 20 mg/ml DPhPC and 4 mg/ml SM. (c) Dose–response relation of the effect of poly(dA)100 on a lysenin channel. Mean fraction of current remaining (I/I0) after addition of DNA was plotted against DNA-concentrations (mean ± SD, n = 3). (d) Inhibition of the lysenin channel current by poly(L-lysine). The current was measured ∼30 sec after addition of poly(L-lysine). Final concentration of poly(L-lysine): 30 µM. Holding potential: +50 mV (upper) and –50 mV (lower). Solution: 100 mM KCl and 40 mM Tris-Hepes pH 7.4. Lipid contents: 20 mg/ml DPhPC and 8 mg/ml SM.
The lysenin channel was also blocked by poly(L-lysine) (PLL, Mw: 5000–15000) as shown in Fig. 3c. Within 10 seconds after adding PLL to the bath solution (final conc; 30 µM), lysenin channels showed gradual inhibition until they were completely blocked under a negative membrane potential. At a positive membrane potential, the blocking phenomena could not be observed. There were two channels in this bilayer membrane and both were shown to be blocked by PLL. PEG4000, on the other hand, did not block the channel current (data not shown).
Discussion
In this study, we measured the single channel properties of lysenin in detail. The average value of the conductance for KCl (∼480 pS) was small compared to a previous report.9) However, this is likely explained by differences in the bilayer formation technique and lipid content. KCl and NaCl conductance values were approximately equal and about twice that of CaCl2 conductance.
From the reversal potential obtained in the presence of 50 mM CaCl2/100 mM NaCl (10 mV), an apparent permeability ratio for PCa/PNa of 1.2 was calculated using the Goldman–Hodgkin–Katz (GHK) equation. These results suggest that the lysenin channel has little selectivity between monovalent cations but does show charge selectivity against divalent cations.
Like the case of Ca2+, TMA+ conductance was about one half that of Na+ and K+, which indicates the ion radius influences channel conductance. Electron microscopy studies of lysenin-containing liposomes have shown that lysenin oligomers spanning the bilayer membrane have an open vestibule of about several nanometers in diameter.4) It has been inferred that there exist “bottleneck” positions within the channel that have diameters comparable to the radius of the TMA ion. Such structures could explain the poor TMA permeability.
According to the reversal potential shift value derived from an asymmetric solution composition between the bilayer, the permeability ratio PNa/PCl was 5.8 according to the GHK relationship, indicating that the channel has an approximately 6-fold preference for cations over anions. Compared to other pore-forming toxins such as Delta-endotoxins CryIA(c) (PK/PCl ∼ 25), CryIIIA (PK/PCl ∼ 25)15) and Actinaria cytolysin St-I (Pcation/Panion ∼ 4),16) lysenin has moderate cation selectivity in its channel function.
We also examined how the lysenin channel can be used as a molecular sensor by observing the channel current fluctuations. α-hemolysin is often cited as the preferred biological channel for a detector pore in a molecular sensor.17,18) However, the lysenin channel has some ideal features too including high stability and silent single currents,8,9,11) which are advantageous for high-sensitive detection of certain stochastic events. Furthermore, we found that the protein can form a stable channel even after incubated for several days at room temperature. This result suggests a potential clinical use.
Because we could block the lysenin channel with either DNA or PLL at opposite potential polarities (Fig. 3a, c), the electrophoretic movement of certain charged macromolecules could be detected by observing current changes. The change in the current inhibition reflects the kinetics of the interaction between channel pore and analytes. As shown in Fig. 3c, the inhibition by DNA was dependent on DNA concentration. Therefore, the channel current of lysenin can be applied to bio-sensing devices in order to detect the concentration of analytes.
It was previously reported that lysenin’s hemolytic activity was inhibited by PEG4000.4) In fact, in our bilayer experiments, PEGs of any molecular weight (Mw: 300–8000) failed to significantly inhibit channel activity. This suggests that the reported hemolysis inhibition activity by PEG seen previously in hemolytic activity experiments was either not the result of completely blocking channel current or those larger molecules of Mw > 8000 are needed to plug the pore when applying PEGs in single channel current experiments.
In conclusion, the lysenin pore is applicable for the detection of biomolecular properties. Further control of the pore chemistry should improve the performance of these bio-sensor devices.
Acknowledgements
We thank our colleagues at Osaka University for valuable discussions and Drs. Sho Carl Shibata and Peter Karagiannis for critical reading of the manuscript. This work was supported by the grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Promotion of Novel Interdisciplinary Fields Based on Nanotechnology and Materials, and Innovative Nanoscience of Supermolecular Motor Proteins Working in Biomembrane).
Abbreviations
- SM
sphingomyelin
- TMA
Trimethylammonium
- DPhPC
diphytanoylphosphatidylcholine
- Cho
cholesterol
- PS
phosphatidylserine
- PG
phosphatidylglycerol
- PC
phosphatidylcholine
- PLL
poly(L-lysine)
- PEG
poly(ethylene glycol)
- poly(dA)
poly(deoxy adenine)100.
References
- 1).Roch P., Canicatti C., Valembois P. (1989) Interactions between earthworm hemolysins and sheep red blood cell membranes. Biochim. Biophys. Acta 983, 193–198 [DOI] [PubMed] [Google Scholar]
- 2).Sekizawa Y., Kubo T., Kobayashi H., Nakajima T., Natori S. (1997) Molecular cloning of cDNA for lysenin, a novel protein in the earthworm Eisenia foetida that causes contraction of rat vascular smooth muscle. Gene 191, 97–102 [DOI] [PubMed] [Google Scholar]
- 3).Kiyokawa E., Makino A., Ishii K., Otsuka N., Yamaji-Hasegawa A., Kobayashi T. (2004) Recognition of sphingomyelin by lysenin and lysenin-related proteins. Biochemistry 43, 9766–9773 [DOI] [PubMed] [Google Scholar]
- 4).Yamaji-Hasegawa A., Makino A., Baba T., Senoh Y., Kimura-Suda H., Sato S.B., et al. (2003) Oligomerization and pore formation of a sphingomyelin-specific toxin, lysenin. J. Biol. Chem. 278, 22762–22770 [DOI] [PubMed] [Google Scholar]
- 5).Yamaji A., Sekizawa Y., Emoto K., Sakuraba H., Inoue K., Kobayashi H., et al. (1998) Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 273, 5300–5306 [DOI] [PubMed] [Google Scholar]
- 6).Ishitsuka R., Yamaji-Hasegawa A., Makino A., Hirabayashi Y., Kobayashi T. (2004) A lipid-specific toxin reveals heterogeneity of sphingomyelin-containing membranes. Biophys. J. 86, 296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Grzybek M., Kozubek A., Dubielecka P., Sikorski A.F. (2005) Rafts—the current picture. Folia Histochem. Cytobiol. 43, 3–10 [PubMed] [Google Scholar]
- 8).Bruhn H., Winkelmann J., Andersen C., Andrä J., Leippe M. (2006) Dissection of the mechanisms of cytolytic and antibacterial activity of lysenin, a defence protein of the annelid Eisenia fetida. Dev. Comp. Immunol. 30, 597–606 [DOI] [PubMed] [Google Scholar]
- 9).Ide T., Aoki T., Takeuchi Y., Yanagida T. (2006) Lysenin forms a voltage-dependent channel in artificial lipid bilayer membranes. Biochem. Biophys. Res. Commun. 346, 288–292 [DOI] [PubMed] [Google Scholar]
- 10).Kwiatkowska K., Hordejuk R., Szymczyk P., Kulma M., Abdel-Shakor A.B., Plucienniczak A., et al. (2007) Lysenin-His, a sphingomyelin-recognizing toxin, requires tryptophan 20 for cation-selective channel assembly but not for membrane binding. Mol. Membr. Biol. 24, 121–134 [DOI] [PubMed] [Google Scholar]
- 11).Fologea D., Krueger E., Lee R., Naglak M., Mazur Y., Henry R., et al. (2010) Controlled gating of lysenin pores. Biophys. Chem. 146, 25–29 [DOI] [PubMed] [Google Scholar]
- 12).Ehrlich B. (1992) Planar lipid bilayers on patch pipettes: Bilayer formation and ion channel incorporation. Methods Enzymol. 207, 463–470 [DOI] [PubMed] [Google Scholar]
- 13).Oiki, S., Kopper, R.E. II and Anderson, O.S. (1997) Voltage-dependent gramicidin channels. In Progress in Cell Research Vol. 6: Towards Molecular Biophysics of Ion Channels (eds. Sokabe, M., Auerbach, A. and Sigworth, F.J.). Elsevier, Amsterdam, pp. 189–201. [Google Scholar]
- 14).Matsumori N., Eiraku N., Matsuoka S., Oishi T., Murata M., Aoki T., et al. (2004) An amphotericin B-ergosterol covalent conjugate with powerful membrane permeabilizing activity. Chem. Biol. 11, 673–679 [DOI] [PubMed] [Google Scholar]
- 15).Slatin S.L., Abrams C.K., English L. (1990) Delta-endotoxins form cation-selective channels in planar lipid bilayers. Biochem. Biophys. Res. Commun. 169, 765–772 [DOI] [PubMed] [Google Scholar]
- 16).Tejuca M., Serra M.D., Ferreras M., Lanio M.E., Menestrina G. (1996) Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry 35, 14947–14957 [DOI] [PubMed] [Google Scholar]
- 17).Ashkenasy N., Sánchez-Quesada J., Bayley H., Ghadiri M.R. (2005) Recognizing a single base in an Individual DNA strand: A step toward DNA sequencing in nanopores. Angew. Chem. Int. Ed. 44, 1401–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Robertson J.W., Rodrigues C.G., Stanford V.M., Rubinson K.A., Krasilnikov O.V., Kasianowicz J.J. (2007) Single-molecule mass spectrometry in solution using a solitary nanopore. Proc. Natl. Acad. Sci. USA 104, 8207–8211 [DOI] [PMC free article] [PubMed] [Google Scholar]