Abstract
Background
Distress may be heightened among members of cancer patient-caregiver dyads that are mismatched on smoking status (either the patient or caregiver smokes, but the other does not), negatively affecting quality of life (QoL). The purpose of this study was to examine associations between patient-caregiver smoking concordance, caregiver psychological adjustment, and caregiver and patient mental and physical QoL.
Methods
Lung and colorectal patient-caregiver dyads (N= 742) were identified from the Cancer Care Outcomes Research and Surveillance (CanCORS) and CanCORS Caregiver studies. The majority of the cancer patients were male (67.0 %) with local (45.6 %) or regional (12.9 %) disease. The majority of the informal caregivers were females (78.6%), under 65 years of age (69.6%), and often spouses (57.8%) of the patients.
Results
Lung and colorectal cancer caregivers who were members of dyads where one or both members continued to smoke, reported worse mental health QoL than non-smoking dyads. For colorectal cancer patients, continuing to smoke when the caregiver did not was associated with worse mental health QoL compared to non-smoking dyads. Dyad smoking was less strongly associated with physical QoL for both caregivers and patients.
Conclusion
Results highlight the importance of assessing smoking in both cancer patients and their caregivers and referring families to appropriate psychosocial and smoking cessation services.
Impact
This is the first study to show associations between cancer patient-caregiver smoking status and QoL for both dyad members. Future studies will need to confirm these associations longitudinally and investigate potential mechanisms linking dyad smoking and QoL.
Keywords: lung cancer, colorectal cancer, smoking, caregivers, quality of life
Introduction
Cigarette smoking increases the risk of a number of cancers (1, 2), and continued smoking after a cancer diagnosis has been linked with adverse outcomes for cancer patients, including treatment complications, diminished treatment efficacy, reduced overall survival, increased risk of second cancers, and poorer quality of life (QoL) (for reviews see (3–5)). Unfortunately, a substantial number of people with smoking-related and non-smoking related cancers continue to smoke after their diagnosis. Estimates vary, with the highest rates reported for people with lung and head and neck cancers (with 24%– 60% smoking) (6–8) and more modest rates among breast, prostate, and colorectal cancer survivors (7%–13% smoking (9)). Population-based data suggest that approximately 20% of long-term cancer survivors smoke after their diagnosis (10, 11), with rates varying greatly by age and cancer site.
Smoking may also be a concern for family members who serve as informal caregivers while the cancer patient undergoes treatment. Smoking-related comorbidities such as cardiovascular disease and chronic obstructive pulmonary disease may comprise the health of family caregivers, diminishing their quality of life. In addition, smoking is linked with negative emotionality, depression, and history of psychiatric disorders in the general population (12, 13), suggesting that smoking caregivers may be a population at risk for poor mental health-related quality of life, especially in the context of increased stress associated with caregiving. Although one study of family members of patients with lung cancer found that symptoms of distress were actually associated with increased intentions to quit smoking (14), findings from the general caregiving literature indicate that, compared with non-caregivers, caregivers are more likely to engage in unhealthy lifestyle behaviors (15). This includes include greater frequency of smoking (16), particularly if they are providing high-levels of care or experiencing high strain. This suggests that the stress and burden of caregiving might undermine smoking cessation efforts or that smoking may increase perceived stress and burden among caregivers. Research shows that cancer patients who smoke are likely to have family members who smoke (14). One cessation trial recruiting smoking relatives of cancer patients reported that on average, cancer patients have two relatives who smoke (17) and a recent study reported that 18% of family caregivers of women with lung cancer continued smoking after the diagnosis(18).
It may be especially important to consider smoking in the context of dyads of cancer patients and their family caregivers. Continued smoking after a cancer diagnosis may be associated with guilt or blame about the cause of the cancer, particularly for cancers that are strongly associated with smoking, such as lung cancer. Patients and caregivers may experience increased distress after a cancer diagnosis if their loved one continues to smoke, particularly if they are not smoking, due to worry and increased recognition of the health risks for both partners(19). If a cancer patient does quit smoking, continued smoking by family members may undermine the patients’ quit attempts (20) and cause contentious interactions among family members. In a qualitative study of lung cancer patients, most of whom quit smoking after their diagnosis, Bottorff and colleagues (21) found that many relationships with smoking family members were characterized by frequent stressful interactions regarding smoking and coercive attempts to get the family member to stop smoking. These types of negative interpersonal encounters would be expected to undermine social support and increase distress, thereby diminishing dyad members’ QoL. Despite the plausible mechanisms connecting cancer caregiver-patient smoking and QoL, few prior studies have examined whether the smoking of one dyad member is related to the QoL of both caregivers and their care recipients diagnosed with cancer. This study sought to build on the prior research by utilizing a large sample of cancer patients and caregivers recruited from multiple sites across the country that included patients with a cancer strongly associated with smoking (lung cancer) and one not commonly associated with smoking (colorectal cancer).
The first aim of the present study was to characterize patterns of smoking among recently diagnosed lung and colorectal cancer patients and their family caregivers, Second, we sought to characterize the context of smoking among lung and colorectal cancer caregivers by examining the association between caregiver smoking and psychosocial adjustment. As prior research has indicated that high-level or more intense caregiving is associated with more smoking among caregivers (16), we hypothesized that smoking caregivers would report greater perceived caregiving burden and greater depression and anxiety. Finally, we examined the concordance of smoking between caregivers and cancer patients and concomitant associations with mental and physical QoL for both cancer patients and caregivers. Based on prior research indicating stronger associations between caregiving strain and emotional, rather than physical QoL(22), we hypothesized that dyad members with discordant smoking status (one member smoked while the other did not) would report poorer mental health, but no difference in physical QoL.
Method
Sample
Cancer patient and caregiver dyads were identified from the National Cancer Institute Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium study (23) and the linked CanCORS Caregiver study (24). The seven CanCORS consortium sites used identical data collection methods and recruited patients from cancer registries (5 sites) and integrated health care systems (2 sites). The caregiver study was started approximately one year after the cohort study began enrolling patients and consecutive CanCORS participants were asked to provide the name and contact information of an informal caregiver (e.g., family, friends, etc.) or the person who was “most likely to care for you should you need it” at either the baseline CanCORS survey (approximately 4 months after diagnosis) or the follow-up survey (approximately 12 months after diagnosis). Only data from the baseline patient and caregiver surveys were used in this analysis. Caregivers received a self-administered questionnaire in the mail approximately one to three weeks after the patient assessment. Patient and caregiver assessments occurred in 2004 and 2005. Study procedures were approved by the institutional review boards of all participating institutions.
Patients in the CanCORS study identified 1,505 caregivers at the baseline assessment. Of these, 75 were not mailed a questionnaire due to a delayed start at some sites. Of the 1,430 mailed, 828 were completed. An additional 113 were returned, but not eligible because the identified patient had never needed care or the respondent had never provided care, and 33 were not eligible because of other reasons (unable to read English, bad contact information, or they were deceased)., The response rate was 65.9% of eligible respondents. We excluded 54 dyads because self-reported patient data were not available and 32 dyads where the patient died before the caregiver completed the survey, resulting in an analytic sample of 742 for models including only caregiver smoking status. For an additional 85 dyads, the patient completed a brief survey that did not contain information about smoking behaviors. Complete patient and caregiver smoking data were available for 637 dyads (313 colorectal and 324 lung).
Measures
Current smoking status was ascertained by self-report for both patients and caregivers. Patients were asked “Do you smoke cigarettes regularly now? [a few cigarettes every day]” and caregivers were asked “Do you now smoke cigarettes?”. Caregivers reporting smoking ”every day” or “some days” were classified as current smokers. QoL was assessed via the Short Form-12 (SF-12) (25), using the physical (PCS) and mental (MCS) component subscales1. For both subscales the general population mean is 50 and the standard deviation is 10. Additional caregiver measures included the short form of the Zarit Burden Inventory (26) to assess perceived caregiving burden, , and the Center for Epidemiological Studies Depression (CES-D 10) scale-short form (27). We used a three item measure of caregiving-related financial strain (“How much do you agree or disagree with the following statements: 1) “My financial resources are adequate to pay for things that are required for caregiving (reversed)”, 2) It is difficult to pay for the things my Care Recipient needs,” and 3) Caring for my Care Recipient puts a financial strain on me.” rated on a scale of 1= Disagree A Lot to 5= Agree A Lot), The Chronbach’s alpha reliability for this scale was .76. We measured anxiety using 4 items from the Hopkins Symptoms Checklist (28) (“In the past four weeks, I have felt… (nervous or shaking inside, suddenly scared for no reason, tense or keyed up, and spells of terror or panic), rated on a four point Likert scale (1= Rarely, or none of the time to 4= Most or all of the time). Chronbach’s alpha was .87 for these items. Cancer stage at diagnosis was ascertained from patient medical records and categorized as local, regional, or distant stage. Demographic characteristics including gender, age, education, race/ethnicity were gathered from the patient and caregiver surveys.
Analytic Plan
First, we examined the prevalence of smoking in both caregivers and patients and for the matched dyads. Next, caregiver smoking and caregiver-patient dyad smoking were considered as predictors of caregiver burden, financial strain, depression, and anxiety in multivariate models statistically adjusting for sociodemographic characteristics (gender, race/ethnicity, age, education, timing of assessment, cancer site, & relationship between patient and caregiver) using multiple linear regression. Finally caregiver-patient dyad smoking (i.e., neither smoked, caregiver only smoked, patient only smoked, both smoked) was examined as a predictor of both patient and caregiver QoL in separate models. As stigma associated with the perceived causal relationship between smoking and cancer might differ between the lung and colorectal cancer samples, we decided to stratify all analyses by cancer type.
Results
Sample Characteristics
Sociodemographic characteristics of participating caregivers and patients are shown in Table 1. The majority of the caregivers were females (78.6%), 65 years of age or younger (69.6%), and often spouses (57.8 %) of the patients. Most of the caregivers were non-Hispanic, White (76.5%), with at least some college education (59.8%), and resided in the same household as the patient (71%). In contrast, the majority of the lung (n=383, 51.6%) and colorectal cancer (n=359, 48.4%) patients were male (67.0%) and older than the caregivers (46.9% 65 years of age and younger). Race/ethnicity and education were similar to the patients. A slight majority of the patients had localized (45.6%) or regional (12.9%) disease, while 41.5% had distant metastases.
Table 1.
Descriptive Characteristics of Cancer Caregivers and Patients
| Caregivers N=742, % |
Cancer Patients N=742 % |
||||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 155 | 21.4 | 497 | 67.0 | |
| Female | 570 | 78.6 | 245 | 33.0 | |
| Age | |||||
| <=50 years | 191 | 26.5 | 44 | 5.9 | |
| 51–65 years | 311 | 43.1 | 304 | 41.0 | |
| 66–80 years | 193 | 26.7 | 321 | 43.3 | |
| >80 years | 27 | 3.7 | 73 | 9.8 | |
| Race/Ethnicity | |||||
| White, non-Hispanic | 530 | 76.5 | 564 | 76.3 | |
| Black | 97 | 14.0 | 113 | 15.3 | |
| Hispanic or Latino | 28 | 4.0 | 24 | 3.3 | |
| Asian/Pacific Islander | 14 | 2.0 | 7 | 1.0 | |
| Other | 24 | 3.5 | 31 | 4.2 | |
| Education | |||||
| <High School | 91 | 12.4 | 133 | 18.1 | |
| High School Graduate | 203 | 27.7 | 260 | 35.3 | |
| Some College | 301 | 41.1 | 202 | 27.4 | |
| 4 year college degree< | 137 | 18.7 | 142 | 19.3 | |
| Relationship of Caregiver | |||||
| Spouse | 428 | 57.8 | |||
| Child | 110 | 14.9 | |||
| Other | 202 | 27.3 | |||
| Living Situation | |||||
| Living with the Patient | 524 | 71.0 | |||
| Not Living with the Patient | 214 | 29.0 | |||
| Cancer Type | |||||
| Lung | 383 | 51.6 | |||
| Colorectal | 359 | 48.4 | |||
| Cancer Stage at Diagnosis | |||||
| Local | 328 | 45.6 | |||
| Regional | 93 | 12.9 | |||
| Distant | 298 | 41.5 | |||
Numbers may not sum to 742 because of missing data.
Smoking Prevalence among Caregivers and Patients
The prevalence of smoking among patients and caregivers is shown in Table 2. Among caregivers, 24.5% of lung cancer caregivers and 19.7% of colorectal cancer caregivers reported being current smokers. Among patients, 18.6% of lung cancer patients and 12.2% of the colorectal cancer patients reported that they were current smokers. Patient and caregiving smoking were significantly associated in the lung (X2 (1)= 11.07, p<.001), but not the colorectal sample.In a large majority of dyads (71.9% of colorectal and 65.1% of lung), neither person currently smoked. There were 105 dyads (16.0% of colorectal and 17.0% of lung) where only the caregiver was smoking, 61 dyads (8.6% of colorectal and 10.5% of lung) where only the patient was smoking, and 35 dyads (3.5% of colorectal and 7.4% of lung) where both caregiver and patient were smoking.
Table 2.
Smoking descriptive information for patients and caregivers
| Caregivers | Cancer Patients | ||||
|---|---|---|---|---|---|
| Lung (n=383) |
Colorectal (n=359) |
Lung (n= 383) |
Colorectal (n=359) |
||
| Current Smoker | |||||
| Yes | 90(24.5%) | 69 (19.7%) | 62 (18.6)%) | 39 (12.2%) | |
| No | 278(75.5%) | 281(80.3%) | 272 (81.4%) | 281(87.8%) | |
| Missing | 15 | 9 | 49 | 39 | |
| History of Regular Smoking | |||||
| Yes | 213 (57.9%) | 169 (48.4%) | 312 (93.4%) | 212 (66.3%) | |
| No | 155 (42.1%) | 180(51.6%) | 22 (6.6%) | 108 (33.8%) | |
| Missing | 15 | 10 | 49 | 39 | |
Smoking data was not available for 47 lung cancer and 38 colorectal cancer patients because they completed a brief survey that did not include health behaviors.
Smoking in the Caregiver Context
Caregiver smoking and psychosocial adjustment
Descriptive statistics for the caregiver and patient outcome variables are shown in Table 3. In multivariate analyses statistically adjusting for gender, age, race/ethnicity, education, relationship with cancer patient, and cancer stage, colorectal cancer caregivers who smoked reported greater depression (b= 1.96, p<.05) and greater anxiety (b= .19, p< .05). Lung cancer caregivers who smoked also reported great anxiety (b= .23, p<.05) and marginally significant levels of depression.
Table 3.
Descriptive Statistics for Outcome Variables
| n | Mean | SD | Range | |
|---|---|---|---|---|
| Cancer Caregivers | ||||
| SF-12 PCS | 689 | 48.18 | 11.17 | 12.30–72.07 |
| SF-12 MCS | 689 | 47.47 | 10.74 | 8.80–69.86 |
| CESD-12 | 728 | 8.55 | 5.71 | 0–30 |
| Hopkins Anxiety (Mean) | 722 | 1.49 | .60 | 1–4 |
| Zarit Burden Inventory (Mean) | 723 | 2.04 | .90 | 1–5 |
| Caregiving Financial Strain | 689 | 2.57 | 1.08 | 1–5 |
| MOS Emotional Support (Mean) | 734 | 3.85 | 1.04 | 1–5 |
| MOS Instrumental Support (Mean) | 731 | 3.55 | 1.12 | 1–5 |
| MOS Informational Support (Mean) | 734 | 3.78 | 1.06 | 1–5 |
| Cancer Patients | ||||
| SF-12 PCS | 692 | 37.47 | 11.37 | 7.02–64.69 |
| SF-12 MCS | 692 | 50.58 | 11.13 | 7.46–74.01 |
Dyad smoking and caregiver psychosocial adjustment
When dyad smoking status, rather than caregiver smoking alone, was included as a predictor of caregiver outcomes, results indicated an interaction between patient and caregiver smoking status. There was a trend towards greater depression and anxiety scores among caregivers in mismatched dyads (either patient or caregiver smoked, but the other did not) reported when compared to caregivers in nonsmoking dyads (see Table 4), but the depression results did not reach statistical significance in the lung cancer sample. In addition, lung cancer caregivers whose care recipient continued to smoke when the caregiver did not also reported the highest levels of perceived caregiving burden and greater financial strain due to caregiving.
Table 4.
Patient-Caregiver Dyad Smoking Status as a Predictor of Caregiving Burden & Caregiver Distress
| Lung Cancer (n= 324) | Colorectal Cancer (n= 313) | ||||
|---|---|---|---|---|---|
| Estimated Marginal Means |
Comparison with Neither Smokes Group b (p) |
Estimated Marginal Means |
Comparison with Neither Smokes Group b (p) |
||
| Zarit Burden Inventory | |||||
| Neither Smokes | 1.92a | Ref | 1.84 | Ref | |
| Patient Smokes | 2.26ab | .34 (p=.04) | 2.06 | .22 (p=.24) | |
| Caregiver Smokes | 1.97b | .05 (p=.71) | 2.13 | .29 (p=.05) | |
| Both Smoke | 2.04ab | .11 (p=.57) | 1.61 | −.23 (p= .41) | |
| Financial Strain due to Caregiving | |||||
| Neither Smokes | 2.32a | Ref | 2.43 | Ref | |
| Patient Smokes | 2.83b | .51 (p=.02) | 2.50 | .07 (p=.76) | |
| Caregiver Smokes | 2.44ab | .12 (p=.49) | 2.65 | .22 (p=.23) | |
| Both Smoke | 2.78ab | .46 (p=.07) | 1.98 | .45 (p=.17) | |
| CESD-10 | |||||
| Neither Smokes | 7.79 | Ref | 7.25a | Ref | |
| Patient Smokes | 9.46 | 1.67 (p=.12) | 10.28b | 3.04 (p=.02) | |
| Caregiver Smokes | 9.44 | 1.65(p=.06) | 10.37b | 3.12 (p=.002) | |
| Both Smoke | 8.79 | 1.00 (p= .43) | 10.67ab | 3.42 (p=.07) | |
| Hopkins Anxiety | |||||
| Neither Smokes | 1.30a | Ref | 1.35a | Ref | |
| Patient Smokes | 1.58b | p=.01 | 1.63b | .28 (p=.03) | |
| Caregiver Smokes | 1.57b | p=.<.01 | 1.61b | .26 (p=.01) | |
| Both Smoke | 1.52ab | p=.11 | 1.62ab | .27 (p=.17) | |
Models adjusted for gender, race/ethnicity, age, education, timing of assessment, cancer site, cancer stage & relationship between patient and caregiver.
Means not sharing the same subscript differ from each other at the p <.05 level. There were no statistically different differences for MOS Instrumental and Informational Support.
Smoking Concordance and QoL in Caregivers and Patients
Mental health QoL
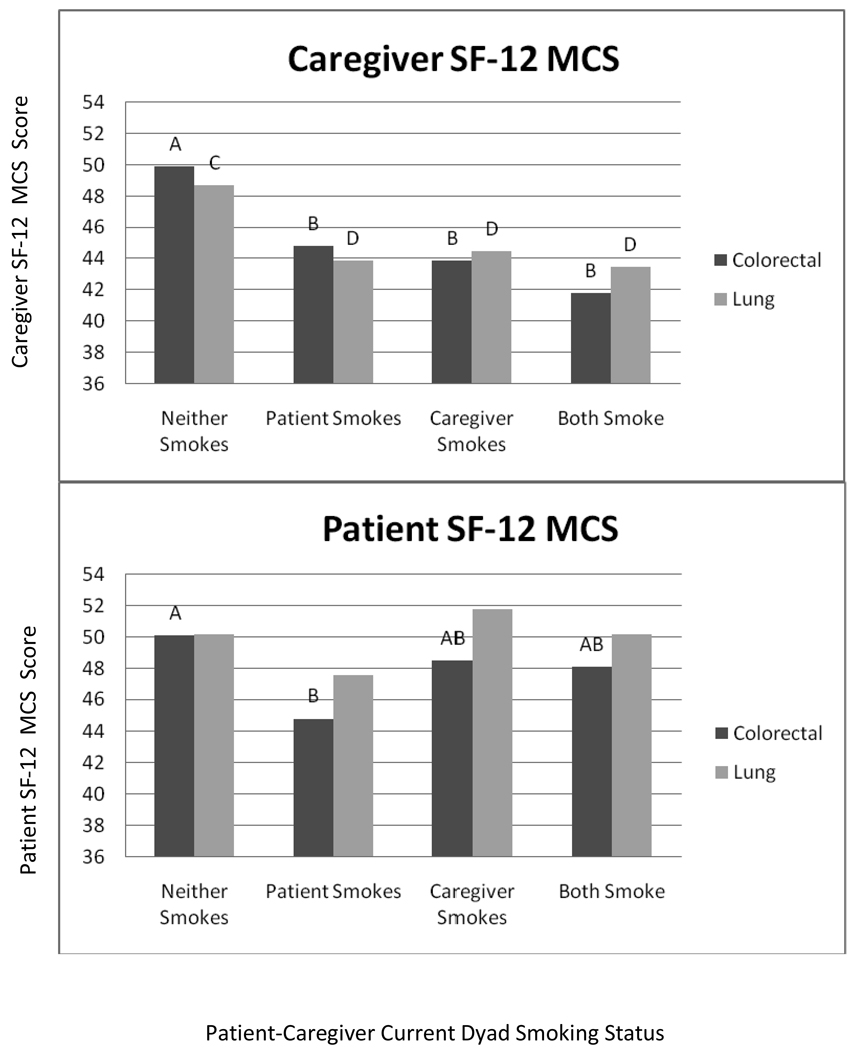
Smoking concordance (i.e., neither smoke, caregiver only smokes, patient only smokes, both smoke) was significantly associated with both caregiver and patient QoL in multivariate models after statistically adjusting for gender, age, race/ethnicity, education, cancer stage and relationship between cancer patient and caregiver (spouse, child, or other). As shown in Figure 1, both lung and colorectal caregivers in dyads where one or more members continued to smoke after cancer diagnosis reported lower mental health QoL compared to dyads where both members did not smoke. A different pattern emerged for patient mental health QoL. Colorectal cancer patients who smoked when their caregiver did not, reported the lowest mental health QoL, and were significantly different from the neither smoking dyads. A similar pattern was observed for lung cancer patients, but did not reach statistical significance.
Figure 1. Estimated Marginal Means for Short Form 12 Mental Components Scale for Lung and Colorectal Cancer Caregivers and Patients.
Models adjusted for gender, race/ethncity, age, education, timing of assessment, relationship between patient and caregiver, and stage of disease. Different letters (A/B for colorectal and C/D for lung) indicate groups that are significantly different (p<.05) from the other caregiver or patient groups. None of the lung cancer patient groups were significantly different (all p>.05)
Physical QoL
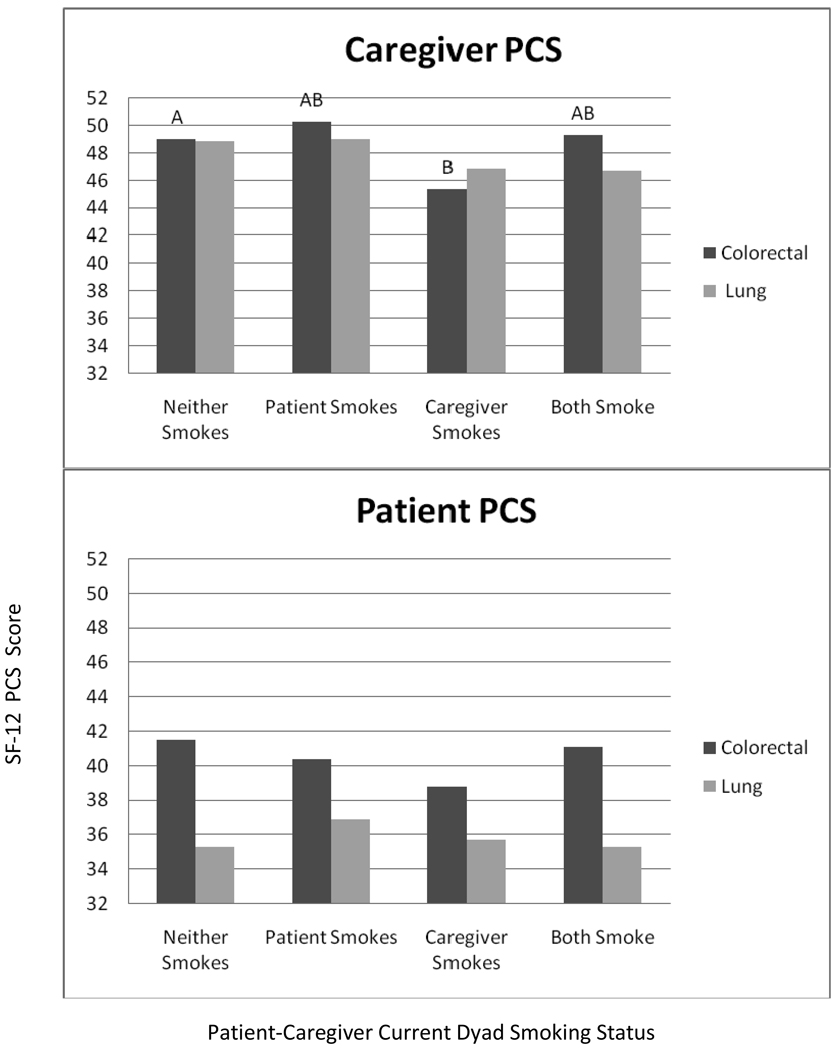
Dyad smoking appeared to be less strongly associated with physical QoL as compared to mental QoL for both patients and caregivers (see Figure 2). Colorectal caregivers who smoked, when their patient did not reported the lowest levels of physical QoL and were significantly different from the dyads where neither member smoked. There were no significant differences among the dyads for lung cancer caregivers or patient physical QoL.
Figure 2. Estimated Marginal Means for Short Form 12 Physical Components Scale Scores for Lung and Colorectal Cancer Caregivers and Patients.
Models adjusted for gender, race/ethncity, age, education relationship between patient and caregiver, and stage of disease. Different letters (A/B for colorectal) indicate groups that are significantly different (p<.05) from the other caregiver groups. There were no significant differences among lung cancer caregivers or both patient groups.
Although, mean mental QoL scores were similar between patients and caregivers, substantial differences between patients and caregivers emerged for the different smoking dyads. The smallest difference was for the neither smoking group, compared to differences of 6–8 points (more than half a standard deviation) for the caregiver only smoking and both smoking groups. In contrast, there were consistent differences between patients and caregivers for physical QoL that did not differ by dyad smoking. Patient physical QoL scores were generally nine to ten points lower than caregiver scores. Lung cancer patient scores were generally low (approximately 1.5 standard deviations below the population mean of 50 and 3–6 points lower than colorectal cancer patient scores) indicating that these patients may have been very ill at the time of the assessment. The effect sizes for dyad smoking group on mental QoL were larger than those for physical QoL. The largest mental QoL difference between dyad smoking groups was 8.1 points for caregivers and 5.3 points for patients. In comparison, the largest physical QoL difference between groups was 4.9 points for caregivers and 2.7 points for patients.
Discussion
To the best of our knowledge, this study is the first to report on associations between smoking concordance and QoL outcomes for both members of dyads including patients with cancer and their caregivers. Partially consistent with our hypotheses, smoking among lung and colorectal cancer caregivers was associated with greater emotional distress, but not caregiving burden and social support. These findings are consistent with the robust association between smoking and negative emotionality in the general population (for example see 12, 13, 29). Given the well-established links between smoking and cancer, the stress of caregiving for loved ones diagnosed with cancer becomes particularly relevant in relation to the smoking behavior of caregivers.
However, focusing on the smoking behavior of only caregivers provides only a partial picture of the relationship between smoking and well-being/QoL of caregivers. By examining patient-caregiver dyads, more complex relationships were revealed for both caregivers and patients. Lung and colorectal cancer caregivers who were members of dyads where one or both members continued to smoke, reported worse mental health QoL. Of note, this is not a simply a main effect for smoking, because caregiver mental health QoL was also lower in dyads where the caregiver did not smoke, but the patient did. For colorectal cancer patients, continuing to smoke when the caregiver did not was associated with worse mental health QoL. A similar pattern was observed for lung cancer patients, but did not reach statistical significance. It is interesting that the association between dyad smoking and patient mental QoL was stronger in the colorectal cancer sample, a disease that patients and caregivers may not associate with smoking. This may have been due to higher mental QoL among recent quitters (who likely comprised a larger proportion of the lung cancer patient sample) and or other unmeasured factors that might strongly influence mental QoL in lung cancer patients (e.g, stigma, self-blame, pain), In addition, we may have had limited power to detect differences among the some of the dyad subgroups, and dyad smoking misclassification bias may have been more of a problem in the lung cancer group because of increased smoking-related stigma. Indeed the self-reported smoking rate of 18.6% among lung cancer patients is lower than that observed in some recent studies of lung cancer patients after diagnosis (8, 30).
Negative affect and poorer mental QoL among dyads with one or more smoking member may be the result of guilt, blame, or other negative cognitions about continued smoking or diminished partner-specific social support resulting from negative dyad interactions regarding smoking. These results contrast with recent studies reporting that smoking behavior was not an independent predictor of psychological distress in either lung cancer patients or their spouses(19) or mental or physical QoL in family caregivers of women with lung cancer (18). Of note, these prior study did not examine interactions between patient and caregiver smoking, as we did in the current study.
Dyadic concordance in smoking behavior showed a different pattern of associations with physical QoL. Colorectal cancer, but not lung cancer caregivers who smoked, but whose partner did not reported worse physical QoL. There were also no significant differences in patient physical QoL among the smoking dyad groups. This is surprising due to the effect of smoking on multiple medical disorders including pulmonary diseases and cardiovascular disease and contrasts with prior research linking persistent smoking after lung or colorectal cancer with poorer QoL and increased symptoms(31, 32). Inconsistent findings may result from use of generic rather than disease specific quality of life measures or use of total QoL scores, rather than subscales. In addition because patient physical QoL scores were generally lower, a floor effect may have limited our ability to detect associations between smoking and QoL. This may have been especially true for the lung cancer patient group.
Strengths of the study include the inclusion of patients and their caregivers from a large national cohort that is broadly representative of US patients with lung and colorectal cancers(33) and collection of data regarding smoking status and QoL from both cancer patients and caregivers. The principal limitation of this study was its cross-sectional nature. The baseline survey was conducted 4–5 months post-diagnosis when many patients may have still been receiving cancer treatment or in the early stages of post-treatment recovery. Physical and emotional distress may be heightened during this period and this may impact the generalizability of our results. In addition, we could not track changes in smoking status over time (i.e., relapse or cessation) which may have increased or decreased dyad distress. Future research should assess smoking over time to better understand the longitudinal association between dyad smoking and patient/caregiver QoL. Other limitations include the restricted data available on smoking history, including the lack of information on recent quit attempts, when the individual quit smoking (prior to their own or their care-recipients’ cancer diagnosis or after), or smoking-related cognitions (e.g, motivation, beliefs about smoking and cancer) for either the cancer patient or caregiver. In addition, cancer patients with more advanced disease may have been excluded from this sample if they were too ill to complete their own survey or identify an informal caregiver. Finally, biochemical verification of current smoking status was not available. Smoking is a stigmatized behavior, especially among cancer patients, and under-reporting may have underestimated the true effect size.
The rate of smoking among cancer caregivers (22.1%) in this study is slightly higher than national rates of smoking among US adults (20.3%) (34) in 2005. Our caregivers were predominantly middle age females, suggesting that these rates may be even higher than expected from the general population (19.2% for women 45–64 years of age)(35). Even if cancer caregivers are not smoking at higher rates, they may represent a vulnerable population because they may share similar environmental exposures as well as negative health behaviors of the cancer patient for whom they are providing care, and in some cases (where children care for parents) even similar genetic makeup. In addition, the stresses of caregiving may place caregivers at increased risk for chronic disease and death(36, 37).
More research is needed to determine if providing care for a loved one with cancer is a “teachable moment” for smoking cessation among cancer caregivers and what cessation interventions might be most effective in helping them quit. Health professionals working with cancer patients and their family members have the opportunity to provide a clear message about the importance of smoking cessation for patients and caregivers and to provide either a brief clinical intervention (as described in Treating Tobacco Use and Dependence(38) Clinical Practice Guidelines) or a referral for more in depth smoking cessation services. In addition, clinician should be aware that smoking among cancer caregivers may be a marker of greater perceived caregiving burden and increased distress. Referrals for psychological or social work services may be helpful to these families as they navigate the stresses of cancer diagnosis and treatment. Overall, the results of this study suggest that smoking among lung and colorectal cancer patients and their informal caregivers has implications for the QoL of both dyad members. Being a member of a dyad where either member is smoking is associated with worse mental QoL for lung and colorectal cancer caregivers. For colorectal cancer patients, smoking when the caregiver is not is associated with worse mental QoL. Longitudinal research is needed to confirm these associations across time and to better understand the cognitive and social mechanisms by which mismatch in smoking status might influence QoL in cancer patients and their caregivers and the role of these interactions on the ultimate health and survival of both.
Acknowledgments
We would like to thank the CanCORS Caregivers and Publications committees for their thoughtful comments on this manuscript. The views expressed represent those of the authors and not necessarily those of the Department of Health and Human Services, National Institutes of Health, or National Cancer Institute.
This work from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Dana Farber Cancer Institute/Cancer Research Network U01 CA093332, Harvard Medical School/Northern California Cancer Center U01 CA093324, RAND/UCLA U01 CA093348, University of Alabama at Birmingham U01 CA093329, University of Iowa U01 CA01013, University of North Carolina U01 CA093326) and by a Department of Veteran’s Affairs grant to the Durham VA Medical Center U01 CDA093344 (MOU) and HARQ 03-438MO-03).
Footnotes
There are no financial disclosures from any of the authors.
The response options for the SF-12 question about pain was changed to match the response options of the other items to streamline the response options and reduce respondent burden. We sought statistical consultation and decided to use the original scoring algorithm, as the number of response options was the same, albeit the descriptors were different.
References
- 1.US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. 2004. [Google Scholar]
- 2.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Smoking Cessation and Continued Risk in Cancer Patients (PDQ) Health Professional Version. 2008 Available from: URL: http://www.cancer.gov/cancertopics/pdq/supportivecare/smokingcessation/healthprofessional.
- 4.Gritz ER, Demark-Wahnefried W. Health behaviors influence cancer survival. J Clin Oncol. 2009;27:1930–1932. doi: 10.1200/JCO.2008.21.3769. [DOI] [PubMed] [Google Scholar]
- 5.Wein RO. Preoperative smoking cessation: impact on perioperative and long-term complications. Arch Otolaryngol Head Neck Surg. 2009;135:597–601. doi: 10.1001/archoto.2009.33. [DOI] [PubMed] [Google Scholar]
- 6.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer Control. 2003;10:325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- 7.Cooley ME, Sarna L, Brown JK, et al. Tobacco use in women with lung cancer. Ann Behav Med. 2007;33:242–250. doi: 10.1007/BF02879906. [DOI] [PubMed] [Google Scholar]
- 8.Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 9.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 10.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 11.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40:702–711. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Laje R, Berman J, Glassman A. Depression and nicotine: Preclinical and clinical evidence for common mechanisms. Current Psychiatry Reports. 2001;3:470–474. doi: 10.1007/s11920-001-0040-z. [DOI] [PubMed] [Google Scholar]
- 13.Kahler CW, Daughters SB, Leventhal AM, et al. Personality, psychiatric disorders, and smoking in middle-aged adults. Nicotine and Tobacco Research. 2009;11:833–841. doi: 10.1093/ntr/ntp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride CM, Pollak KI, Garst J, et al. Distress and motivation for smoking cessation among lung cancer patients' relatives who smoke. J Cancer Educ. 2003;18:150–156. doi: 10.1207/S15430154JCE1803_08. [DOI] [PubMed] [Google Scholar]
- 15.Schulz R, Beach SR, Lind B, et al. Involvement in caregiving and adjustment to death of a spouse: findings from the caregiver health effects study. Jama. 2001;285:3123–3129. doi: 10.1001/jama.285.24.3123. [DOI] [PubMed] [Google Scholar]
- 16.Burton LC, Newsom JT, Schulz R, Hirsch CH, German PS. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26:162–169. doi: 10.1006/pmed.1996.0129. [DOI] [PubMed] [Google Scholar]
- 17.Schilling A, Conaway MR, Wingate PJ, et al. Recruiting cancer patients to participate in motivating their relatives to quit smoking. A cancer control study of the Cancer and Leukemia Group B (CALGB 9072) Cancer. 1997;79:152–160. doi: 10.1002/(sici)1097-0142(19970101)79:1<152::aid-cncr22>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Sarna L, Cooley ME, Brown JK, et al. Quality of life and health status of dyads of women with lung cancer and family members. Oncol Nurs Forum. 2006;33:1109–1116. doi: 10.1188/06.ONF.1109-1116. [DOI] [PubMed] [Google Scholar]
- 19.Carmack Taylor C, Badr H, Lee J, et al. Lung Cancer Patients and Their Spouses: Psychological and Relationship Functioning Within 1 Month of Treatment Initiation. Ann Behav Med. 2008;36:129–140. doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnoll RA, Malstrom M, James C, et al. Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns. 2002;46:137–145. doi: 10.1016/s0738-3991(01)00157-4. [DOI] [PubMed] [Google Scholar]
- 21.Bottorff JL, Robinson CA, Sullivan KM, Smith ML. Continued family smoking after lung cancer diagnosis: the patient's perspective. Oncol Nurs Forum. 2009;36:E126–E132. doi: 10.1188/09.ONF.E126-E132. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Given BA. Quality of life of family caregivers of cancer survivors: across the trajectory of the illness. Cancer. 2008;112:2556–2568. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- 23.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 24.van Ryn M, Sanders S, Kahn K, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology. 2010 doi: 10.1002/pon.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware JE. A 12 item short form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Bedard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: A New Short Version and Screening Version. 2001:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 27.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 28.Lipman RS, Covi L, Shapiro AK. Hopkins Symptom Checklist (Hscl) - Factors Derived from the Hscl-90. J Affect Disord. 1979;1:9–24. doi: 10.1016/0165-0327(79)90021-1. [DOI] [PubMed] [Google Scholar]
- 29.Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- 30.Cooley ME, Sarna L, Kotlerman J, et al. Smoking cessation is challenging even for patients recovering from lung cancer surgery with curative intent. Lung Cancer. 2009;66:218–225. doi: 10.1016/j.lungcan.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard CM, Courneya KS, Stein K. Cancer Survivors' Adherence to Lifestyle Behavior Recommendations and Associations With Health-Related Quality of Life: Results From the American Cancer Society's SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 33.Catalano P. Representativeness of CanCORS Participants Relative to Surveillance, Epidemiology, and End Results (SEER) Cancer Registries. 2008 [Google Scholar]
- 34.National Center for Health Statistics. Health, United States, 2007. 2009. [PubMed] [Google Scholar]
- 35.Schoenborn C, Adams P. Health Behaviors of Adults: United States, 2005–2007. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Centter for Health Sttistics; 2010. [Google Scholar]
- 36.von Kanel R, Mausbach BT, Patterson TL, et al. Increased Framingham Coronary Heart Disease Risk Score in dementia caregivers relative to non-caregiving controls. Gerontology. 2008;54:131–137. doi: 10.1159/000113649. [DOI] [PubMed] [Google Scholar]
- 37.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. Jama. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 38.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update US Public Health Service Clinical Practice Guideline executive summary. Respiratory Care. 2008;53:1217–1222. [PubMed] [Google Scholar]